Abstract
Aim
To assess target volume coverage during prostate image-guided radiotherapy based on bony anatomy alignment and to assess possibility of safety margin reduction.
Background
Implementation of IGRT should influence safety margins. Utilization of cone-beam CT provides current 3D anatomic information directly in irradiation position. Such information enables reconstruction of the actual dose distribution.
Materials and methods
Seventeen prostate patients were treated with daily bony anatomy image-guidance. Cone-beam CT (CBCT) scans were acquired once a week immediately after bony anatomy alignment. After the prostate, seminal vesicles, rectum and bladder were contoured, the delivered dose distribution was reconstructed. Target dose coverage was evaluated by the proportion of the CTV encompassed by the 95% isodose. Original plans employed a 1 cm safety margin. Alternative plans assuming a smaller 7 mm margin between CTV and PTV were evaluated in the same way. Rectal and bladder volumes were compared with the initial ones. Rectal and bladder volumes irradiated with doses higher than 75 Gy, 70 Gy, 60 Gy, 50 Gy and 40 Gy were analyzed.
Results
In 12% of reconstructed plans the prostate coverage was not sufficient. The prostate underdosage was observed in 5 patients. Coverage of seminal vesicles was not satisfactory in 3% of plans. Most of the target underdosage corresponded to excessive rectal or bladder filling. Evaluation of alternative plans assuming a smaller 7 mm margin revealed 22% and 11% of plans where prostate and seminal vesicles coverage, respectively, was compromised. These were distributed over 8 and 7 patients, respectively.
Conclusion
Sufficient dose coverage of target volumes was not achieved for all patients. Reducing of safety margin is not acceptable. Initial rectal and bladder volumes cannot be considered representative for subsequent treatment.
Keywords: Prostate, Image-guided radiotherapy, Cone-beam computed tomography, Dose reconstruction, Dose-volume histogram
1. Background
Imaging technology drives advancement in radiation therapy. Different styles of image-guided radiation therapy (IGRT) are frequently discussed.1,2 Unpredictable prostate position variation is the challenge for comparison of different IGRT strategies. Techniques of patient setup relative to external beam's isocenter have developed during the last decade. Historically, skin marks and setup lasers have been used. These are not adequate surrogates for prostate position and require extensive safety margins, which are incompatible with the delivery of the high radiation doses above 70 Gy which are currently used in routine practice.3,4 Planar X-ray imaging techniques have enabled registration with skeletal anatomy, but recent studies have shown a poor correlation of prostate position and bony anatomy.5 Prostate location variations were studied relative to the adjacent bony anatomy by Schallenkamp et al.6 with the conclusion that a significant interfractional motion exists between the prostate and the pelvic bony anatomy. These move independently, therefore, the pelvic bony anatomy should not be used as a surrogate for prostate motion. Authors also suggest that fiducial markers are stable within the prostate and allow significant margin reduction when used for on-line localization of the prostate. The limited interuser variability and the marker stability make markers an ideal surrogate for the prostate position.4 Another promising way of prostate image guidance is the use of in-room CT – helical on-rail CT or cone-beam CT (CBCT) – which provides 3D anatomic information directly in irradiation position. Compared to fiducial markers detected with planar imaging, the acquisition time is longer with CBCT and patient is exposed to a significantly larger additional radiation dose. Also the 3D image registration is more difficult and time-consuming.
Substantial positional variation of prostate over a 1-h period is caused by a variety of factors. The most significant predictor for intrafraction prostate motion is the status of rectal filling.7 A full rectal state is invariably associated with mobile gas pockets responsible for elevated levels of prostate motion. While the apex is largely immobile, prostate motion is well described by rotation, but does undergo deformation due to rectal distension.8 Effects of rectal motion during prostate radiotherapy with regard to rectal dose and clinical target volume (CTV) dose coverage were studied by Sripadam et al.9 This study revealed instances of insufficient CTV coverage.
IGRT systems provide more information than is required for simple patient positioning. Utilization of cone-beam CT (CBCT) can provide 3D anatomic information directly in irradiation position. Such information enables reconstruction of a current dose distribution. CBCT was evaluated for treatment planning by Yoo and Yin10 and Yang et al.11 with the conclusion that CBCT could be used for verification planning to verify treatment delivery retrospectively.
Evaluation of the ‘dose of the day’ using post-treatment CBCT for IMRT prostate cancer patients with implanted markers was described by van Zijtveld et al.12 The actual IMRT fluence maps delivered to a patient were derived from measured EPID-images acquired during treatment. Retrospective IMRT dose reconstruction based on CBCT and MLC log-file was described by Lee et al.13
2. Aim
The aim of the present study was to utilize the CBCT scans acquired before treatment for dose reconstruction purposes and hereby to assess target volume coverage during prostate image-guided radiotherapy based on bony anatomy alignment.
3. Materials and methods
3.1. Patient characteristics
Seventeen patients with adenocarcinoma of the prostate staged T2a–T3b N0 M0 were evaluated. Patients were treated using intensity modulated radiotherapy (IMRT) to the prostate with simultaneous integrated boost to the proximal part of seminal vesicles. Daily bony anatomy image-guidance was performed based on 2 orthogonal kV images. In order to assess target volume coverage, CBCT scans were acquired once a week in the treatment position immediately after bony anatomy alignment.
3.2. Validation of dose calculation on CBCT
A Siemens Somatom Sensation CT scanner (Siemens Medical Solutions, Erlangen, Germany) was used for acquisition of CT images (512 × 512 matrix, 0.98 mm pixel size, 3 mm slice thickness). CBCT images were acquired using Varian On-board imaging system (OBI®, Varian Medical Systems, Palo Alto, CA) and reconstructed using about 700 images in a “half-fan” projection with a bowtie filter acquired over 360° rotation. For CBCT reconstruction, 45 cm diameter and 12 cm axial length with 3 mm slice thickness and 512 × 512 matrix was used. The technique used was 125 kV, 80 mA, 25 ms.
The method to reconstruct the actually delivered dose based on pretreatment CBCT was first validated using phantom measurements. Dose profiles were compared for CBCT and CT images of inhomogeneous antropomorfic RANDO® phantom (The Phantom Laboratory, Salem, NY, USA). The images were imported into the Eclipse™ treatment planning system, version 8.1 (Varian Medical Systems, Palo Alto, CA). Plans based on CBCT and CT images were generated using (1) one 10 cm × 10 cm field and (2) five coplanar IMRT fields. 6 MV photon energy was used. Dose calculations were performed by a pencil beam convolution algorithm with Modified Batho heterogeneity correction. The dose calculation grid used was 0.25 cm. Dose was normalized to the isocenter with a prescription of 2 Gy. The resulting dose distributions and depth dose profiles on the central beam axis were compared.
3.3. Radiotherapy planning and delivery
Intensity modulated radiotherapy (sliding window technique) with five coplanar fields to the prostate plus the proximal 2/3 of seminal vesicles was planned and delivered. CT slices of 3 mm thickness were acquired. Patients were scanned and treated in a supine position with Dual Leg Positioner (Civco Medical Solutions, IA, USA) to immobilize their legs and pelvis.
The patients were instructed to empty their rectum before the planning CT as well as before each irradiation session. Patients also obtained glycerin suppositories, but their application was voluntary. Application of suppositories was strongly recommended only when the planning CT had to be repeated by reason of rectum volume. The planning CT scan was repeated in case the rectum volume exceeded 120 cm3 (for organs at risk delineation see below). Patients were instructed to have their bladders slightly filled at the time of planning CT. This can be achieved by drinking 500 ml of water 45 min before planning CT. Instructions for the rectum and bladder filling management were similar at time of irradiation.
CT images were transferred to the Eclipse™ treatment planning system. Clinical target volumes (CTVs) were delineated as follows: CTV1 represents the prostate plus the proximal part of seminal vesicles, CTV2 represents the prostate alone, thus CTV1–2 represents the proximal part of seminal vesicles. Then, planning target volumes (PTVs) were created: to obtain the planning target volume for the prostate (PTV2), a 10-mm margin was applied to CTV2 in all directions. PTV1 was constructed in the same manner. PTV1–2 was obtained by subtraction PTV1–PTV2. Organs at risk (OARs) – rectum and bladder – were delineated just 1 cm in excess of PTV1 both in the cranial and caudal directions.
A five-field intensity modulated radiotherapy (IMRT) with simultaneous integrated boost using 6 MV photons was used to deliver 78 Gy to PTV2 and 72.15 Gy to PTV1–2 in 39 fractions. This regimen corresponds to 2 Gy/fr. and 1.85 Gy/fr. to the prostate and proximal part of seminal vesicles, respectively. The IMRT plan was optimized to fulfill criteria presented in Table 1.
Table 1.
Prescription doses for planning target volumes and acceptable doses for organs at risk.
| Structure | Prescription |
|---|---|
| Prostate (PTV2) | Prescribed dose 78 Gy = mean dose for PTV2 |
| Minimally 95% of the prescribed dose (i.e. 74.1 Gy) to 95% of the PTV2 | |
| Maximal dose ≤107% of the prescribed dose (i.e. 83.5 Gy) | |
| Seminal vesicles (PTV1–2) | Prescribed dose 72.15 Gy |
| Minimally 95% of the prescribed dose (i.e. 68.5 Gy) to 95% of the PTV1–2 | |
| Maximal dose ≤107% of the prescribed dose (i.e. 77.2 Gy) | |
| Rectum | Maximally 50% can receive 50 Gy |
| Maximally 25% can receive 70 Gy | |
| Maximally 15% can receive 75 Gy and maximally 15 cm3 can receive 75 Gy | |
| Maximum dose 78 Gy | |
| Bladder | Maximally 30% can receive 70 Gy |
| Maximally 15% can receive 75 Gy and maximally 15 cm3 can receive 75 Gy | |
| Maximum dose 78 Gy | |
In order to evaluate the possibility of margin reduction, alternative plans assuming a smaller 7 mm margin between the clinical and planning target volumes were prepared. These plans were not intended for irradiation, but were made for reconstruction purposes. Alternative plans were optimized for maximal OARs sparing allowed for PTV2 dose coverage between 95 and 96%.
3.4. On-treatment CBCT acquisition and assessment
Patients were treated using Varian Clinac® 2100C/D linear accelerator equipped with On-board imager® (OBI) kV imaging system version 1.3 with CBCT option. After an initial skin marks setup, two orthogonal kV images were acquired and patient's position was corrected based on bony anatomy. Immediately after the bony anatomy set-up, CBCT scan was acquired. The resulting images were subsequently sent to the Eclipse™ TPS where the treatment isocenter was identified. CTVs and OARs were outlined by a single observer at the same range as on the planning CT. CBCT scans were acquired once a week, during treatment fractions no. 2, 5, 10, 15, 20, 25, 30 and 35. CBCTs were not used for set-up of the patient, just for retrospective analysis. Original treatment plan based on planning CT was then reconstructed on each CBCT. Reconstruction of the actually delivered dose distribution was performed based on planned fluences and MUs. The isocenter of the reconstructed plan was set to the isocenter of the CBCT scan, which corresponded to the online matched treatment isocenter. Alternative plans assuming a smaller 7 mm margin were reconstructed in the same way.
3.5. DVH analysis
The reconstructed dose distributions were compared with the planned dose distribution by evaluating the dose-volume histograms (DVHs) for the prostate (CTV2), seminal vesicles (CTV1–2), rectum and bladder. For the prostate and seminal vesicles, the relative volumes that received at least 95% of the prescribed dose were derived. Situations where less than 95% of the CTV was covered by 95% of the prescribed dose were considered as underdosed.
For each patient, variation coefficients were calculated for rectal and bladder volumes based on planning CT and CBCTs together. Correlations between the delineated rectal volume and relative rectal volume irradiated with doses higher than 75 Gy, 70 Gy, 60 Gy, 50 Gy and 40 Gy were tested. Correlations between delineated bladder volume and absolute volume irradiated with doses higher than 75 Gy, 70 Gy, 60 Gy, 50 Gy and 40 Gy were tested similarly.
4. Results
4.1. Validation of dose calculation on CBCT
Comparison of dose distributions of CBCT-based and CT-based plans using one conventional photon beam showed 1% difference in the maximum dose. Comparison of depth dose profiles on the central beam axis showed the agreement better than 1.3%. Higher difference was observed in the range of 2 mm below the body surface, where the beam entered into the body.
Comparison of 5-field IMRT dose distributions showed 0.2% difference in the maximum dose. Comparison of depth dose profiles on central beam axis showed the agreement better than 3%. Higher difference was observed in the range of 1 cm below the body surface, where the beam entered into the body.
4.2. DVH analysis
Number of CBCT scans acquired for one patient during treatment course was between 4 and 8, median was 6 scans. Total number of CBCT acquisitions was 103. Lower number of CBCT scans than expected was caused by technical problems with the OBI system or by excessive accelerator workload.
There were 12 reconstructed plans, out of 103, where 95% of the CTV2 was not covered by the 95% isodose, and these were distributed over 5 patients. Sufficient coverage of CTV1–2 was not achieved in 3 cases, and these were observed in 1 patient. Evaluation of alternative plans assuming a smaller 7 mm margin revealed 23 of 103 cases of CTV2 underdosage distributed over 8 patients and 11 cases of CTV1–2 underdosage distributed over 7 patients. Overall CTV coverage by 95% of the prescribed dose is shown in Fig. 1.
Fig. 1.
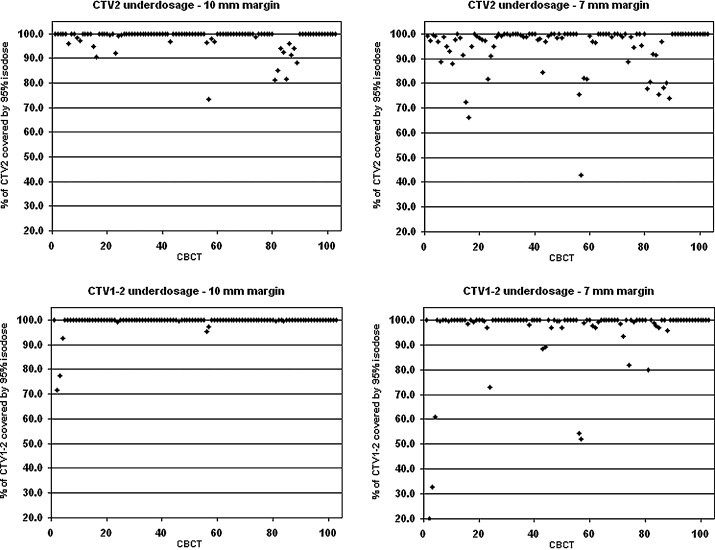
Coverage by 95% of the prescribed dose: the upper part of the figure concerning the prostate (CTV2) and the lower part concerning the seminal vesicles (CTV1–2). 10-mm margin left and alternative 7-mm margin right.
Variability of rectal and bladder volumes is shown in Figs. 2 and 3, respectively. Variation coefficients for rectal volumes were between 0.05 (Patient 17) and 0.60 (Patient 15). Maximum rectal volume observed was 267 cm3 (Patient 10), which is more than three times higher than corresponding planning CT volume (81 cm3). Variation coefficients for bladder volumes were between 0.08 (Patient 17) and 0.48 (Patient 3).
Fig. 2.
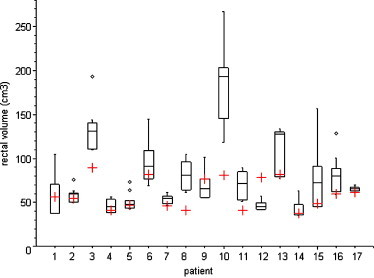
Comparison of rectal volume on CBCT scan (box plot) with planning CT scan (red cross). The box represents the 25th and 75th percentiles, with the central line showing the median value. Two lines extending from the central box of maximal length 3/2 the interquartile range but not extending past the range of the data. Outliers are points that lie outside the extent of the previous elements.
Fig. 3.
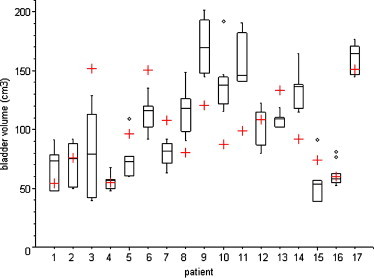
Comparison of bladder volume on CBCT scan (box plot) with planning CT scan (red cross). The box interpretation is the same as in Fig. 2.
As mentioned above, there were 5 patients with underdosed CTV2. This underdosage can be mostly explained by excessive rectal or bladder filling:
In Patient 3, the CTV2 underdosage was observed twice. In the first case the rectal volume was 192 cm3 (2 times larger than initial volume) and in the second case the rectal volume was 131 cm3 (1.5 times larger than initial volume). Both of these volumes were higher than our limit value for planning CT rectal volume (120 cm3).
In Patient 5, the CTV2 underdosage seems to be caused by bladder filling.
In Patient 10, the CTV2 underdosage was caused by too high rectal volume of 267 cm3 (more than three times higher than initial CT rectal volume). This value was the biggest observed as well. Prostate was significantly moved anteriorly out of 95% isodose.
In Patient 14, the CTV2 underdosage was observed in all five CBCT acquisitions. We consider the initial rectal volume to be representative for subsequent CBCT rectal volumes (Fig. 2). The underdosage can be explained by a higher bladder volume (115–164 cm3) than the volume at initial planning CT scan (92 cm3). In these cases CBCT bladder volumes represent 125–178% of the initial bladder volume (Fig. 3).
In Patient 15, the CTV2 underdosage was observed three times. In the first case the rectal volume was 157 cm3 (more than 3 times larger than initial volume) and in the second case the rectal volume was 91 cm3 (almost 2 times larger than initial volume).
Correlations between rectal volume (bladder volume resp.) and its volume irradiated to various specific doses are summarized in Table 2. A medium correlation can be seen between rectal volume and the proportion of rectal volume irradiated with doses higher than 75 Gy.
Table 2.
Pearson's correlation coefficient between rectal volume (bladder volume resp.) and its volume irradiated to various specific doses.
| Rectal volume |
Bladder volume |
||||
|---|---|---|---|---|---|
| 10 mm margin | 7 mm margin | 10 mm margin | 7 mm margin | ||
| V75 (%) | 0.50 | 0.45 | V75 (cm3) | −0.01 | −0.11 |
| V70 (%) | 0.43 | 0.42 | V70 (cm3) | 0.11 | 0.00 |
| V60 (%) | 0.28 | 0.30 | V60 (cm3) | 0.22 | 0.11 |
| V50 (%) | 0.14 | 0.15 | V50 (cm3) | 0.33 | 0.20 |
| V40 (%) | −0.08 | −0.20 | V40 (cm3) | 0.46 | 0.33 |
Abbreviations: Vdose (%), relative organ volume receiving higher dose than specified; Vdose (cm3), absolute organ volume receiving higher dose than specified.
5. Discussion
Feasibility of CBCT-based dose calculation was evaluated previously by Yoo and Yin10 by comparing CBCT-based treatment plans to conventional CT-based plans for both phantoms and patients. CBCT-based treatment plans were dosimetrically comparable to CT-based treatment plans: up to 3% of dosimetric errors were observed to occur in the plans for the inhomogeneous phantom. Usability of CBCT for dose reconstruction was also investigated by Yang et al.11 For the static phantom, doses computed based on conventional CT and CBCT agreed to within 1%. Based on prostate patient and lung patient studies, authors concluded that the CBCT can be employed directly for dose calculation for a disease site such as the prostate, where there is little motion artefact. Our comparison of dose distributions computed on an inhomogenous antropomorfic phantom revealed that CBCT-based plans were comparable to CT-based plans, thus the CBCT can be used to verify treatment delivery retrospectively.
A daily cone-beam CT study of the effect of rectal motion on CTV coverage during prostate radiotherapy was performed by Sripadam et al.9 CBCT scans were acquired from 15 patients immediately after daily treatment. Daily off-line electronic portal imaging verification of bony anatomy positioning was carried out, with an intervention level of 5 mm. To obtain the PTV, a 10-mm margin was applied in all directions, except posteriorly where a 7-mm margin was added. A four-field conformal technique was used with the PTV covered by the 95% isodose. Fields were shaped with multileaf collimators with a penumbra margin of 7 mm. Sripadam revealed instances of insufficient CTV coverage occurring in 38% of the fractions delivered to six patients. These only occurred in the upper regions corresponding to the prostate base and seminal vesicles. Sripadam indicated no statistical correlation between initial rectal volume from planning CT scan and insufficient coverage. However, for patients with large planning rectal volumes, mean dose to the rectum was less than predicted, whereas for patients with small planning rectal volumes, mean dose was greater than predicted.
In a recent paper, Hatton et al.16 assessed the accuracy of the initial CT plan dose-volume histograms for the prostate, rectum and bladder by comparison to delivered doses determined from CBCT scans acquired immediately following conformal treatment delivery. For the group of 12 prostate patients, daily online implanted fiducial guidance was carried out, with a uniform margin of 7 mm around CTV to determine the PTV. Prostate dose coverage was assessed by the proportion of the CTV fully encompassed by the 95% and 98% isodose lines. Four patients showed marginally compromised CTV coverage by the 95% isodose at all CBCT plans. Hatton reports 88% of all plans where more than 95% of the prostate volume is covered by the 98% isodose. Hatton suggests that margin size of 7 mm is not enough to ensure sufficient prostate coverage at all treatment levels.
Our group of 17 patients is not large enough to represent entire patient population, but it is comparable with previously published studies.13–15 However, it was helpful to identify general issues of the CTV insufficient dose coverage. Using IMRT technique with 10-mm CTV-to-PTV margin, we observed insufficient prostate coverage in 12 cases distributed over five patients. Converted into the proportion of delivered fractions for purpose of comparison with the Sripadam's study,9 this represents 43% of the fractions delivered to five patients. However, the comparison can be affected by a lower number of CBCT scans (range 4–8 scans) than in Sripadam's study (range 10–16 scans). We observed similar behaviour of prostate displacement in cases of prostate insufficient coverage caused by excessive rectal volume – prostate apex seems to be relatively fixed, while the prostate base undergoes rotational movement around the apex anteriorly.8,9
Using Hatton's methodology of counting cases fully encompassed by the 95% isodose, i.e. 100% of the CTV2 should receive 95% of the prescribed dose, our results of prostate coverage using 10-mm margin and bony anatomy alignment are poorer than the Hatton's using 7-mm margin and implanting fiducial guidance. We observed 23% of all plans where the CTV2 is not fully encompassed by the 95% and these were distributed over 9 patients. However, in half of these cases the CTV2 coverage was compromised marginally, where more than 95% of the CTV2 was covered by the 95% isodose (Fig. 1, upper left). Moreover, our CBCT scans were acquired before treatment. If the CBCTs were acquired after treatment, the results would be worse in terms of prostate coverage because of the potential intrafraction motion.17
6. Conclusions
Our findings are in agreement with the literature data and confirm insufficient prostate coverage during IGRT based on bony anatomy alignment. Moreover, reconstruction of dose distribution assuming a smaller margin between clinical and planning target volumes leads to significantly worse results in prostate coverage. Initial rectal and bladder volumes cannot be considered representative for subsequent treatment. Especially excessive rectal filling can cause prostate movement out of irradiated volume. We suggest that clearly defined diet instructions could be helpful. Reproducible bladder filling could be achieved by asking patients to empty their bladder 30 min before planning CT examination and each treatment fraction and then to drink 500 ml of water.7 Of course, advanced IGRT techniques like CBCT and fiducials should be considered for daily prostate alignment.
Conflict of interest
None declared.
Acknowledgements
This work was supported by the Grant Agency of Charles University, Grant GAUK 144210 and by the Research Project MZO 00179906 of the Ministry of Health, Czech Republic.
References
- 1.Bindhu J., Supe S.S., Ramachandra A. Cyberknife: a double edged sword? Rep Pract Oncol Radiother. 2010;15(4):93–97. doi: 10.1016/j.rpor.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Herk M. Different styles of image-guided radiotherapy. Semin Radiat Oncol. 2007;17:258–267. doi: 10.1016/j.semradonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ceylan C., Kucuk N., Bas Ayata H., Guden M., Engin K. Dosimetric and physical comparison of IMRT and CyberKnife plans in the treatment of localized prostate cancer. Rep Pract Oncol Radiother. 2010;15(6):181–189. doi: 10.1016/j.rpor.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupelian P.A., Langen K.M., Willoughby T. Image-guided radiotherapy for localized prostate cancer: treating a moving target. Semin Radiat Oncol. 2008;18:58–66. doi: 10.1016/j.semradonc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Balter J.M., Sandler H.M., Lam K. Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys. 1995;31:113–118. doi: 10.1016/0360-3016(94)00382-U. [DOI] [PubMed] [Google Scholar]
- 6.Schallenkamp J.M., Herman M.G., Kruse J.J. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2005;63:800–811. doi: 10.1016/j.ijrobp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Bajon T., Piotrowski T., Antczak A., Bąk B., Błasiak B., Kaźmierska J. Comparison of dose volume histograms for supine and prone position in patients irradiated for prostate cancer–a preliminary study. Rep Pract Oncol Radiother. 2011;16(2):65–70. doi: 10.1016/j.rpor.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghilezan J.M., Jaffray D.A., Siewerdsen J.H. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI) Int J Radiat Oncol Biol Phys. 2005;62:406–417. doi: 10.1016/j.ijrobp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Sripadam R., Stratford J., Henry A.M. Rectal motion can reduce CTV coverage and increase rectal dose during prostate radiotherapy: a daily cone-beam CT study. Radiother Oncol. 2009;90:312–317. doi: 10.1016/j.radonc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Yoo S., Yin F.F. Dosimetric feasibility of cone-beam CT-based treatment planning compared to CT-based treatment planning. Int J Radiat Oncol Biol Phys. 2006;66:1553–1561. doi: 10.1016/j.ijrobp.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Schreibmann E., Li T. Evaluation of on-board kV cone beam CT (CBCT)-based dose calculation. Phys Med Biol. 2007;52:685–705. doi: 10.1088/0031-9155/52/3/011. [DOI] [PubMed] [Google Scholar]
- 12.van Zijtveld M., Dirkx M., Breuers M. Evaluation of the ‘dose of the day’ for IMRT prostate cancer patients derived from portal dose measurements and cone-beam CT. Radiother Oncol. 2010;96:172–177. doi: 10.1016/j.radonc.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee L., Le Q.T., Xing L. Retrospective IMRT dose reconstruction based on cone-beam CT and MLC log-file. Int J Radiat Oncol Biol Phys. 2008;70:634–644. doi: 10.1016/j.ijrobp.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Wang W., Wu Q., Yan D. Quantitative evaluation of cone-beam computed tomography in target volume definition for offline image-guided radiation therapy of prostate cancer. Radiother Oncol. 2010;94:71–75. doi: 10.1016/j.radonc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlowski J.M., Yang E.S., Malcolm A.W. Reduction of dose delivered to organs at risk in prostate cancer patients via image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:924–934. doi: 10.1016/j.ijrobp.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 16.Hatton J.A., Greer P.B., Tang C. Does the planning dose-volume histogram represent treatment doses in image-guided prostate radiation therapy? Assessment with cone-beam computerised tomography scans. Radiother Oncol. 2011;98:162–168. doi: 10.1016/j.radonc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Kasaova L., Sirak I., Jansa J. Daily prostate volume and position monitoring using implanted gold markers and on-board imaging during radiotherapy. Acta Med. 2011;54:149–152. doi: 10.14712/18059694.2016.39. [DOI] [PubMed] [Google Scholar]


