Abstract
Background
Hepatocyte growth factor plays an important role in tumor growth, metastasis and angiogenesis. C-met is HGF's high affinity receptor.
Aim
The aim of the study was to assess the correlations between c-met expression and clinic-pathological factors in breast cancer tissues. Furthermore, the purpose of the study was to evaluate the prognostic value of the hepatocyte growth factor receptor (HGFR, c-met) expressions in homogenous group of breast cancer patients.
Materials and methods
Tumor samples were collected from 302 patients with breast carcinoma treated with primary surgery. We have assessed the percentage of tumor cells with c-met expression, the intensity of reaction and the ratio of these two factors—immunoreactivity according to the Remmele score.
Results
We have observed no correlations between HGFR immunoreactivities and clinical parameters (tumor size, grade, axillary lymph node status, age). In 5-year observation we have found prognostic value of assessing c-met immunoreactivity in primary tumor.
Conclusion
Our study has revealed prognostic value of c-met. Unlike in other authors’ studies, our patients’ group is very homogenous which might contribute to obtained results.
Keywords: c-met, Breast cancer, Prognostic factor
1. Introduction
In breast cancer patients, tumor size and lymph node stage are two main prognostic factors.1 As a standard, the patient's age, cancer histological type, its malignancy grade, steroid receptor expression and expression of HER-2 receptor are included in the group of prognostic factors.2 The detection of selected immunohistochemical markers combined with the assessment of some pathoclinical features may separate the subgroup of patients with favorable prognosis.3
Hepatocyte growth factor (HGF) is a cytokine that plays an important role in tumor growth, invasion, metastasis and angiogenesis and c-met is HGF's high affinity receptor. Hypoxia promotes HGF's anti-apoptosis, invasion and metastasis activities via HIF-1α.4 Furthermore, c-met signaling is involved in the regulation of tumor angiogenesis, either directly through the proangiogenic activity of HGF that induces formation of new vessels and sprouting of the pre-existing ones or indirectly through the regulated secretion of angiogenic factors.5
The c-met receptor is expressed selectively in several normal human epithelial tissues as well as in carcinoma. The c-met encoded receptor is a 190-kilodalton (kDa) glycoprotein comprised of a transmembrane 145-kDa β subunit and an extracellular 50-kDa α subunit and belongs to the tyrosine kinase family of receptors.6
Several studies have shown that c-met has a prognostic role in breast cancer patients but their results are contradictory. The aim of this study was to assess the prognostic value of determining the c-met immunoreactivity in breast cancer tissue.
2. Materials and methods
Expression of c-met in 302 archival breast carcinoma specimens from patients with invasive breast carcinoma was evaluated using a standard immunoperoxidase technique. Formalin-fixed paraffin embedded, freshly cut 4 μm tissue sections were mounted on Superfrost slides (Menzel Glaeser, Germany), dewaxed with xylene, and gradually rehydrated. The sections were incubated with citrate buffer at 98 °C to unmask the epitopes and treated with 1% hydrogen peroxide (H2O2) for 10 min to block endogenous peroxidase. The sections were then incubated with human hepatocyte growth factor receptor mouse monoclonal antibody (from Novocastra Laboratories Ltd.). The sections were further incubated with biotin-labeled secondary antibody and streptavidin–biotin–peroxidase for 20 min each. The tissues were stained for 5 min with 0.05% 3.3′-diaminobenzidine tetrahydrochloride (DAB), counterstained with haematoxylin, dehydrated and mounted (Figs. 1–3).
Fig. 1.
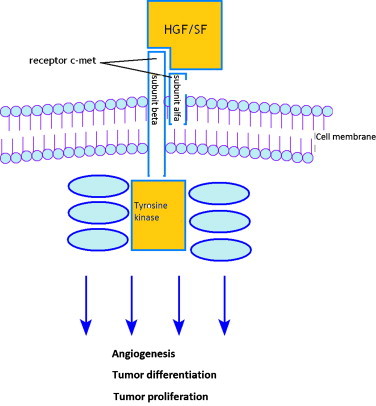
The pattern of activation of hepatocyte growth factor.
Fig. 2.
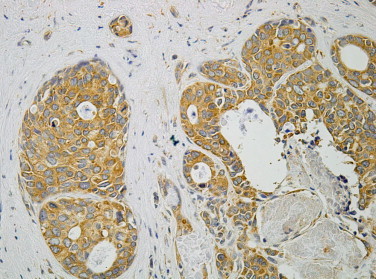
Immunohistochemical reaction with c-met antibody IRS = 12.
Fig. 3.

Correlation between c-met expression and OS.
Each case was assessed by calculating the percentage of cells with expression and intensity of reaction using the Remmele score.7
The age of the patients at the diagnosis ranged from 29 to 83 years (median 57 years). According to TNM system, T1 was recognized in 203 patients, T2 in 93 and higher stage of tumor in 7. Axillary lymph node metastases were diagnosed in 126 patients. Histologically, 217 out of 302 tumors were categorized as infiltrating ductal carcinomas and 55 as infiltrating lobular carcinomas. The expression of estrogen receptor was found in 193 patients and the expression of progesterone receptor was found in 187 (Tables 1 and 2).
Table 1.
Patients characteristics: stage of disease and G score.
| pT stage | pN stage | Stage of disease | G score | ||||
|---|---|---|---|---|---|---|---|
| T1 | 198 (65.57%) | N0 | 182 (60.27%) | DCIS | 3 (0.99%) | G1 | 31 (10.27%) |
| T2 | 90 (29.80%) | N1 | 54 (17.88%) | I | 131 (43.38%) | G2 | 172 (56.95%) |
| T3 | 2 (0.66%) | N2 | 42 (13.90%) | IIA | 75 (24.83%) | G3 | 90 (29.80%) |
| T4 | 9 (2.98%) | N3 | 24 (7.95%) | IIB | 21 (6.95%) | Gx | 9 (2.98%) |
| Tis | 3 (0.99%) | IIIA | 41 (13.58%) | ||||
| IIIB | 6 (1.99%) | ||||||
| IIIC | 25 (8.28%) | ||||||
Table 2.
Patients characteristics: expression of hormonal and HER2/neu receptors according to Remmele score.
| ER% | ER I | ER IRS | PgR % | PGR I | PgR IRS | HER2% | HER2 I | HER2 IRS | |
|---|---|---|---|---|---|---|---|---|---|
| No assess. | 5 | 5 | 5 | 5 | 5 | 5 | 21 | 21 | 21 |
| 0 | 106 | 106 | 106 | 111 | 111 | 111 | 53 | 53 | 53 |
| 1 | 32 | 37 | 21 | 35 | 20 | 10 | 18 | 76 | 15 |
| 2 | 44 | 126 | 29 | 38 | 114 | 30 | 24 | 96 | 13 |
| 3 | 77 | 28 | 1 | 63 | 52 | 7 | 74 | 56 | 30 |
| 4 | 38 | – | 24 | 50 | – | 21 | 112 | – | 34 |
| 6 | – | – | 70 | – | – | 56 | – | – | 39 |
| 8 | – | – | 21 | – | – | 23 | – | – | 42 |
| 9 | – | – | 8 | – | – | 13 | – | – | 4 |
| 12 | – | – | 17 | – | – | 26 | – | – | 51 |
The univariate significance of differences in the studied marker expressions was assessed by the chi-square test for binary or categorical covariates, by the Pearson test and by the Spearman rank correlation for ordered covariates. Cancer-specific overall survival (OS) was estimated using the Kaplan–Meier method, and the comparison between study groups was performed with a log-rank test. The survival time was measured from date of diagnosis to date of death or last follow up. In all tests, the significance level was set at 0.05 and all were two-sided tests. Statistical analyses were performed using the R CRAN version 2.10 (http://cran.r-project.org).
3. Results
Expression of c-met was not shown in 3% of patients, in 70% weak immunoreaction was observed and 27% exhibited strong immunoreaction. There was no correlation between c-met expression and tumor size, lymph node status, stage of disease or histological type of carcinoma. Moreover, we did not find any relations between the expression of hormone receptors, HER2/neu and c-met. Details are shown in Tables 3–6.
Table 3.
The correlation between c-met expression and tumor size.
| T1 vs T2 | T1 vs T3 | T1 vs T4 | T2 vs T3 | T2 vs T4 | T3 vs T4 | |
|---|---|---|---|---|---|---|
| c-met % | p = 0.72 | p = 0.74 | p = 0.85 | p = 0.77 | p = 0.98 | p = 1 |
| c-met I | p = 0.41 | p = 0.57 | p = 0.98 | p = 0.66 | p = 0.8 | p = 0.8 |
| c-met IRS | p = 0.61 | p = 0.8 | p = 0.9 | p = 0.9 | p = 95 | p = 1 |
Table 4.
The correlation between c-met expression and lymph node status.
| N0 vs N1 | N0 vs N2 | N0 vs N3 | N1 vs N2 | N1 vs N3 | N2 vs N3 | %N+ | |
|---|---|---|---|---|---|---|---|
| c-met % | p = 0.78 | p = 0.67 | p = 0.65 | p = 0.24 | p = 0.68 | p = 0.76 | p = 0.43 |
| c-met I | p = 0.54 | p = 0.87 | p = 0.45 | p = 0.34 | p = 0.67 | p = 0.23 | p = 0.35 |
| c-met IRS | p = 0.22 | p = 0.056 | p = 0.35 | p = 0.64 | p = 1 | p = 0.7 | p = 0.36 |
Table 5.
The correlation between c-met expression and stage of disease.
| c-met % | c-met I | c-met IRS | |
|---|---|---|---|
| I vs IIA | p = 0.3 | p = 0.91 | p = 0.4 |
| I vs IIB | p = 0.87 | p = 0.77 | p = 0.9 |
| I vs IIIA | p = 0.73 | p = 0.2 | p = 0.69 |
| I vs IIIB | p = 0.42 | p = 0.56 | p = 0.53 |
| I vs IIIC | p = 0.71 | p = 1 | p = 0.76 |
| IIA vs IIB | p = 0.43 | p = 0.85 | p = 0.55 |
| IIA vs IIIA | p = 0.55 | p = 0.34 | p = 0.3 |
| IIA vs IIIB | p = 0.65 | p = 0.62 | p = 0.83 |
| IIA vs IIIC | p = 0.34 | p = 0.96 | p = 0.45 |
| IIB vs IIIA | p = 0.71 | p = 0.6 | p = 0.89 |
| IIB vs IIIB | p = 0.41 | p = 0.55 | p = 0.56 |
| IIB vs IIIC | p = 0.91 | p = 0.83 | p = 0.94 |
| IIIA vs IIIB | p = 0.46 | p = 0.25 | p = 0.44 |
| IIIA vs IIIC | p = 0.59 | p = 0.37 | p = 0.94 |
| IIIB vs IIIC | p = 0.4 | p = 0.64 | p = 0.55 |
Table 6.
The correlation between c-met expression and expression of hormonal and HER2/neu receptors.
| ER | PgR | HER2/neu | |
|---|---|---|---|
| c-met % | p = 0.175 | p = 0.0357 | p = 0.588 |
| c-met I | p = 0.139 | p = 0.377 | p = 0.89 |
| c-met IRS | p = 0.0911 | p = 0.134 | p = 0.447 |
The correlation between c-met expression and patients’ 5-year overall survival established by the Kaplan–Meier method showed increased survival in patients with a high marker expression (p = 0.0089).
Furthermore, the multivariate analysis revealed that pT (p < 0.0001), stage (p = 0.041) and the expression of c-met (p = 0.013) were independent prognostic factors.
4. Discussion
In the majority of tumors deriving from epithelium, c-met overexpression is observed in the cytoplasm of cancer cells. In ductal carcinoma in situ (DCIS), Lindemann et al.8 observed the expression of c-met in both cancer and normal breast tissue. However, in the majority of histological specimens the expression of c-met protein was more intense in cancer compared to surrounding physiological mammary gland tissue, though in the quarter of cases the activity level was equal in both.
In the current study, the immunoreactivity for c-met protein was retrospectively evaluated in 302 patients, 3% of whom did not stain for c-met protein, in 70.5% only weak immunoreactivity was seen, and 26.5% showed a strong c-met expression. Our results were similar to those achieved by Lengyel et al.,9 who used the same scoring system, Remmele, evaluating c-met expression. In the group of 40 patients with breast cancer and axillary lymph node involvement, they showed weak immunoreactivity for this protein in 69% of patients and strong one in 31%. Camp et al.10 also evaluated the percentage of cells, as well as the intensity of c-met immunoreactivity, however, they used a scale other than Remmele. Their results were comparable with ours, as 25% and 75% of breast cancer tissues showed strong and weak immunoreactivity for c-met protein, respectively. In other reports describing the pattern of c-met expression in breast cancer tissue, only the percentage of cells with positive immunohistochemical staining for c-met was showed, and multifaceted cut-off limits were used.11,12 Immunohistochemical staining of breast cancer tissues performed by Carracedo et al.11 demonstrated the expression of HGF's receptor in only insignificant percentage of patients (65/168). In this study, the c-met receptor expression was observed slightly more often in the group of preinvasive cancers. Ghoussoub et al.13 divided patients with breast cancer only into two groups: those with weak c-met expression (immunoreactivity for c-met expression detected in less than 30% of cells) and strong c-met expression (more than 70% found positive for c-met protein) and compared them.
Other authors evaluating c-met expression in head and neck,5,14 lung,15 and esophagus cancer,4 examined only the percentage of cells stained and assumed different values for the intensity of c-met immunoreactivity.
The chosen method of immunoreactivity scoring and analysis for c-met in breast cancer tissue by assessing both the percentage of stained cells and the intensity of expression in accordance with the Remmele scoring system is very precise. It also provides much more information about the pattern of immunoreactivity. However, it limits the possibility of comparing our results with those yielded by other authors, as few evaluated both the percentage of stained cells and the intensity of expression.
In our study, we did not find any relation between c-met expression and tumor size, lymph node status, stage of disease or histological type of carcinoma. Most researchers have not found any relationship between the intensity of c-met expression and the acknowledged prognostic factors, such as tumor size, lymph nodes status or hormonal receptor expression.10,13,16–19 However, in a small group of 31 patients with invasive breast cancer, Greenberg et al.12 demonstrated a correlation between the intensity of c-met expression and tumor size: expression was observed in all tumors with more than 2 cm of diameter, whereas in only 50% of cases when tumor size was less than 1 cm in diameter. In patients with axillary lymph nodes metastases, c-met expression was observed in all specimens. However, only half of the patients with axillary lymph node involvement presented c-met positive staining.
Moreover, we did not find any relations between the expression of hormone receptors, HER2/neu and c-met. Lindemann et al.8 also did not observe the correlation between c-met and Her2/neu expression. In a considerably larger group of 200 patients diagnosed with DCIS, Gotte et al.5 showed a strong correlation between the intensity of c-met and Her2/neu receptor expression. Carracedo et al.11 demonstrated that c-met expression positively correlated with estrogen receptor expression and negatively with progesterone receptor expression.
The prognostic value of c-met expression was confirmed: significantly better survival was observed in patients with overexpression of this marker. This is in accordance with results yielded by Nakopoluou et al.,20 as he also showed better survival for patients with strong immunoreactivity for c-met. In opposition to our results stand other reports in which overexpression of c-met was associated with worse prognosis in patients with breast cancer. Ghoussoub et al.13 found a significant negative association between the c-met staining intensity and 5- and 10-year survival among patients with invasive breast cancer, the predictive value of this parameter was showed to be nearly equivalent to lymph node status. In a group of patients with invasive breast cancer without nodal involvement. In Lengyel et al.9 study overexpression of c-met was a statistically significant adverse prognostic factor (p = 0.045); 5 years DFS was 30% in patients with overexpression and 54% in those with weak staining for c-met, respectively. Camp et al.10 showed a decreased 5-year overall survival in patients with overexpression of c-met. Similar results were yielded by Parr et al.,19 who described a worse prognosis in patients with invasive breast cancer and overexperssion of c-met, during a median follow-up of 6 years. In unison with above mentioned reports, Ocal et al.18 described, based on 10-year follow-up, a worse prognosis in breast cancer patients with overexpression of c-met.
We could not compare directly the results of above-mentioned authors with ours because of the large differences in study methodologies. As far as patients are concerned, our sample is the biggest; compared with 40 patients in Lengyel et al.,9 113 patients in Camp et al.10 and 91 in Ghoussoub et al.13 Furthermore, Ghoussoub et al.13 divided their material in two subgroups: cases with <20–30% positive or >70–80% positive expressions of c-met; however, this division could not be accurate and univocal as the subgroup with 30% to 70% of cells with c-met positive reactivity may be assigned to both subgroups. On the other hand, in Camp et al.10 H-score was calculating by assessing the immunoreactivity of c-met in tumor and this score could not be compared with the Remmele score. In Lengyel et al.,9 the reactivity of c-met was assessed both in cells cytoplasm and cells membrane. For those reasons, we could not compare our results with other research works and further studies must be conducted to confirm the prognostic significance of c-met expression.
5. Conclusion
The expression of c-met in patients with invasive carcinoma of the breast is a strong, independent prognostic factor. It may be useful to identify a subset of patients with less aggressive disease.
Conflict of interest statement
None declared.
References
- 1.Tubiana M., Koscielny S. The natural history of breast cancer and the link between local recurrence and distant metastases: implications for therapy. Rep Pract Oncol Radiother. 2001;6:181–195. [Google Scholar]
- 2.Grodecka-Gazdecka S., Gryczka R., Musial M. Expression of selected markers in patients treated for breast cancer-based on our own data. Rep Pract Oncol Radiother. 2006;11:229–234. [Google Scholar]
- 3.Gisterek I., Frydecka, Swiatoniowski G. Tumour-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Rep Pract Oncol Radiother. 2008;13:205–208. [Google Scholar]
- 4.Tuyman J.B., Lagarde S.M., ten Kate F.J.W. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer. 2008;98:1102–1108. doi: 10.1038/sj.bjc.6604251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotte M., Kersting C., Radke I. An expression signature of syndecan (CD138) E-cadherin and c-met is associated with factors of angiogenesis and lymphangiogenesis in ductal breast carcinoma in situ. Breast Cancer Res. 2006;9:R8–R19. doi: 10.1186/bcr1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C.H., Moon S.K., Bae J.H. Expression of hepatocyte growth factor and c-met in hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2006;126:88–94. doi: 10.1080/00016480510037014. [DOI] [PubMed] [Google Scholar]
- 7.Remmele W., Stenger H.E. Vorschlag zum eine zur einheitlichen Definition eines Immunreaktiven Score (IRS) fuer den immunohistochemischen Oestrogenrezeptor-Nachweis (ER-ICA) im Mammarkarzinomgewebe. Der Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 8.Lindemann K., Resau J., Nahrig J. Differential expression of c-met, its ligand HGF/SF and HER2/neu in DCIS and adjacent normal breast tissue. Histopathology. 2007;51:54–62. doi: 10.1111/j.1365-2559.2007.02732.x. [DOI] [PubMed] [Google Scholar]
- 9.Lengyel E., Prechtel D., Resau J.H. c-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 10.Camp R.L., Rimm E.B., Rimm D.L. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer. 1999;86:2259–2265. doi: 10.1002/(sici)1097-0142(19991201)86:11<2259::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Carracedo A., Egervari K., Salido M. FISH and immunohistochemical status of the hepatocyte growth factor receptor (c-met) in 184 invasive breast tumors. Breast Cancer Res. 2009;11:R402–R403. doi: 10.1186/bcr2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg R., Schwartz I., Skornick Y. Detection of hepatocyte growth factor/scatter factor receptor (c-met) in axillary drainage after operations for breast cancer using reverse transcriptase-polymerase chain reaction. Breast Cancer Res. 2002;5:R71–R76. doi: 10.1186/bcr588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoussoub R.A.D., Dillon D.A., D’Aquila T. Expression of c-met is a strong independent prognostic factor in breast carcinoma. Cancer. 1998;82:1513–1520. doi: 10.1002/(sici)1097-0142(19980415)82:8<1513::aid-cncr13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Yucel O.T., Sungur A., Kaya S. c-Met overexpression in supraglottic laryngeal squamous cell carcinoma and its relation to lymph node metastases. Otolaryngol Head Neck Surg. 2004;130:698–703. doi: 10.1016/j.otohns.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Masuya D., Huang C., Liu D. The tumour-stromal interaction between intratumoral c-met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br J Cancer. 2004;90:1555–1562. doi: 10.1038/sj.bjc.6601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgibbons P.L., Page D.L., Weaver D. Prognostic factors in breast cancer College of American Pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 17.Gisterek I., Matkowski R., Suder E. Correlation between hepatocyte growth factor receptor and vascular endothelial growth factor-A in breast carcinoma. Folia Histochem Cytobiol. 2010;48:78–83. doi: 10.2478/v10042-010-0016-0. [DOI] [PubMed] [Google Scholar]
- 18.Ocal I.T., Dolled-Filhart M., D’Aquila T.G. Tissue microarray-based studies of patients with lymph node negative breast carcinoma show that met expression associated with worse outcome but is not correlated with epidermal growth factor family receptors. Cancer. 2003;97:1841–1848. doi: 10.1002/cncr.11335. [DOI] [PubMed] [Google Scholar]
- 19.Parr C., Watkins G., Mansel R.E. The hepatocyte growth factor regulatory factors in human breast cancer. Clin Cancer Res. 2004;10:202–211. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- 20.Nakopoluou L., Gakiopoluou H., Keramopoulos A. C-met tyrosine kinase receptor expression is associated with abnormal β-catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology. 2000;36:313–325. doi: 10.1046/j.1365-2559.2000.00847.x. [DOI] [PubMed] [Google Scholar]


