Abstract
Background
The most often found complications in patients with breast cancer who received radiotherapy are cardiac and pulmonary function disorders and development of second malignancies.
Aim
To compare the intensity modulated radiotherapy with the 3D tangential beams technique in respect of dose distribution in target volume and critical organs they generate in patients with early-stage breast cancer who received breast-conserving therapy.
Materials and methods
A dosimetric analysis was performed to assess the three radiotherapy techniques used in each of 10 consecutive patients with early-stage breast cancer treated with breast-conserving therapy. Radiotherapy was planned with the use of all the three techniques: 3D tangential beams with electron boost, IMRT with electron boost, and intensity modulated radiotherapy with simultaneous integrated boost.
Results
The use of the IMRT techniques enables more homogenous dose distribution in target volume. The range of mean and median dose to the heart and lung was lower with the IMRT techniques in comparison to the 3D tangential beams technique. The range of mean dose to the heart amounted to 0.3–3.5 Gy for the IMRT techniques and 0.4–4.3 for the tangential beams technique. The median dose to the lung on the irradiated side amounted to 4.9–5 Gy for the IMRT techniques and 5.6 Gy for the 3D tangential beams technique.
Conclusion
The application of the IMRT techniques in radiotherapy patients with early-stage breast cancer allows to obtain more homogenous dose distribution in target volume, while permitting to reduce the dose to critical organs.
Keywords: Early-stage breast cancer, Radiotherapy, Breast-conserving therapy, Intensity modulated radiotherapy, Tangential beams technique
1. Background
Radiotherapy is an integral component of breast-conserving therapy in patients with early-stage breast cancer. In these cases, radiotherapy has the effect of reducing locoregional failures in 70% of patients and increasing disease-free survival rates (in 85–90%).1,2
This favourable influence on treatment results leads to improved survival. Therefore, the aim of therapy should also be to minimise the risk of complications which may develop in critical organs. In patients with early-stage breast cancer, the critical organs in radiotherapy are: lungs, heart, left anterior descending coronary artery (LAD) and contralateral breast. The most often found complications in these patients are cardiac and pulmonary function disorders and development of second malignancies.3–6
Cardiac complications may develop after 10 years following radiotherapy and they are most frequently observed in women with left-sided breast cancer.2,7–12 These complications cause a 30% increase in cardiovascular deaths after the period of 10 years following radiotherapy.7
Other complications related to radiotherapy are those affecting the lungs. Pulmonary complications are confined to antero-lateral peripheral (subpleural) region of the lung on the irradiated side. They are usually divided into early and late complications. Immediately after radiotherapy, patients may develop radiation pneumonitis which later evolves into lung fibrosis.3
The lower risk of complications connected with radiotherapy correlates with reduction of dose and irradiated volume in critical organs.11–13 This effect has been observed for radiotherapy techniques using 3D CT-based dosimetry methods (conformal radiotherapy, intensity modulated radiotherapy (IMRT), moderate deep inspiration breath hold (mDIBH)).4,14–16 At the same time, improvements in radiotherapy planning afford possibilities for obtaining more homogenous dose distribution in target volume.
Patients with early-stage breast cancer are at increased risk of second primary malignancies.3,12 The most often diagnosed are lung cancer, ovarian cancer, sarcomas and contralateral breast cancer.17–20 The development of second malignancies depends not only on radiotherapy but also on other carcinogenous factors.
2. Aim
The purpose of this paper is to compare the intensity modulated radiotherapy (IMRT) with the 3D tangential beams technique in respect of dose distribution in target volume and critical organs they generate in patients with early-stage breast cancer who received breast-conserving therapy.
3. Materials and methods
A dosimetric analysis was performed at the Oncology Centre in Cracow to assess the three radiotherapy techniques used in patients with left-sided early-stage breast cancer treated with breast-conserving therapy:
-
1.
3D tangential beams (covering the breast) with electron boost to the tumour bed;
-
2.
intensity modulated radiotherapy (IMRT) irradiation to breast with electron boost to the tumour bed;
-
3.
intensity modulated radiotherapy with simultaneous integrated boost (IMRT-SIB).
In each of 10 consecutive patients after breast-conserving therapy the postoperative radiotherapy was planned with the use of all three of the above techniques. All plans performed for linear accelerator Clicac 2300 C/D (applying of 6MV photon beam and 12 or 16 MeV electron beams) were prepared employing the Eclipse treatment planning system with Helio module.
Fig. 1 presents the arrangement of radiotherapy beams in the three techniques being compared.
Fig. 1.
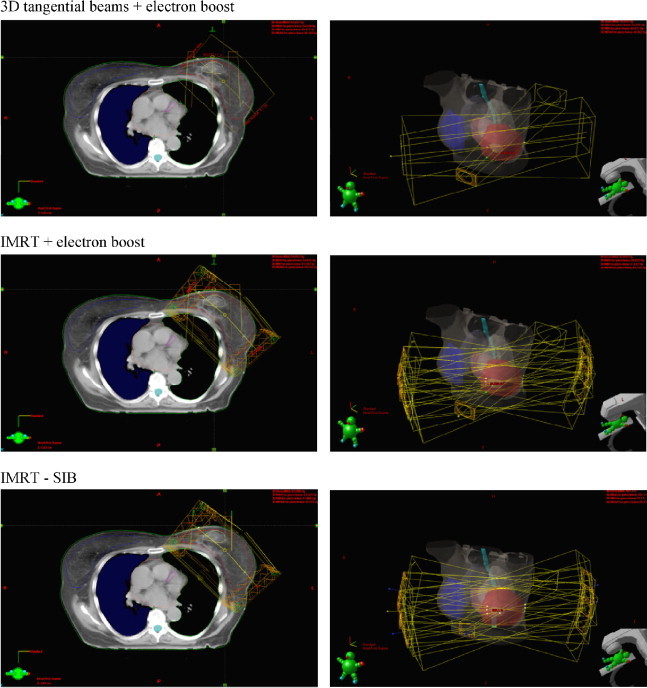
The arrangement of radiotherapy beams in three techniques used in postoperative treatment of patients with early-stage breast cancer treated with breast-conserving therapy.
The target volume (CTV) covered the breast with regard to the tumour bed. The margin of 1 cm was added to CTV to create PTV.
The critical organs for this analysis were: the heart, LAD, the lung on the irradiated side and the contralateral breast.
Table 1 presents the range and median volume of target, the contralateral breast and the other critical organs.
Table 1.
The range and median volumes of target, contralateral breast and critical organs in 10 consecutive patients with early-stage breast cancer treated with breast-conserving therapy.
| Anatomical structures | Volume [cm3] |
|
|---|---|---|
| Range | Median | |
| Irradiated breast (CTV) | 290.3–630.8 | 486.4 |
| Tumour bed (boost target) | 13.7–20.2 | 17.1 |
| Contralateral breast | 307.6–709.8 | 524.6 |
| Heart | 573.4–593.5 | 584.1 |
| LAD | 0.9–1.7 | 1.2 |
| Lung on irradiated side | 1131.0–1270.8 | 1206.4 |
| Contralateral lung | 1272.5–1677.2 | 1423.1 |
In all cases, the total dose was 42.5 Gy given to the whole breast with boost of 10 Gy given to the tumour bed. The fraction dose amounted to 2.5 Gy.
The energy and gantry of electron beams for boost were individually established depending on a patient's anatomy and localisation of the tumour bed in the breast.
The optimal radiotherapy plans were prepared on the basis of CT scans.
The dose values (range of mean and median) and dose distribution in target volume and critical organs were compared for the three radiotherapy techniques.
4. Results
Table 2 presents details of dosage and Fig. 2 shows the dose distribution in target volume for each of the three radiotherapy technique.
Table 2.
The range of mean and median doses to target volume in three techniques in 10 consecutive patients with early-stage breast cancer treated with breast-conserving therapy.
| Target volume | Radiotherapy techniques dose [Gy] (range/median) |
||
|---|---|---|---|
| 3D + electron boost | IMRT + electron boost | IMRT-SIB | |
| Breast (CTV) | 40.4–54.6 | 41.0–54.9 | 41.2–53.3 |
| 46.3 | 45.8 | 45.3 | |
| Tumour bed | 51.8–54.5 | 52.6–54.9 | 49.1–53.3 |
| 53.7 | 53.7 | 52.2 | |
Fig. 2.
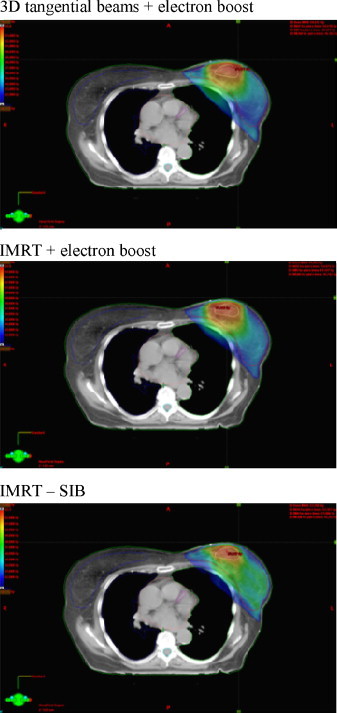
The comparison of dose distribution in target volume in three techniques used in postoperative treatment of patients with early-stage breast cancer treated with breast-conserving therapy.
The use of the IMRT techniques enables more homogenous dose distribution in target volume. Furthermore, the use of IMRT-SIB leads to shortening of the treatment time.
The information on doses in target volume and in critical organs in each of the three radiotherapy techniques used are presented in Table 3, while Fig. 3 presents the curves of dose-volume histograms (DVH).
Table 3.
The range of mean and median doses to contralateral breast and critical organs in three techniques in 10 consecutive patients with early-stage breast cancer treated with breast-conserving therapy.
| Critical organs | Radiotherapy techniques dose [Gy] (range/median) |
||
|---|---|---|---|
| 3D + electron boost | IMRT + electron boost | IMRT-SIB | |
| Contralateral breast | 0.1–1.8 | 0.0–1.4 | 0.0–1.5 |
| 0.4 | 0.3 | 0.2 | |
| Heart | 0.4–43.1 | 0.3–35.9 | 0.3–35.0 |
| 1.7 | 1.6 | 1.6 | |
| LAD | 0.7–7.4 | 0.6–8.0 | 0.7–7.6 |
| 2.7 | 3.0 | 2.8 | |
| Lung on irradiated side | 0.3–50.1 | 0.1–49.4 | 0.1–44.5 |
| 5.6 | 5.0 | 4.9 | |
| V 20 value (for lung on irradiated side) | 9–14% | 4–8% | 5–7% |
| 10.7% | 6% | 5.7% | |
| V 20 value (for both lungs) | 4–6% | 2–3% | 1–3% |
| 5% | 2.3% | 2% | |
Fig. 3.
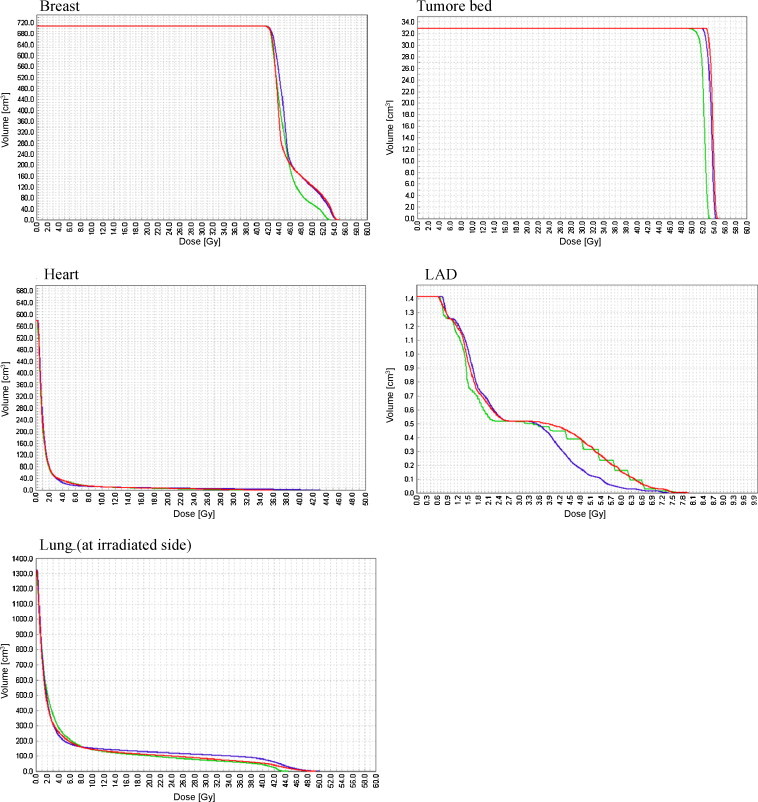
The comparison of dose-volume histograms (DVH) in target volume and critical organs in three techniques used in postoperative treatment of patients with early-stage breast cancer treated with breast-conserving therapy (3D tangential beams + electron boost, IMRT + electron boost, IMRT-SIB).
It was observed that the range of mean and median dose to LAD was similar in all the three radiotherapy techniques. However, the range of mean and median dose to the heart and the lung (on irradiation side) was lower with the IMRT techniques than with the classical 3D tangential beams technique. Especially marked was the difference observed in the value of V20 for the lung on the irradiated side. This median value amounted to 10.7% in the 3D tangential beams technique and 6% in the IMRT techniques. The medium value of V20 evaluated for both lungs amounted to 5% in the 3D tangential beams technique and 2–2.3% in the IMRT techniques.
Similar observation refers to doses received by the contralateral breast. For IMRT, the dose in this volume amounted to 0.2–0.3 Gy and was lower than that of the classical 3D tangential beams technique.
Table 4 presents the range of mean and median dose to 50% of volume of the contralateral breast and critical organs.
Table 4.
The range of mean and median doses to 50% of volume of contralateral breast and critical organs in three techniques in 10 consecutive patients with early-stage breast cancer treated with breast-conserving therapy.
| Critical organs | Radiotherapy techniques dose [Gy] (range/median) |
||
|---|---|---|---|
| 3D + electron boost | IMRT + electron boost | IMRT-SIB | |
| Contralateral breast | 0.3–0.4 | 0.2–0.3 | 0.2–0.3 |
| 0.3 | 0.2 | 0.2 | |
| Heart | 1.0–1.8 | 0.9–1.0 | 0.9–1.2 |
| 1.3 | 0.9 | 0.9 | |
| LAD | 2.6–3.1 | 1.9–3.0 | 1.6–3.1 |
| 2.9 | 2.3 | 2.4 | |
| Lung on irradiated side | 1.5–2.0 | 0.9–1.7 | 1.2–2.0 |
| 1.7 | 1.3 | 1.5 | |
These data show that the dose in 50% of volume of critical organs was lower in the IMRT techniques than in the classical 3D tangential beams technique. Especially marked were the differences concerning the heart and the lung on irradiation side.
5. Discussion
The role of postoperative radiotherapy in the treatment of patients with early-stage breast cancer has been identified and confirmed in some clinical studies.1 The results of metaanalysis performed by Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed that adjuvant radiotherapy has improved treatment effects in patients with breast cancer. This improvement is expressed by significant decrease in locoregional relapse and increase in overall survival rates.2,5 The therapeutic gain for postoperative radiotherapy amounts to 70% reduction of locoregional failures and 5.3% increase of survival.5
Patients with early-stage breast cancer receive radiotherapy after a breast-conserving surgery. The total dose given to the whole breast amounts to 50 Gy, and it is classically fractionated (average fraction dose 1.8–2.0 Gy, is given once-a-day, five days in a week). Furthermore, the boost is given to increase dose to the tumour bed. Data from clinical studies confirmed the necessity to apply boost in these patients. Clinical observations showed that majority of patients developed local relapse in the tumour bed.1,21 The results of EORTC study confirmed that the employment of boost had the effect of decreasing local relapse ratio from 7.3% to 4.3%.22
The average boost dose is 10–20 Gy and depends on diameter of the primary tumour and microscopic completeness of surgical therapy. The boost may be given using electron beams, brachytherapy or photon beams.1
Randomised EORTC study was performed to evaluate the impact of boost dose on reduction of local control. It was observed that local recurrences appeared in 10.8% of patients who received 26 Gy, whereas after the dose of 10 Gy, the incidence of failures was higher and averaged 17.5%. These differences were not statistically significant. However, in patients who received high boost dose, significantly more frequent occurrence of fibrosis in subcutaneous tissues was noted. This complication led to unfavourable cosmetic consequence.23
As already mentioned, in patients with early-stage breast cancer, radiotherapy with classical fractionation is usually employed both for breast irradiation and boost to the tumour bed.
In our study, patients received radiotherapy in a shortened overall treatment time (the fraction dose average of 2.5 Gy) under the study conducted on Polish population since 2003. In this study the whole breast dose amounts to 42.5 Gy, given in 17 fractions, and the boost dose to the tumour bed is increased by 10 Gy in 4 fractions. The results obtained up to date show the similar efficiency as in the case of 50 Gy given in 2-Gy fractions.24
Radiotherapy improves treatment outcome in patients with breast cancer, but is connected with a risk of complications. The most serious late side effects include cardiac and pulmonary complications and second malignancies localized in the lung or the contralateral breast.3,5
The cardiac complications occur over 10 years after treatment and are responsible for 30% increase in cardiovascular deaths in the period of more than 10 years after radiotherapy. These data apply to both breast-conserving therapy and post-mastectomy irradiation.7,10,12 These complications develop more frequently in women treated for left-sided breast cancer.2,8–11 In these patients the cardiac mortality ratio was 1.2 for up to 10 years, increased to 1.42 for the 10–14 years, and averaged 1.58 for over 15 years after radiotherapy.12 Radiation can damage the myocardium and endothelium of LAD, thus becoming responsible for development of cardiac dysfunctions. Injury to endothelium of coronary arteries leads to microcirculary disturbances and in consequence to changes in the myocardial perfusion.11,13,25–28 Radiotherapy dependent perfusion defects occurred 6 months after treatment and were observed in approximately 40% of patients within 2 years after treatment.28,29 The development of cardiac complications is related to the following factors: total dose, fractionation scheme, irradiated volume, patient age, earlier usage of cardiotoxic chemotherapy, as well as coexistence of diabetes and smoking habit, which promote the development of circulatory disturbances.11,28
The literature shows that the method of conducting radiotherapy (technique applied, arrangement of beans) can indirectly affect the development of cardiac complications. The most important factors are dose distribution, maximal dose in critical organs and dose per fraction.11–13,15,16
Pulmonary complications are the second important group of complications which may develop in patients treated for breast cancer. Immediately after radiotherapy, patients may develop radiation pneumonitis which later evolves into lung fibrosis in the irradiated volume. The clinical consequence of this complication is respiratory insufficiency. The estimation of lung volume which received a dose equal or higher than 20 Gy (value V20) is an important factor for minimizing the risk of complication. In patients with early-stage breast cancer the above changes are localized in the antero-lateral subpleural part of the lung on the irradiated side.3,4 The lowering of lung dose is possible with the use of improved radiotherapy techniques such as: tangential beams, conformal radiotherapy, moderate deep inspiration breath hold or intensity modulated radiotherapy.4,15,16,30
IMRT techniques afford possibilities for obtaining more homogenous dose distribution in target volume (breast and tumour bed) and at the same time reducing the dose in critical organs. In IMRT, the volume which receives lower dose is greater than in the other radiotherapy techniques.31–34 Reduction of acute reaction intensity is observed which, in turn, largely affects patients quality of life.33,35,36
Data published and own results confirm that IMRT allows to decrease dose to critical organs, especially to the lungs.37–39
We have observed that maximal and medium dose in the heart and the lung and V20 in the lung on the irradiated side and V20 evaluated for both lungs were lower for IMRT than for 3D tangential beams. Unfortunately, this effect was not found in relation to the dose received by LAD.
Apart from the influence on dose distribution in target volume and critical organs, IMRT also offers the reduction of dose to the contralateral breast.40,41
As suggested by published data, when IMRT was used we observed a decrease of maximal and medium doses to the contralateral breast.
Some authors suggest that using the IMRT techniques can increase the risk of second malignancies. This particularly concerns patients with early-stage breast cancer and refers to the risk of contralateral breast and lung cancers.6 It should be pointed out that using IMRT involves more treatment fields and, in consequence, larger volume of critical organs is exposed to lower doses. Moreover, in the IMRT technique the number of monitor units is increased which can have an impact on incidence of cancers localized in critical organs.17,22,42
It is believed that in patients with early-stage breast cancer, development of second malignancies depends on: age, genetic factors, exposition to radiotherapy as well as other carcinogens.18–20 In patients treated for early-stage breast cancer, the increased risk of second malignancies begins 5 years after therapy. The incidence after 10 years is 16%.18,19 The risk of lung cancer is 2 at 10 years and increases to 2.7 at over 15 years after radiotherapy.12
6. Conclusion
The application of the IMRT techniques in radiotherapy patients with early-stage breast cancer treated with breast-conserving therapy affords possibilities to obtain more homogenous dose distribution in target volume.
IMRT techniques have permitted to reduce the dose to critical organs, i.e. the heart, the lung (on irradiated side) and the contralateral breast.
References
- 1.Perez C.A., Taylor M.E., Bradley J., Mansur D., Sanchez-Aragon M.M. In: Principles and practice of radiation oncology. Perez C.A., Brady L.W., Halperin E.C., Schmidt-Ullirich R.K., editors. Lippincott Williams &Wilkins; Philadelphia: 2004. pp. 1331–1345. Breast tumors. [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trialsLancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 3.Senkus-Konefka E., Jassem J. Complications of breast-cancer radiotherapy. Clin Oncol. 2006;18:229–235. doi: 10.1016/j.clon.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Korreman S.S., Pedersen A.N., Aarup L.R., Nottrup T.J., Specht L., Nystrom H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2006;65(5):1375–1380. doi: 10.1016/j.ijrobp.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed R.S., de los Santos J.F., Fiveash J.B., Keene K.S., Popple R.A. An IMRT technique to increase therapeutic ratio of breast irradiation in patients with early-stage left breast cancer: limiting second malignancies. Med Dosim. 2008;33:71–77. doi: 10.1016/j.meddos.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Taylor C.W., McGale P., Darby S.C. Cardiac risks of breast-cancer radiotherapy: a contemporary view. Clin Oncol. 2006;18:236–246. doi: 10.1016/j.clon.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Harris E.E., Correa C., Hwanq W.T., Liao J., Litt H.I., Ferrari V.A. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 9.Giordano S.H., Kuo Y.F., Freeman J.L., Buchholz T.A., Hortobagyi G.N., Goodwin J.S. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszat L.F., Mackillop W.J., Groome P.A., Boyd C., Schulze K., Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in surveillance, epidemiology, and end-results cancer registers. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 11.Lind P.A., Pagnanelli R., Marks L.B., Borges-Neto S., Hu C., Zhou S.M. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys. 2003;55:914–920. doi: 10.1016/s0360-3016(02)04156-1. [DOI] [PubMed] [Google Scholar]
- 12.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registers. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 13.Gaya A.M., Ashford R.F.U. Cardiac complications of radiation therapy. Clin Oncol. 2005;17:153–159. doi: 10.1016/j.clon.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Hurkmans C.W., Cho B.C., Damen E., Zijp L., Mijnheer B.J. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62:163–171. doi: 10.1016/s0167-8140(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor C.W., Povall J.M., McGale P., Nisbet A., Dodwell D., Smith J.T. Cardiac dose from tangential breast cancer radiotherapy in year 2006. Int J Radiat Oncol Biol Phys. 2008;72:501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 16.Remouchamps V.M., Vicini F.A., Sharpe M.B., Kestin L.L., Martinez A.A., Wong J.W. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 17.Hall E.J., Phil D. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Galper S., Gelman R., Recht A., Silver B., Kohli A., Wong J.S. Second nonbreast malignancies after conservative surgery and radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:406–414. doi: 10.1016/s0360-3016(01)02661-x. [DOI] [PubMed] [Google Scholar]
- 19.Fowble B., Hanlon A., Freedman G., Nicolaou N., Anderson P. Second cancers after conservative surgery and radiation for stages I–II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 20.Gao X., Fisher S.G., Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast caner: a population study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 21.Slampa P., Ruzickova J., Ondrova B., Ticha H., Dolezelova H. Sole conformal perioperative interstitial brachytherapy of early stage breast carcinoma using high-dose rate aftreloading: longer-term results and toxicity. Rep Pract Oncol Radiother. 2008;13(2):62–68. [Google Scholar]
- 22.European Organization for Research and Treatment of Cancer Radiotherapy and Breast Cancer Groups Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;245:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 23.Poortmans P.M., Collette L., Horiot J.C., van den Bogaert W.F., Fourquet A., Kuten A. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol. 2009;90:80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Gałecki J., Hliniak A., Bujko K., Grudzień-Kowalska M., Hicer-Grzendowicz J., Załucki W. Wczesne wyniki pooperacyjnej radioterapii e skróconym czasie u chorych na raka piersi – badania populacyjne. Nowotwory. 2007;57:20–28. [Google Scholar]
- 25.Ziółkowska E., Woźniak-Wiśniewska A., Wiśniewski T., Makarewicz R., Simkiewicz W. Wpływ radioterapii na zaburzenia pracy serca. Współczesna Onkologia. 2009;13:16–21. [Google Scholar]
- 26.Gyenes G., Fornander T., Carlens P., Glas U., Rutqvist L.E. Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 1996;36:899–905. doi: 10.1016/s0360-3016(96)00125-3. [DOI] [PubMed] [Google Scholar]
- 27.Yu X., Prosnitz R.R., Zhou S., Hardenberg P.H., Tisch A., Blazig M.A. Symptomatic cardiac events following radiation therapy for left-sided breast cancer: possible association with radiation therapy-induced changes in regional perfusion. Clin Breast Cancer. 2003;4:193–197. [PubMed] [Google Scholar]
- 28.Hardenbergh P.H., Munley M.T., Bentel G.C., Kedem R., Borges-Neto S., Hollis D. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys. 2001;49:1023–1028. doi: 10.1016/s0360-3016(00)01531-5. [DOI] [PubMed] [Google Scholar]
- 29.Marks L.B., Yu X., Prosnitz R.G., Zhou S.M., Hardenbergh P.H., Blazing M. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Mayo C.S., Urie M.M., Fitzgerald T.J. Hybrid IMRT plans – concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys. 2005;61:922–932. doi: 10.1016/j.ijrobp.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Beckham W.A., Popescu C.C., Patenaude V.V., Wai E.S., Olivotto I.A. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys. 2007;69:918–924. doi: 10.1016/j.ijrobp.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 32.Vicini F.A., Sharpe M., Kestin L., Martinez A., Mitcheli C.K., Wallace M.F. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–1344. doi: 10.1016/s0360-3016(02)03746-x. [DOI] [PubMed] [Google Scholar]
- 33.Saibishkumar E.P., MacKenzie M.A., Severin D., Mihai A., Hanson J., Daly H. Skin-sparing radiation using intensity-modulated radiotherapy after conservative surgery in early-stage breast cancer: a planning study. Int J Radiat Oncol Biol Phys. 2008;70:485–491. doi: 10.1016/j.ijrobp.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 34.Morganti A.G., Cilla S., Valentini V., Digesu C., Macchia G., Deodato F. Phase I-II studies on accelerated IMRT in breast carcinoma: technical comparison and acute toxicity in 332 patients. Radiother Oncol. 2009;90:86–92. doi: 10.1016/j.radonc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Pignol J.P., Olivotto I., Rakovitch E., Gardner S., Sixel K., Beckham W. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:1–8. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 36.Bindhu J., Supe S., Pawar Y. Intensity modulated radiotherapy (IMRT) the white, black and grey: a clinical perspective. Rep Pract Oncol Radiother. 2009;14(3):95–103. [Google Scholar]
- 37.Horton J.K., Halle J.S., Chang S.X., Sartor C.I. Comparison of three concomitant boost techniques for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2006;64:168–175. doi: 10.1016/j.ijrobp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Donovan E., Bleakley N., Denholm E., Evans P., Gothard L., Hanson J. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82:254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Kestin L.L., Sharpe M.B., Fraizer R.C., Vicini F.A., Yan D., Matter R.C. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int J Radiat Oncol Biol Phys. 2000;48:1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 40.Bhatnagar A.K., Brandner E., Sonnik D., Wu A., Kalnicki S., Deutsch M. Intensity modulated radiation therapy (IMRT) reduces the dose to the contralateral breast when compared to conventional tangential fields for primary breast irradiation. Breast Cancer Res Treat. 2005;95:35–39. doi: 10.1007/s10549-005-9032-8. [DOI] [PubMed] [Google Scholar]
- 41.Borghero Y.O., Salehpour M., McNeese M.D., Stovall M., Smith S.A., Johnson J. Multileaf field-in-field forward-planned intensity-modulated dose compensation for whole-breast irradiation is associated with reduced contralateral breast dose: a phantom model comparison. Radiother Oncol. 2007;82:324–328. doi: 10.1016/j.radonc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Hall E.J. Second malignancies following radiotherapy. In: Levitt S.H., Purdy J.A., Perez C.A., Vijayakumar S., editors. Technical basis of radiation therapy. Practical clinical applications. Springer-Verlag; Berlin: 2008. pp. 33–38. [Google Scholar]


