Abstract
Background
Radical nephrectomy is the gold standard for treatment of renal cell carcinoma (RCC), but even for localized disease the survival rates are still unsatisfactory. Identification of prognostic factorsl is the basis for future treatment strategies for an individual patient.
Aim
The aim of our study was to assess the usefulness of the concentration of IL-6 and CRP as prognostic factors in patients after nephrectomy due to localized RCC.
Materials and methods
Our prospective study included 89 patients (55 men and 34 women) who had been surgically treated for RCC. The examined group included patients with localized advanced disease (from T1 to T3) with no metastases in lymph nodes (N0), and with no distant metastases (M0). All patients had blood samples drawn three times during the study (one day before surgery, six days after surgery and 6 months after surgery) to evaluate the concentration of CRP and IL-6. In each patient RCC of the kidney was removed during radical nephrectomy. Statistical analysis was conducted using statistica v.7.0.
Results
Statistically significant relationships were found between the concentration of CRP before the operation and OS (p = 0.0001). CRP concentration at baseline was statistically significantly correlated with CSS (p = 0.0004). The level of IL-6 assessed before the surgery was significantly correlated with survival times such as OS (p = 0.0096) and CSS (p = 0.0002). The concentration of IL-6 and CRP measured 6 days after surgery and 6 months after surgery were not statistically significantly correlated with survival times.
Conclusions
Results of our study showed that elevated levels of IL-6 and CRP in peripheral blood before surgery of RCC were correlated with worse OS and CSS.
Keywords: Renal cell carcinoma, Interleukin-6, C-reactive protein, Prognostic factors, Nephrectomy
1. Background
Renal cell carcinoma (RCC) accounts for about 3–4% of malignant neoplasms in adults. In Poland, in 2008, RCC was diagnosed in 2469 men and 1695 women. The number of deaths caused by the disease amounted to 1574 in men and 989 in women.1 Radical nephrectomy is the gold standard for treatment of RCC, but even for localized disease the survival rates are still unsatisfactory. Identification of good predictors of survival is the basis for future treatment strategies for an individual patient. Prognostic factors are universally utilized in the management of many cancers and take an important role in their treatment.2,3 Prognostic factors can allow in the future to establish more efficient supplementary methods of therapy for RCC. Generally, prognostic factors in RCC are divided into anatomical, histological and molecular. Many molecular factors have been examined in terms of their clinical usefulness in the course of RCC. Among them were carbonic anhydrase 9 (CA9), vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF), p53 protein, E-cadherin and many others. Unfortunately, none of them has been confirmed to be a reliable prognostic factor and they have not been used in daily clinical practice.4–6 The usefulness of other prognostic factors in RCC with potential clinical usability, such as IL-6 and CRP, is yet to be confirmed.
2. Aim
The aim of our study was to assess the usefulness of the concentration of IL-6 and CRP as prognostic factors in patients after nephrectomy due to localized RCC.
3. Materials and methods
89 patients (55 men and 34 women) aged from 30 to 81 years (mean age 60.08 ± 9.99 years) were included into this prospective study. All patients had been surgically treated for RCC in the Clinic of Urology and Urologic Oncology of the Poznan University of Medical Sciences from 2004 to 2005 year. All patients with primary renal tumor were diagnosed by ultrasonographic (US) examination and computer tomography (CT). The examined group included patients with localized advanced disease (from cT1 to cT3) without metastases in lymph nodes (cN0) and no distant metastases (M0). All patients had blood samples drawn three times during the study: one day before surgery as baseline, six days after surgery, and the last one six, months after surgery (Table 1). The patients’ performance status according to the Eastern Cooperative Oncology Group (ECOG) was 0 or 1 and none of them suffered from chronic inflammatory disease nor had undergone prior surgery hemodialysis, blood transfusion, radiotherapy, chemotherapy, and immunotherapy. In each case, the kidney removed during nephrectomy was histopathologically examined in the Department of Histopathology of the General Municipal Hospital in Poznan. To assess pathological tumor stage, the TNM classification from 2002 was used. All patients with histologically confirmed metastases to lymph nodes and/or pT4 stage were excluded from the study. Patient's clinical and pathological characteristics are presented in Table 2.
Table 1.
Blood tests results before surgery, after 6 days and after 6 months for 89 patients with RCC after radical nephrectomy.
| Variables | Baseline (prior surgery) | After 6 days | After 6 months | Significance level |
|---|---|---|---|---|
| N | 89 | 89 | 69 | |
| WBC | 7.48 (3.15–18.43) | 8.38 (3.2–15.4) | 7.48 (3.55–30.8) | 0.00294 |
| RBC | 4.57 (3.13–5.78) | 3.94 (2.77–4.91) | 4.70 (2.56–7.62) | <0.0001 |
| HGB | 8.29 (7.1–10.7) | 7.20 (7.5–12.4) | 8.65 (7.6–12.4) | <0.0001 |
| PLT | 259.60 ± 103.78 (78.0–651.0) | 313.39 ± 103.48 (121.0–583.0) | 242.16 (119.0–562.0) | <0.0001 |
| LDH | 346.36 (169.0–846.0) | 435.42 (208.0–895.0) | 34,280 (228.0–597.0) | <0.0001 |
| FE | 17.48 (3.5–39.0) | 7.66 (2.0–21.4) | 17.64 (3.1–44.5) | <0.0001 |
| CHOL | 5.24 (3.2–8.8) | 4.88 (3.0–9.4) | 5.84 (3.7–8.9) | <0.0001 |
| Cre | 84.58 (48.2–216.5) | 110.72 (55.3–331.7) | 105.71 (3.2–242.1) | <0.0001 |
| CRP | 19.13 (0.5–178.1) | 61.40 (11.4–301.6) | 6.53 (0.2–50.3) | <0.0001 |
| IL-6 | 11.63 (0.0–291.8) | 17.47 (0.0–135.8) | 3.59 (0.0–63.68) | <0.0001 |
Table 2.
Clinical and pathological characteristic of 89 patients with RCC after radical nephrectomy.
| Parameters | Value Number of patients N = 89(100%) |
|---|---|
| Gender | |
| Male | 55(62) |
| Female | 34(38) |
| Tumor stage | |
| pT1 | 47(53) |
| pT2 | 23(26) |
| pT3 | 19(21) |
| Tumor grade | |
| G1 | 27(30) |
| G2 | 47(53) |
| G3 | 15(17) |
| Mean age | 60.8 |
| Survival | |
| Alived | 74(83) |
| Deceased | 15(17) |
| Cancer related | 11(12) |
| Non-cancer related | 4(5) |
All patients were followed after the operation at the Urology Clinic in Poznan according to the following schema: first visit after surgery was at 1 month, then at 3-month intervals up to one year, and thereafter, at 6-month intervals up to the end of follow-up. During each visit, the following examinations were conducted: general examination, US of abdomen and/or CT, chest X-ray (once per year) and additional examinations in any case of suspected disease progression. CRP was measured using the rocket immunoelectrophoresis method with the established reference value of up to 5 mg/l. Quantitative analysis of human IL-6 was conducted with the use of the sandwich ELISA technique (Enzyme-Linked Immunosorbent Assay) with a complete analysis set by R&D Systems with the method sensitivity of 0.70 pg/ml. The test procedure was conducted according to the manufacturer's recommendations, and the results were analyzed using KC Junior software by Bio-Tek.
Statistical analysis was conducted using Statistica version 7.0 where the following tests were applied: Wilcoxon, Friedman and ANOVA tests. Additionally for survival times survival, curves were generated using the Kaplan–Meier method. The study was approved by the Bioethics Commission at the University of Medical Sciences, Poznań.
4. Results
During the follow-up, 4 patients died for reasons unrelated to cancer: myocardial infarction (n = 3), cerebral stroke (n = 1). On the other hand, death caused by development of RCC was observed for 11 patients. Statistically significant relationships were found between the higher concentration of CRP at baseline (before the operation) and shorter survival time (p = 0.0001). The higher level of IL-6 measured before the operation correlated with worst survival times (p = 0.0096), too. An analysis of IL-6 showed higher mean values in the group of patients with death related to RCC (23.8 units) in comparison with the group of living patients (9.2 units). In the group of patients who died due to RCC, mean CRP concentration at baseline was 49.7 units. On the other hand, mean CRP level in the group of living patients was 12.9 units. However, 3 patients in the group of 52 patients with initially normal results of CRP test died. The examination of IL-6 concentration before the operation showed that 9 (38%) patients of the group of 24 patients with an elevated level of IL-6 died, while in the group of 74 patients with normal results only 6 (9%) patients died. Longer OS time was demonstrated in the group of patients with RCC, in whom the CRP concentration before the beginning of therapy was normal, in comparison to the group of patients with an increased CRP concentration (log-rank test p = 0.001). Longer survival time was noted in the patients in whom the interleukin-6 level before the surgery was normal when compared to the group with initially elevated IL-6 level (log-rank test, p = 0.001) (Figs. 1 and 2).
Fig. 1.

Kaplan–Meier survival probability analysis in relation to the concentration of interleukin-6.
Fig. 2.

Kaplan–Meier survival probability analysis in relation to the concentration of C-reactive protein.
We analyzed also CSS depending on CRP and IL-6. Statistically significant correlations were found for both. A longer survival time was observed in the patients in whom CRP levels before the operation were normal when compared to the patients with an initially increased levels of the protein (log-rank test, p = 0.0004) (Fig. 3). A longer CSS time was found also in the patients with a normal initial IL-6 concentration as compared to the patients with IL-6 concentration above normal (Fig. 4).
Fig. 3.

Probabilities of carcinoma-specific survival for patients with localized RCC according to concentration of CRP (normal level versus higher level) prior to radical nephrectomy.
Fig. 4.
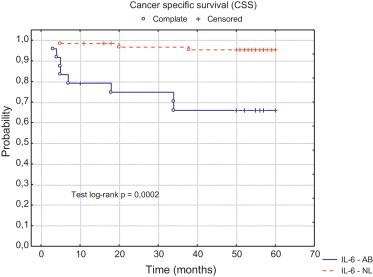
Probability of carcinoma specific survival for patients with localized RCC (normal level vs. abnormal level) according to IL-6 concentration prior radical nephrectomy.
In our analysis, there were no correlation between concentration of IL6 and CRP after surgery (6 days after surgery and 6 months after surgery) in terms of CSS and OS.
5. Discussion
RCC derived from epithelium of renal tubules accounts for about 90% of primary renal carcinomas in adults.7 It is a heterogeneous cancer and, in addition, the one with complex biology which does not allow to follow clearly the course of the disease; therefore, prognostication in many patients is very difficult. In most locally advanced tumors, recovery after radical nephrectomy or partial tumor removal in the case of small tumors, can be expected. Unfortunately, one has to be aware that studies demonstrate that distant metastases may develop in about 30% of these patients.8,9 Ongoing research attempts to identify factors that could help in a more precise determination of the course of RCC and enable to distinguish the groups of patients for whom complementary treatment should be offered. Therefore, maybe in a future, the ability to recognize the risk groups for the disease progression will allow to administer treatment adequate to the degree of disease development. At present, prognostic factors in locally advanced RCC can be divided into anatomical, histological, clinical and molecular. Clinical prognostic factors for OS in RCC are extensively discussed in the literature and come under review in most scientific papers.10 In most papers, the assessment of acute phase parameters in prognostication of RCC is limited to CRP and proinflammatory cytokines. In our present study, we focused on searching for prognostic factors that can help in prognosis in patients after nephrectomy due to locally advanced RCC. The study included results of assessment of CRP and IL-6 concentrations one day before the surgery at baseline. At present CRP is considered a sensitive indicator that suggests the presence of an inflammatory state and reflects its intensity. An increased CRP concentration is an undeniable evidence of a disease process. CRP monitoring seems to be a good diagnostic test of a disease process.11,12 In the last few years, assessment of CRP concentration has been carried out in numerous clinical studies on many diseases of inflammatory origin. The following disease entities can be named here: bone and joint inflammation, rheumatoid arthritis, psoriasis vulgaris, arthropathic psoriasis, endocarditis, Alzheimer's disease and eye diseases where increased concentration of CRP is observed in intraocular inflammation, and in patients with cellulitis, keratitis and uveitis.13 Increase CRP concentration has also been observed in the course of the neoplastic process. The available literature describes cases of an increased CRP concentration in such cancers as: breast, lung, ovarian or esophageal.14–19 In our study, we assessed prognostic factors (CRP and IL-6), in particular time periods in RCC patients. Distinctly higher levels of CRP and IL-6 were observed before the operation. Our study demonstrated that the lower CRP concentration before the operation was correlated with the longer survival times (OS, CSS) of RCC patients. In addition, the analysis conducted in the study showed that a higher IL-6 level before surgery was associated with shorter survival times (OS, CSS), too. So far, many studies have been conducted on RCC survival time. Lamb et al. showed on a group of 100 patients with RCC the influence of CRP concentration before the operation on the course of the disease and on CSS patients with RCC. They demonstrated that in patients with an initial CRP level above 10 mg/dl, CSS was 71 months versus 96 months in the group of patients with CRP below 10 mg/dl (p = 0.001).20 The same research team, two years later, conducted another analysis on a group of 60 patients with RCC. A relationship between CRP, IL-6, and expression of cyclooxygenase (COX-2), concentration of CD4+ and CD8+, T-lymphocytes and probability of CSS was analyzed. The association between CRP concentration and CSS and probability of recurrence-free survival was demonstrated.21 Masuda et al. in their study on a group of 111 patients found that CRP concentration is a negative prognostic factor in RCC dependent on TNM and Robson staging.22 Komai et al. conducted research on a group of 101 patients subjected to radical nephrectomy due to RCC (pT1-3N0M0). They assessed CRP concentration as a prognostic factor in RCC. An elevated CRP level (>0.5 mg/dl) was observed in 26 patients, of whom 12 (46%) and 3 of the other group of 75 patients died due to RCC. They demonstrated significantly lower survival in the group of patients with an increased CRP level than in the normal concentration of the protein (p < 0.001).23 Fujikawa et al. in their study suggested that a reduction in CRP level in response to cytoreductive nephrectomy and complementary immunotherapy predicts better prognosis.24
Another candidate as a prognostic factor for RCC patients, which deserves much attention, is IL-6. IL-6 is a pleiotropic cytokine with a wide range of activity, evident especially in the immune, hematopoietic and nervous system. Miki et al. on the basis of their research demonstrated that mRNA expression of IL-6, IL-6 secretive activity of tumor tissues and anti-IL-6 antibodies inhibiting growth of the tumor can be observed in freshly isolated tissues of RCC.25 Other research by Takenawa et al. shows that both primary RCC and RCC cell lines express mRNA of IL-6 and IL-6 receptor and that patients with increased expression have greater incidence of metastases to lymph nodes and an increased level of CRP.26 There are many reports proving the role of IL-6 as an autocrine growth factor in RCC. Blay et al. confirmed the role of IL-6 in RCC. They found that 48% of RCC patients had a considerably higher IL-6 level that correlated with an increased concentration of CRP.27 Tsukamoto et al. demonstrated that an increased level of IL-6 occurred in 25% of patients with RCC, while Hamao et al. observed an increase in IL-6 in 53% of cases.28,29 In the literature, there are also studies on the role of CRP assessed after nephrectomy in patients with locally advanced RCC. Johnson et al. of the Emory University in Atlanta (USA) monitored CRP concentration before nephrectomy due to clear-cell renal cell carcinoma and one year after the surgery on a group of 110 patients (T1-3N0M0). The multifactorial analysis revealed a statistically significant relationship between postoperative CRP level, T-stage and recurrence-free survival and OS (p < 0.001). The researchers demonstrated that postoperative CRP level can facilitate identification of patients with a high risk of progression more than preoperative level of the protein, which will allow to propose a complementary therapy with anti-angiogenic drugs.30 A study by Kamemoto on patients with clear-cell renal cell carcinoma (stage T1–2,N0M0) showed a normal level of IL-6 in blood serum. The concentration of serum IL-6 was higher in patients with advanced RCC (T3N0M0) and correlated with an increased CRP level.31
The problem of identification of prognostic factors in RCC, raised in many studies, seems to be of great importance. The ongoing research conducted in numerous scientific centers, allow clinicians to determine more and more precisely the most significant prognostic factors that influence the choice of therapy and ability to determine further prognostication. Our study is an attempt at emphasizing and presenting the role of the most important parameters that seem to play a role in prognostication in patients with clear-cell renal cell carcinoma. So, the conclusion we drew on the basis of the research conducted is that an increased preoperative levels of CRP and IL-6 are negative prognostic factors for OS and CSS in patients subjected to radical nephrectomy due to RCC. On the other hand, a decrease in the level of IL-6 and CRP in peripheral blood before nephrectomy due to locally advanced RCC may be a valuable diagnostic factor in later qualification of patients for complementary therapy.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Wojciechowska U, Didkowska J, Zatoński W. Nowotwory złośliwe w Polsce w 2006. [PubMed]
- 2.Gisterek I., Lata E., Halon A. Prognostic role of c-met expression in breast cancer patients. Rep Pract Oncol Radiotherapy. 2011;16:173–177. doi: 10.1016/j.rpor.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carman J., Strojan P. Nasopharyngeal carcinoma in Slovenia 1990–2003 (results of treatement with conventional two diemensional radiotherapy) Rep Pract Oncol Radiotherapy. 2012;17:71–78. doi: 10.1016/j.rpor.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G., Feng G., Gentil-Perret A., Genin C., Tostain J. Serum carbonic anhydrase 9 level is associated with postoperative recurrence of conventional renal cell cancer. J Urol. 2008;180(2):510–513. doi: 10.1016/j.juro.2008.04.024. discussion 513–4. [DOI] [PubMed] [Google Scholar]
- 5.Sabatino M., Kim-Schulze S., Panelli M.C. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(June (16)):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H., Ljungberg B., Grankvist K. Gene expression profiling predicts survival in conventional renal cell carcinoma. PLoS Med. 2006;3(January (1)):e13. doi: 10.1371/journal.pmed.0030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elis W.J. In: Epidemiology and etiology of renal cell carcinoma. Principles and practice of genitourinary oncology. Red. Raghavan D., Scher H.I., Leibel S.A., Lange, editors. Lippincott-Raven; Philadelphia, PA: 1997. [Google Scholar]
- 8.Ljungberg B. Prognostic markers in renal cell carcinoma. Curr Opin Urol. 2007;17:303–308. doi: 10.1097/MOU.0b013e328277f180. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey S., Lamb G.W., Aitchison M. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patient undergoing potentially curative resection for renal cancer. BJU Int. 2008;101(8):959–963. doi: 10.1111/j.1464-410X.2007.07363.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa I., Saito Y., Shikura N. Ten-year prospective study of the development of renal cell carcinoma in dialysis patient. Am J Kidney Dis. 1990;16:452–458. doi: 10.1016/s0272-6386(12)80058-8. [DOI] [PubMed] [Google Scholar]
- 11.Pepys M.B. C-reactive protein fifty years on. Lancet. 1981;317:653–657. doi: 10.1016/s0140-6736(81)91565-8. [DOI] [PubMed] [Google Scholar]
- 12.Mackiewicz A. Badanie mechanizmów regulujących glikozylację białek ostrej fazy. Immun Pol. 1989;14:103–127. [Google Scholar]
- 13.Szalai A.J., Agrawal A., Greenhough T.J. C-reactive protein: structural biology, geneexpression, and host defense function. Immunol Res. 1997;16:127–136. doi: 10.1007/BF02786357. [DOI] [PubMed] [Google Scholar]
- 14.Kato K., Hitsuda Y., Kawasaki Y. The value of serum C-reactive protein as a survival determinant in patients with advanced non-small-cell lung cancer. Nihon Kokyuki Gakkai Zasshi. 2000;38:575–580. [PubMed] [Google Scholar]
- 15.Kodama J., Miyagi Y., Seki N. Serum C-reactive protein as a prognostic factor in patients with epithelial ovarian cancer. Eur J Obste Gynecol Reprod Biol. 1999;82:107–110. doi: 10.1016/s0301-2115(98)00227-9. [DOI] [PubMed] [Google Scholar]
- 16.Nozoe T., Saeki H., Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 17.Nozoe T., Korenaga D., Futatsugi M. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus – significance as a tumor marker. Cancer Lett. 2003;192:89–95. doi: 10.1016/s0304-3835(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 18.Shimada H., Nabeya Y., Okazumi S. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003;83:248–252. doi: 10.1002/jso.10275. [DOI] [PubMed] [Google Scholar]
- 19.Zakrzewska I., Kozłowski L., Wojtukiewicz M. Ocena zmian stężeń interleukiny 6 i białka C−reaktywnego u chorych na nowotwory piersi. Pol Merk Lek. 2003;86:115–117. [PubMed] [Google Scholar]
- 20.Lamb G.W., McMillan D.C., Ramsey S. The relationship between the pre-operative systemic inflammatory response and cancer specific survival in patients undergoing potentially curative resection for renal clear cell cancer. Br J Cancer. 2006;94:781–784. doi: 10.1038/sj.bjc.6603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb G., McArdle P., Ramsey S. The relationship between the local and systemic inflammatory responses and survival in patients undergoing resection for localized renal cancer. BJU Inter. 2008;102(6):756–761. doi: 10.1111/j.1464-410X.2008.07666.x. [DOI] [PubMed] [Google Scholar]
- 22.Masuda H., Kurita Y., Fukuta K. Significant prognostic factors for 5-year survival after curative resection of renal cell carcinoma. Int J Urol. 1998;5:418–422. doi: 10.1111/j.1442-2042.1998.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 23.Komai Y., Saito K., Sakai K. Increased preoperative serum C-reactive protein level predicts poor prognostic in patients with localized renal cell carcinoma. BJU Int. 2007;99:77–80. doi: 10.1111/j.1464-410X.2006.06497.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujikawa K., Matsui Y., Oka H. Serum C-reactive protein level and the impact of cytoreductive surgery in patients with metastatic renal cell carcinoma. J Urol. 1999;162:1934–1937. doi: 10.1016/S0022-5347(05)68072-X. [DOI] [PubMed] [Google Scholar]
- 25.Miki S., Iwano M., Miki Y. Interleukin-6-functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 26.Takenawa J., Kaneko Y., Fukumato M. Enhanced expression of interleukin-6 in primary human renal cell carcinoma. J Natl Cancer Inst. 1991;83:1668–1672. doi: 10.1093/jnci/83.22.1668. [DOI] [PubMed] [Google Scholar]
- 27.Blay J.Y., Negrier S., Combaret V. Serum level of interleukin 6 as a prognostic factor in metastatic renal cell carcinoma. Cancer Res. 1992;15(52):3317–3322. [PubMed] [Google Scholar]
- 28.Tsukamoto T., Kumamoto Y., Miyao N., Masumori N., Takahashi A., Yanase M. Interleukin-6 in renal cell carcinoma. J Urol. 1992;148:1778–1782. doi: 10.1016/s0022-5347(17)37026-x. [DOI] [PubMed] [Google Scholar]
- 29.Hamao T., Kanayama H., Kan M. Serum level and gene expression of interleukin-6 and tumor necrosis factor – alpha in human renal cell carcinoma. Nippon Hinyoukika Gakkai Zasshi. 1994;85:563–570. doi: 10.5980/jpnjurol1989.85.563. [DOI] [PubMed] [Google Scholar]
- 30.Johnson T.V., Abbasi A., Owen-Smith A. Absolute preoperative C-reactive protein predicts metastasis and mortality in the first year following potentially curative nephrectomy for clear cell renal cell carcinoma. J Urol. 2010;183(2):480–485. doi: 10.1016/j.juro.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Kamemoto H. Significance of plasma interleukin-6 in the diagnosis of renal cell carcinoma. Hinyokika Kiyo. 1993;39:301–306. [PubMed] [Google Scholar]


