Abstract
Background
During a proper execution of dMLC plans, there occurs an undesired but frequent effect of the dose locally accumulated by tissue being significantly different than expected. The conventional dosimetric QA procedures give only a partial picture of the quality of IMRT treatment, because their solely quantitative outcomes usually correspond more to the total area of the detector than the actually irradiated volume.
Aim
The aim of this investigation was to develop a procedure of dynamic plans verification which would be able to visualize the potential anomalies of dose distribution and specify which tissue they exactly refer to.
Materials & methods
The paper presents a method discovered and clinically examined in our department. It is based on a Gamma Evaluation concept and allows accurate localization of deviations between predicted and acquired dose distributions, which were registered by portal as well as film dosimetry. All the calculations were performed on the self-made software GammaEval, the γ-images (2-dimensional distribution of γ-values) and γ-histograms were created as quantitative outcomes of verification.
Results
Over 150 maps of dose distribution have been analyzed and the cross-examination of the gamma images with DRRs was performed.
Conclusions
It seems, that the complex monitoring of treatment would be possible owing to the images obtained as a cross-examination of γ-images and corresponding DRRs.
Keywords: IMRT, QA, Portal dosimetry, Gamma Evaluation, Plan verification, DRR
1. Background
Although the IMRT was first implemented into the clinical practise several years ago and many papers have ever since been published on dynamic plans verification, it is still unclear why the local dose deviations between predicted and acquired dose distribution are observed.1 It is believed that only the pre-treatment control of actually generated fluence is able to provide essential information about the quality of irradiation. Usually, it is limited to dosimetric verification which is typically performed using the gamma evaluation method.2–4 As a result of the comparison between the acquired dose distribution and the predicted one, the matrix of is obtained. The measurement point passes the criteria of correctness if . A quantitative estimation of dose delivery is possible owing to γ-histograms which combine the information about a γ-index value with that of the area of corresponding part of the field.5,6 In our previous paper,7 we discussed the difference between the global gamma conception and its variety, local gamma, when the acceptable dose deviation (Dmax) was proportional to the expected dose value for each element of the calculated dose matrix . The advantages and disadvantages of both approaches have been presented.
Sometimes, quantitative outcomes of dynamic plans verification performed by commercial instruments (score, average gamma and γ-histograms) correspond to the total area of detector matrix or its regular, usually rectangular part, rather than the actually irradiated volume (field area). It makes the verification outputs hard to use and imprecise. See exemplary results presented in Fig. 1. The average gamma reported by the system for presented field is about 0.096, even though only a small number of points (marked in white colour) correspond to γ < 0.096. The underestimation of the parameter was possible, because the statistical report had been prepared for a total matrix of EPID and the unexposed points, which in fact do not belong to the field, had not been excluded. We believe that the key to obtain more reliable and useful outcomes of IMRT verification is to specify precisely the area of interest (define a border of therapeutic field).
Fig. 1.
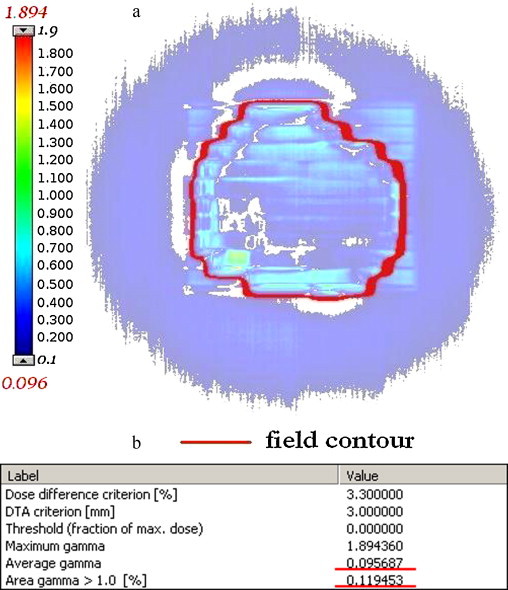
Exemplary results of gamma evaluation obtained with Portal Dosimetry (Varian). (a) γ-Image and (b) quantitative outcomes.
The conventional solution based on the gamma approach shows the level of local dose variations only, which is certainly very important from the dosimetric point of view, but is in fact unable to localise irregularities or specify what kind of tissue they refer to. It will be demonstrated in this paper that the fusion of γ-image (graphical representation of γ-matrix) and corresponding DRR for each field is a sufficient procedure to estimate the process of specified organs irradiation (especially target and organs at risk) during IMRT treatment.
2. Aim
The aim of this investigation was to develop an IMRT verification procedure based on the Gamma Evaluation. When developed, the procedure could demonstrate how the differences in actual and expected dose distribution correspond to the target volume and organs at risk. Precise specification of therapeutic field borders makes the quantitative outcomes of verification more reliable.
3. Materials and methods
In our radiotherapy department the gamma evaluation method was adapted to dynamic plans verification in 2002. The IMRT treatment plans are prepared using the Eclipse treatment planning system (Varian Medical Systems Inc., Palo Alto, CA) and Varian linear accelerators (2300CD and 23Ex) with Mark II 80MLC and Millennium 80MLC collimators are used for treatments. The gamma verification was executed for each therapeutic field and the portal dosimetry (Varian aS500) and films (Kodak X-Omat V) were applied for intensity fluence recording.8,9
The traditional comprehension of therapeutic field border (50% of maximum dose in a total plane perpendicular to beam axis following 3D CRTs example) could entail the loss of information about part of the IMRT field, because of the intentional diversification of dose distribution. To have an undistorted view of plan delivery, the (Dmin) criterion has to be reduced, however, it is difficult to define its correct value.
It must be emphasized that there is a difference between the method of determining a static field aperture and IMRT field, when dynamic mode of MLC is in operation. When the border of the static field is calculated, the system is looking for the farthest point from the centre of the field which accumulates no less than Dmin and no direction is preferred. To obtain the contour of the field generated by dMLC, the procedure is performed along the leaves direction only and much more regular shape is obtained (compare Fig. 2).
Fig. 2.
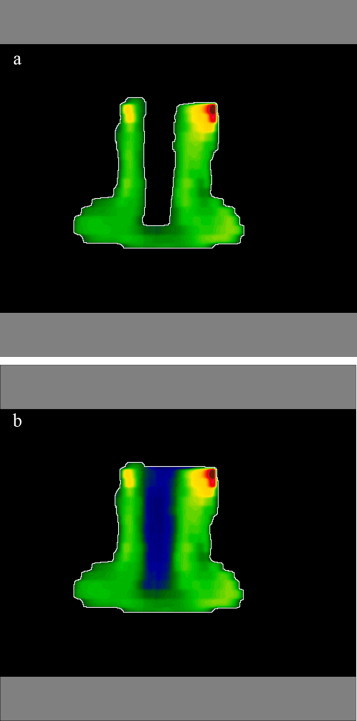
Therapeutic field border calculation: (a) static MLC (3D CRT) and (b) dynamic MLC (IMRT-sliding window).
Depending on the spatial dimension of the fluence which is being created, it is sometimes necessary to split the delivery into two or even three partially overlapping subfields. Verification of this kind of fields (MCG – multiple carriage group fields) is more complicated than single carriage group fields (SCG), especially when portal imager is in operation. The treatment management system (Varis/Aria in the case of Varian radiotherapy solution) stores the portal-acquired images whenever a beam-off signal occurs. Using the Portal Dosimetry workspace (Varian/Eclipse), examination can be performed only for each subfield independently. This approach could entail the loss of information about the resultant dose deviations in overlapping parts of the subfields. We propose to sum up dose distributions corresponding to each subfield to obtain a global image of a treatment field. The procedure has to be performed for both predicted and acquired portal images. The effective (measured) dose distribution is simply created by summing up the EPID readings for all subfields. However, when creating the predicted (resultant) portal image, only relative dose distributions are available for exporting from TPS. Due to the limitation, the contribution of separate subfields, which is simply proportional to the number of their MUs, must be taken into consideration when the expected dose distribution for a total therapeutic field is being calculated. The approach has been presented in Fig. 3.
Fig. 3.
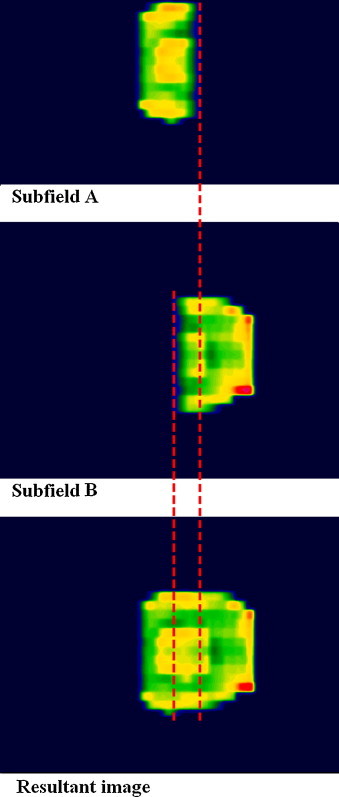
Superposition of portal-acquired images for Multiple Carriage Group Field.
Over 150 maps of dose distribution have recently been precisely analysed using gamma evaluation: 52 maps of SCG fields and 104 maps of MCG fields. To determine optimal value of the parameter defining a border of IMRT field, a set of gamma analyses has been performed for each map. We have been observing a correlation between results of γ-examination and the value of Dmin which has been changing from 50% down to 10% of maximal dose in the total plane perpendicular to the beam axis . The investigation let us come to the conclusion that Dmin should be valued for each field independently. The value depends on field characteristics (level of intensity modulation) and only one question has to be answered before the calculation starts: How many of all irradiated points in a total plane perpendicular to the beam axis have to be taken under consideration to get the full information about the quality of a dMLC field? What is the area of interest? We have assumed that no less than 80% (CR – cover ratio) of all irradiated points must be taken under consideration, to have a reliable overview of field execution.
First step of border drawing is to estimate the highest dose within the field – in our solution, only 2% of points exceed this value. The total area of the field S0 and its contour are obtained for , due to the disregard for the influence of noise. The value of Dmin is then successively increasing and the action is stopped when:
| (1) |
where S(Dmin) is the area of the field corresponding to Dmin and CR is defined arbitrarily by the user.
The comparison of the dose plane measured on the treatment unit with the expected dose distribution is performed on the self-made GammaEval software based on a gamma algorithm.7 Distance-to-agreement (DTA) and acceptable dose deviation (Dmax) are set at 3 mm and 3.3% of the local dose. As a result of the comparison, a 2-D matrix of γ-values is obtained for each field, which is typically to a coloured γ-image transformed and then the histograms, which combine the γ-index value with area of corresponding part of field, are created.
To estimate the dose accumulated by specified tissues and localise hypothetical irregularities (against a background of body structures), the cross-examination of the γ-image with DRR is performed.10 The algorithm employs the original DRRs which are created by the treatment planning system on the basis of patient's CT examination. The images are exported from the Eclipse using Dicom RT file format and have to be converted to ASCII before use. The GammaEval software then translates the γ-image into the isocentrical plane, where the DRR was created – see Fig. 4a.
Fig. 4.
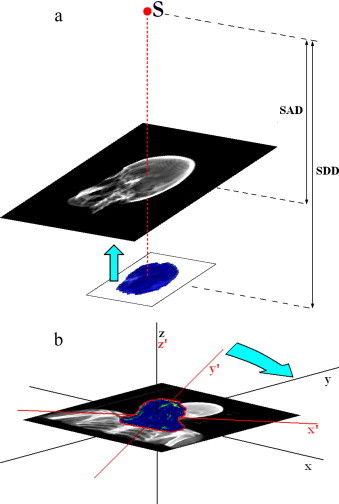
Transformation of DRR image. (a) Vertical translation: S – source, SDD – source-detector distance, SAD – distance from source to the plane where DRR image is created and (b) DRR rotation: x′, y′, z′ – gamma image’ coordination system and x, y, z – DRR image’ coordination system.
Due to a simplification, which is typically made during intensity fluence registration on a treatment unit (reset of collimator settings), the γ-image has to be rotated to stay in the same coordination system with a corresponding DRR (see Fig. 4b). As an optional result of verification the γ-diagrams used to be created. They present the correlation between γ-index value and the expected dose or the deviation of dose, which is observed.
4. Results
Our investigation shows that the quantitative outcomes of γ-examination are strongly determined by the value of dose, which defines the contour of the IMRT field. Regardless of the number of subfields, the results of verification are getting better while the value of Dmin is going higher, however, the outcomes obtained for single carriage group fields are always better than the results for multiple carriage group fields (Fig. 5). The ratio of field area, which does not pass the criteria of correctness (γ > 1) for different values of Dmin, is shown in Fig. 6. In the case of SCG fields, average results are always about 8% better than the outcomes obtained for MCG fields. We have observed for both types of dose delivery that the field area corresponding to γ = 1 is about 8% smaller for than for .
Fig. 5.
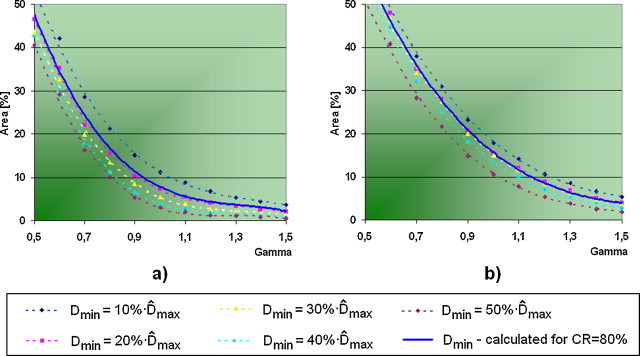
Cumulative histograms of γ-value vs. corresponding field area for different values of Dmin: (a) SCG and (b) MCG.
Fig. 6.
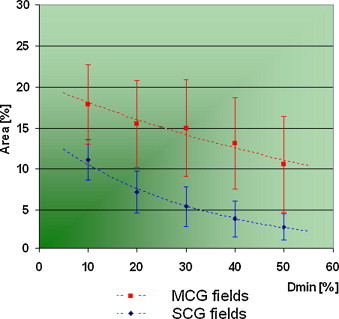
The ratio of field area which does not pass the criteria of correctness (γ > 1) for different values of Dmin (standard deviation outlined).
We have furthermore performed the verification using field border calculated independently for each field where CR = 80% was assumed – Fig. 7 presents averaged results obtained for this approach. We observed that the mean histogram of γ-value obtained, when the value of Dmin had been calculated independently for every field, looks similar to the average histogram obtained for (compare Fig. 5).
Fig. 7.
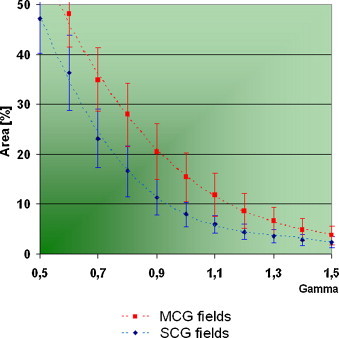
Average histograms obtained for Dmin calculated independently for each field (standard deviation outlined) – compare Fig. 5.
In our clinical practice, the IMRT technique is usually used for brain, head & neck and sometimes also for prostate treatments. Depending on the type of tumour and its volume the cross-examination of γ-images and DRRs would bring essential information about target irradiation and dose accumulated by critical organs situated very close to the tumour. Fig. 8 presents typical results of the fusion for the most frequently cured locations. When head (face region) or brain treatments are performed, not only the dose accumulated by eyeball, lenses and optical nerve needs to be controlled. Owing to the fusion-images, we have the possibility to monitor the dose absorbed by respiratory system, paranasal sinuses, glands and other non specified tissues (compare Fig. 8a–f).
Fig. 8.
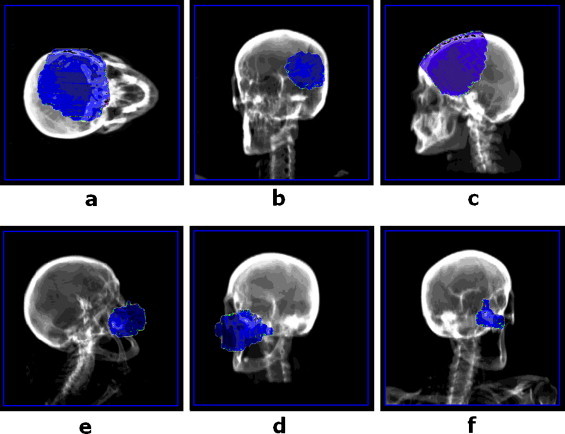
Examples of fusion images obtained for head treatments: (a–c) brain tumour; (d–f) face skin tumour.
The cross-examination of the images is a very useful instrument in the case of head & neck radiotherapy, when the dose to spinal-cord is a critical parameter of the treatment. Fig. 9 presents two examples of H&N treatments examination performed in our department. There was not a great difference between quantitative outcomes of γ-verification observed for them: about 20% of the total number of points within the field (calculated for ) have not passed the criteria of correctness. However, the cross-examination showed that the dose deviations were positioned in very different places. Due to the observation that the dose exceeds expected value only for the target and non critical tissues the treatment of “Patient B” could be initiated (compare Fig. 9b). Otherwise the γ-index will not provide the knowledge about the character of dose deviation, but will inform only of aberration's amplitude. The missing information is given by γ-diagrams which combine the value of the γ-index with the corresponding dose difference (Fig. 10).
Fig. 9.
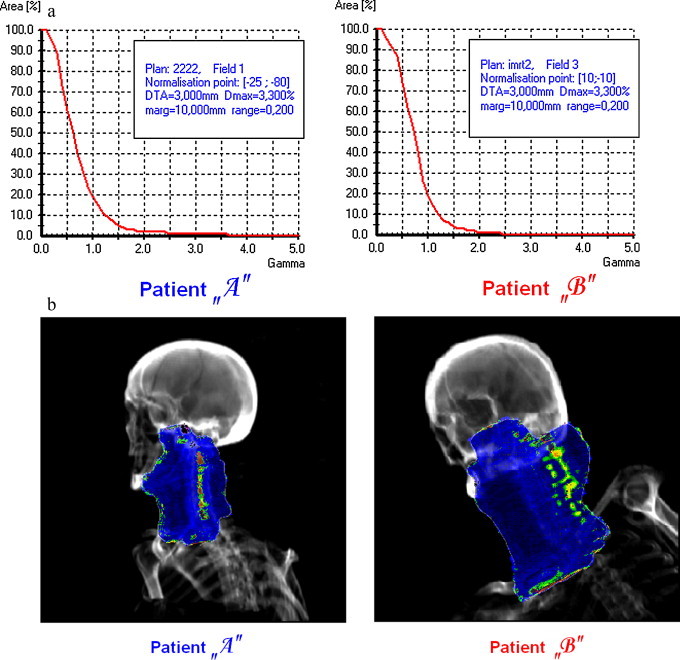
Exemplary results of verification obtained for two different H&N fields: (a) cumulative histograms of γ-index value and (b) the cross-examination of γ-image and the corresponding DRR.
Fig. 10.
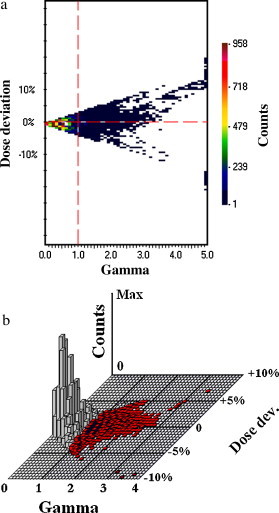
The γ-diagrams combining the γ-value with the corresponding deviation of dose: (a) 2D-view and (b) 3D-view.
When prostate treatment was performed (typically SCG fields), we observed that more than 90% of points within the field (for ) passed the criteria of correctness. Unfortunately we are afraid that the procedure described above is actually unable to sufficiently monitor the quality of prostate treatment, because the real position of the target and organs-at-risk (bladder) is not strictly limited to bone structures, which are visible in DRR. Similarly, the gamma-results obtained for the breast-trial IMRT usually do not give a clear idea of the precision of the treatment (consider Fig. 11a and 13b).
Fig. 11.
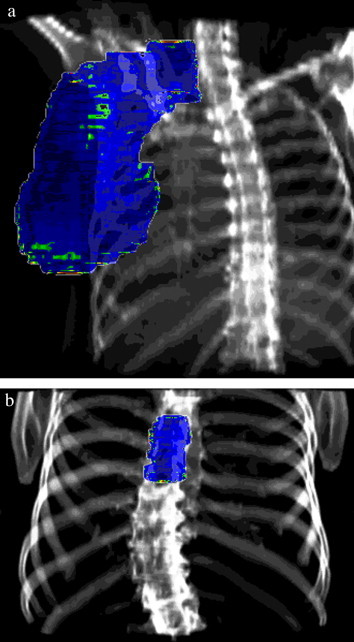
Exemplary results of verification obtained for other tumour locations: (a) breast and (b) spine.
Fig. 13.
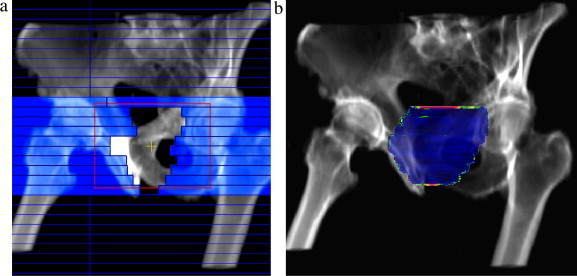
Example of prostate treatment: (a) screenshot of dMLC plan delivery – the leaves used for intensity modulation have been highlighted (the red lines represent the position of jaws) and (b) result of the verification – observe the irregularities in the boundary parts of the field.
The consequence of verification with the γ-parameter calculated locally7 are the high values of it usually obtained for points where a very low dose was expected (e.g. air cavities and discontinuities of the body). Dose deviations occur in this situation very often, firstly, because the leaves move faster to minimize the time of exposure and secondly – the big difference of speed between two adjacent leaves makes the “tongue and groove” effect much more possible. Fig. 12 presents an example of H&N treatment with a collimator rotation equal to 90°. Observe significant irregularities in the peripheral part of the field (left and right side of the field, above the collarbone). However, the quantitative outcomes of verification were relatively bad, the cross-examination of DRR and the γ-image showed that most of the errors were located outside the body and will not affect the success of radiotherapy.
Fig. 12.
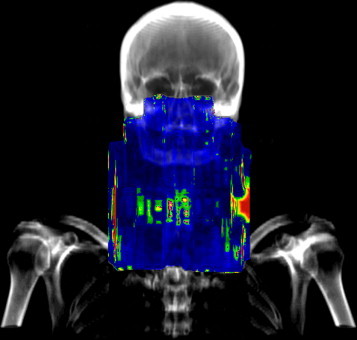
Results of verification obtained for H&N IMRT field with collimator rotation 90°: the irregularities observed outside the body contour.
A special attention has also to be paid when a field dimension defined by the position of jaws in the direction perpendicular to the leaves’ motion is smaller than the width of all leaves being employed to dynamic plan delivery. In order to reduce the dose leakage through a slit between adjacent leaves, the jaws are usually positioned as close as possible to the target. For that reason it is sometime observed that only a very narrow part of the marginal leaf (for example 2 mm) is exposed, the rest (8 mm) is covered by the jaw. We found that the “tongue and groove” effect modifies the local dose in the situation described above much more intensively, which has been presented in Fig. 13. However, the differences are observed only at the top and at the bottom of the field, very close to the its border, decreasing the field width.
5. Discussion
When the Sliding Window method is used, the diversification of dose distribution is obtained by smooth shifting of MLC leaves during the treatment. The local dose value is proportional to the time of exposure determined by opposite leaves position. It is reasonable to monitor the total operating range of MLC motion (including also low-dose regions placed in the central part of the field), firstly, because the low value of dose is strongly determined by actual accuracy of MLC (dosimetrical leaf gap, minimal physical distance between opposite leaves) and, secondly, because hypothetical irregularities in leaf sequence observed for low-dose regions influence the value of dose for high-dose parts of the field. Paradoxically, it is much easier for MLC to escalate the dose in a specified region (the velocity of leaves is then low and the notable distances between opposite leaves are observed) than to minimize it (the leaves go faster and very close to each other). However, while analysing the 3D CRT field, the low-dose parts of the field, typically shielded by blocks or static MLC, are excluded.
In the case of the MCG field, we suggest to perform the analysis for a global image obtained as a superposition of fluencies acquired for all subfields. Firstly, to make sure we do not lose any information about low-dose regions (they may be excluded when subfield by subfield verification is performed) and for the quantitative outcomes to become reliable. Secondly, the influence of dose leakage effect is much more significant for this area and produces additional dose for organs-at-risk, which has to be controlled (e.g. spinal cord). However, summing up of subfields has also disadvantages. Our investigation shows that the dose errors appear often at the position where the motion of the leaf starts and also very close to the place where it stops. In a hypothetical situation, one of the subfields in the junction area would generate a dose that is too low, while the other one would produce a dose that is too high. Analyzing these subfields separately would show differences, however summing them up leads to averaging errors and the gamma verification would unfortunately give good results.
Depending on tumour shape and its location the significant differences between a level of intensity modulation are observed. The static value of Dmin, arbitrarily chosen by the examiner, can be only set for simple IMRT fields, typically used for a small area of irradiation (e.g. part of the brain or prostate), when a quasi uniform dose in a total plane perpendicular to the beam axis is expected. Changing the value of Dmin in this case will not result in essential deviations in quantitative outcomes of verification, because all the contours obtained for different Dmin are close to each other and nearly the same area is analysed. Realizing this condition, we came to the conclusion that the field contour should always be calculated independently for each therapeutic field to take under consideration relatively the same number of points (compare Eq. (1)) and a required algorithm has been designed and developed.
6. Conclusion
As it was reported, the traditional dosimetric verification of dynamic plans, because of its quantitative outcomes, give only a partial picture of the quality of IMRT treatment. Complex monitoring of dose delivery process is possible by owing to images obtained as a result of the γ-bitmaps and corresponding DRRs cross-examination. There have been at least two patients treated in our department, when the presented solution allowed us to continue the radiotherapy, however the quantitative outcomes of gamma verification looked formally bad. Because the IMRT planning is a relatively time consuming procedure, a patient usually continues to be treated with a conventional 3D CRT plan, once the dosimetric verification of dynamic plan has failed.
The method is rather dedicated to head or head & neck treatments, because the typically irradiated volumes are strongly determined by the position of bone structures, which are usually presented on DRR. It can be performed regardless of whether portal dosimetry is available or not, however, the conventional film dosimetry is a very time consuming procedure and makes the verification of all therapeutic fields practically impossible.
Consider, that the presented algorithm is only the conception of treatment plan verification. It allows to estimate the dose accumulated by target and organs-at-risk during proper dMLC plan execution. The procedure assumes no significant changes in patient's anatomy (CT examination conformity) and, this way, it can be only used as an additional source of information during plan-to-treatment acceptation activity. We are currently investigating the proceedings which will allow to combine the map of the γ-index with the image acquired on board (OBI, EPID, CBCT) during the treatment, which is especially promising for irradiation of soft and moving tissues (e.g. the prostate).
References
- 1.Deng J., Pawlicki T., Chen Y., Li J.S., Jiang S.B., Ma C.M. The MLC tongue-and -groove effect on IMRT dose distributions. Phys Med Biol. 2001;46:1039–1060. doi: 10.1088/0031-9155/46/4/310. [DOI] [PubMed] [Google Scholar]
- 2.Depuydt T., van Esch A., Huyskens D.P. A quantitative evaluation of IMRT dose distributions: refinement and clinical assessment of the gamma evaluation. Radiat Oncol. 2002;62:309–319. doi: 10.1016/s0167-8140(01)00497-2. [DOI] [PubMed] [Google Scholar]
- 3.Low D.A., Harms W.B., Mutic S., Purdy J.A. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656–661. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 4.Low D.A., Dempsey J.A. Evaluation of the gamma dose distribution comparison method. Med Phys. 2003;30:2455–2464. doi: 10.1118/1.1598711. [DOI] [PubMed] [Google Scholar]
- 5.Spezi E., Levis D.G. Gamma histograms for radiotherapy plan evaluation. Radiat Oncol. 2006;79:224–230. doi: 10.1016/j.radonc.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Anjum M.N., Parker W., Ruo R., Afzal M. Evaluation criteria for film based intensity modulated radiation therapy quality assurance. Phys Med. 2010;26:38–43. doi: 10.1016/j.ejmp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Winiecki J., Morgaś T., Majewska K., Drzewiecka B. The gamma evaluation method as a routine QA procedure of IMRT. Rep Prac Oncol Radiother. 2009;14(5):162–168. [Google Scholar]
- 8.Dogan N., Leybovich L.B., Sethi A. Comparative evaluation of Kodak EDR2 and XV2 films for verification of intensity modulated radiation therapy. Phys Med Biol. 2002;47(22):4121–4430. doi: 10.1088/0031-9155/47/22/314. [DOI] [PubMed] [Google Scholar]
- 9.Roberson P.L., Moran M.M., Lulasekere R. Radiographic film dosimetry for IMRT fields in the near-surface buildup region. J Appl Clin Med Phys. 2008;9(4) doi: 10.1120/jacmp.v9i4.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winiecki J. Target and organ-at-risk dose estimation in case of IMRT treatment. Zesz Nauk WCO. 2009;6(S1) [Google Scholar]


