Abstract
In the recent years, application of nanoparticles in diagnosis and treatment of cancer has been the issue of extensive research. Among these studies some have focused on the dose enhancement effect of gold nanoparticles (GNPs) in radiation therapy of cancer. On the other hand, some studies indicated energy dependency of dose enhancement effect, and the others have studied the GNP size effect in association with photon energy. However, in some aspects of GNP-based radiotherapy the results of recent studies do not seem very conclusive in spite of relative agreement on the basic physical interaction of photoelectric between GNPs and low energy photons. The main idea behind the GNP dose enhancement in some studies is not able to explain the results especially in recent investigation on cell lines and animal models radiation therapy using GNPs. In the present article the results of the available reports and articles were analyzed and compared and the final status of the GNP-RT was discussed.
Keywords: Gold nanoparticle, Radiation therapy, Monte Carlo method, Dose enhancement effect
1. Aim
The purpose of the current paper was to assess the current status of gold nanoparticles (GNP) application in radiation therapy. The studies on GNP and its applications in radiotherapy beams were reviewed and the advantages and controversies were discussed.
2. Introduction
Nanoparticles are defined as microscopic particles between 1 and 100 nm but definitions including particles of up to 1 μM have also been reported.1 In cancer treatment they have provided better penetration ability for substances used for therapy and diagnosis with lower risk compared to conventional drugs.2,3 Nanoparticles distribution is influenced by different parameters, like size and their ability to use cancerous cells features for own inactivation.4 Radiation therapy with ionizing radiations including X-rays, gamma rays and high energy particles is employed extensively for treatment of almost all types of solid tumors. Unfortunately, ionizing radiations do not discriminate between cancerous and normal cells. Thus, normal tissue damage is still the dose limiting factor that diminishes tumor cells eradication in radiation therapy. Application of tumor-specific nanoparticles in radiation therapy has aimed to improve the radiation therapy outcomes by inducing more toxicity for tumors and less for normal tissues. Among various nanoparticles, preclinical studies have reported gold nanoparticles (GNPs) radiosensitization effect in conjunction with different photon beams.5–9 Although Monte Carlo simulations of GNPs have demonstrated physical dose enhancement of about 60% for low energy photons of 192Ir brachytherapy sources and also X-rays in kilovoltage range,10 the biological study of Jain et al. found comparable sensitization effect at kilovoltage and megavoltage X-ray energies.11 It was suggested that physical dose enhancement based on increased X-ray absorption could not be the main mechanism of sensitization. However, it should be noted that the used GNP dimensions have been different in these studies. In other words, in the MC study GNPs with diameter of 100 nm, while in the biological study the diameter of 1.9 nm were used.
In the pioneering study of Heinfeld et al., the GNP with the diameter of 1.9 nm was injected intravenously into mammary tumor-bearing mice in combination with 250 kVp X-ray.6 Results showed a 86% one-year survival for new method compared to 20% for X-rays alone. Another study was conducted by Chang et al. on melanoma tumor-bearing mice using 13 nm GNP in conjunction with a single dose of 25 Gy of 6 MeV electron beam.5 It resulted in significant reduction in tumor volume compared to a control group. Additionally, the number of apoptotic cells in GNP plus irradiation animals was two times higher than irradiation alone.
It is believed that interactions of X-rays and GNP result in the release of photoelectrons from high Z gold atoms as well as generation of auger electrons. The range of these electrons is very short relative to photons and a pronounced energy is deposited in cells containing GNP or in direct proximity to gold atoms.
The controversial results concerning GNP radiosensitization could be originated from the differences in performed investigations in terms of key parameters including GNP shape, size, concentration and type of cell lines, and radiation energy and type. To address the problem, the affecting parameters in GNP X-ray radiosensitization were comprehensively evaluated by Burn et al. The most efficient factors were found to be large-sized GNP, high molar concentration and 50 KeV photons with possible dose enhancement factor of 6.4
In the present review, the principles behind the GNP radiosensitization will be discussed and the results of the related studies will be reviewed.
3. Interaction of X-ray and gamma radiations with GNPs
Before clinical application of GNP-based radiation therapy, it is a requisite to comprehend the GNP interactions on the cellular level and its molecular partners in biochemical reactions for further optimizations. On the other hand, the X-ray interaction with high-Z GNP and subsequent mechanisms which lead to dose enhancement should be explained for further applications.
The irradiation stability and cytotoxicity of GNPs for radiotherapy purposes was evaluated by Zhang et al. They found no obvious instability and size variation in spherical GNPs with the diameter of 15 nm following gamma radiation of 2000–10,000 Rontgen. Cytotoxicity results showed that the extremely high concentration of GNP could cause a sharp drop off in K562 cell viability, while the low concentration did not affect the cell viability.12
Based on the energies of ionizing photons, different types of interactions occur between photons and GNPs. The photoelectric effect is the predominant process for photons with energy from 10 to 500 keV. The result of this process is the production of electrons, characteristic X-ray of gold atoms or auger electrons. In photoelectric interaction, between photons and GNPs, a vacancy in a K, or L, M shell following photoelectric absorption results in a de-excitation of the atomic system, either by characteristic X-ray or Auger-electron emission. The relative probability of these de-excitation processes is given by the fluorescence yield. Fluorescence yield is strongly dependent on atomic number (Z), being small for light atoms and large for heavy atoms such as gold.
For photons above 500 keV, the Compton scattering and excitation are observed. The Compton scattering will result in atom re-excitation and production of Compton electrons which leads to subsequent photoelectric effect. There are certain selection rules which prohibit photon emission entirely after atom excitation and phonon emission occurs. In phonon emission, the excitation energy flows into the host lattice as low grade heat. This is referred to as a quenching process. The high energy excitation in gold, induces lots of phonons and less photons, because the dominant transition of GNPs is photon–phonon transition processing.12,9
For photon energies higher than 1.02 MeV, pair production process dominates and results in positron and electron pairs. For all of these interactions, except Compton scattering, the cross section of photon interactions depends strongly on Z, when the photoelectric and pair production effect probabilities are proportional to Z3 and Z2 of atoms. Consequently, it is expected that the interaction of X-and γ ray with gold atoms deliver considerable energy GNPs which are transformed to energetic, free electrons and thermal energy.
4. Monte Carlo modeling of GNP dose enhancement effect
Using the GEANT4 MC code a 192Ir brachytherapy source, BEBIG was simulated and benchmarked against thee available published dosimetric data for model validation.10 Two geometries, including parallel beam geometry and 4π beam geometry, were used for MC simulations. The parallel beams were used to resemble the external beam irradiations using two parallel beams. The GNP with a diameter of 100 nm was uniformly distributed into a nanoparticle region with a grid of 450 nm. Moreover, dose enhancement by a volume of gold–water mixture with the same concentration was also simulated. For two parallel beams with 380 keV photons, the maximum dose enhancement of 28% and 36% were seen for gold nanoparticle and gold–water mixture which lead to 16.2% overestimation for gold–water mixture. However, it should be noted that as photons pass through the nanoparticle region, the dose values fall below those for non-gold case. Additionally, it gets more pronounced for lower energies that have been used in majority of previous studies on GNP dose enhancement effect. Also, the self-absorption of the high atomic region could be a problem in creating a uniform dose distribution for treatments using GNPs. According to the MC simulation for GNPs dose enhancement, the GNPs effect comes mainly from higher photoelectric interactions which are inversely proportional to the photon energy (∝E−3). As we know from basic radiation physics, the photoelectric interaction coefficient is significantly raised in energies just above gold's K-edge binding energy, i.e. 80.7 keV. In the study of Zhang et al., they explained the enhancement effect by the fact that as the energy of photoelectrons was about 300 keV for 380 keV photon, the range of photoelectrons was about 85 μm, much longer than the gold diameter of 100 nm. Consequently, the photoelectrons had the chance to deposit their energy in the water surrounding the nanoparticles.
In the study of Cho, the MC method was used to evaluate the dose enhancement effect of GNPs-based radiation therapy by 140 kVp X-rays, 4 and 6 MV photon beams, and 192Ir gamma rays.13 The dose enhancement ratio of 2 was obtained for the 140 kVp X-ray case with gold concentration of 7 mg Au/g tumor assuming no gold outside the tumor. The tumor dose enhancement ratio for the 4 and 6 MV photon beams ranged from about 1% to 7%, depending on the amount of gold within the tumor and photon beam qualities. For the 192Ir case, the dose enhancement ratio of 5–31%, depending on radial distance and gold concentration were reported.
In another study by Cho et al.,14 the feasibility of gold nanoparticle-aided radiation therapy (GNRT) using low energy photons was studied by MC calculations. Brachytherapy sources of 50 kVp X-rays, 125I and 169Yb were used to calculate macroscopic dose enhancement factors (MDEF), defined as the ratio of the average dose in the tumor region with and without the presence of gold nanoparticles during the irradiation of the tumor. A significant tumor dose enhancement was found of more than 40%, using 50 kVp X-rays, 125I, and 169Yb sources and gold nanoparticles. For a tumor loaded with 18 mg Au g−1, the respective MDEFs of 116%, 92% and 108% were reported for 125I, 50 kVp and 169Yb at the distance of 1.0 cm from the center of the source. Whereas, for the concentration of 7 mg Au g−1, it decreased to 68%, 57% and 44%, respectively, for the same sources. They concluded that GNRT using the clinically used brachytherapy sources is feasible and could be exploited for brachytherapy with low energy photons, especially with a high dose rate 169Yb source.
5. GNP sensitization in cell line and animal models
In the first study by Hainfeld, GNPs were employed to enhance radiotherapy toxicity for cancerous cells in mice.6 Mice with subcutaneous breast cancers were divided into three groups. The first one received GNP injection prior to 250 kVp X-ray radiotherapy. The second group received radiation only, and the last group received GNPs only. The one-year survival rates were found to be 86% for the GNP and radiation group versus 20% for the radiation alone and 0% for the gold alone groups. The increased radiosensitivity was attributed to high-Z radio-enhancement by GNPs.
In the study by Chang et al. the increased apoptotic and dose-enhancing effects of GNP in combination with single dose clinical electron beams on tumor-bearing mice were reported.5 Murine B16F10 melanoma cells lines were cultured and then also transplanted into mice leg. The cell lines and tumor-bearing mice were irradiated with a single dose of 25 Gy using 6 MV electron beam in combination with GNPs. Moreover, the accumulation of GNPs in cell lines and mice were detected and quantized. The cell survival results showed that GNP radiosensitized B16F10 melanoma cells in the colony formation assay meaningfully, with P value of 0.02. For tumor-bearing mice it was revealed that the tumor growth was retarded more considerably comparing to the control and radiation only group (P less that 0.05). Additionally, the extent of apoptosis was found (almost two times) higher for the GNP plus radiation group relative to the radiation alone group. They suggested the application of GNP-based radiation therapy with electrons for melanoma treatments. Chang et al.5 used GNP with an average size of 13 nm to benefit from higher sensitization effect, according to the previous study of Chithrani et al.15
In study of Burn et al., DNA plasmid solution combined with GNP was irradiated to investigate the response of this key molecule for GNP radiation therapy. GNPs with diameters of 8.1, 20.2, 37.5, 74, and 92 and photon effective energies from 15 to 70 keV were used.4 With a GNP:DNA ratio of 1:5 and 49 keV effective energy, a linear relationship was observed for enhancement factor in terms of loss of supercoiled DNA and GNP diameter. For diameters from 8 to 92 nm, the enhancement factor raised to 3 for the largest GNP diameter in their study.
Zhang et al. studied the enhancement in radiation sensitivity in prostate cancer by gold nanoparticles.8 Human prostate carcinoma DU-145 cells were exposed to 200 kVp X-rays and 15 nM TGS–GNPs, or 15 nM Glucose–GNPs, or GNPs plus irradiation. The cytotoxicity induced by GNPs, irradiation, or GNPs plus irradiation was measured using a standard colorimetric MTT assay. The results indicated that either TGS–GNPs or X-ray induced an inhibition of cell growth by approximately 14% or 16% individually. However, a combination of TGS–GNPs and X-ray produced an inhibition of cell growth of 30.57%, while the combination of Glucose–GNPs plus X-ray induced an inhibition of cell growth by 46%.
In the in vitro study of Kong et al., two functional molecules of GNPs, including cysteamine (AET) and thioglucose (Glu), were synthesized and cell uptake and radiation cytotoxicity enhancement in a breast-cancer cell line) MCF-7) versus a nonmalignant breast-cell line (MCF-10A) were studied. Transmission electron microscopy (TEM) results showed that cancer cells take up functional Glu-GNPs significantly more than naked GNPs. The results showed that these functional GNPs have little or no toxicity to these cells. Different radiations such as 200 kVp X-rays and gamma-rays were applied to radiation therapy of the cells, with and without functional GNPs. The results showed that the radiotherapy in association with GNPs killed significantly more breast-cancer cells compared to those without GNP.16
6. Impact of radiation energy
The beam energy is one of the major factors influencing the radiation therapy effectiveness. In radiation therapy of tumors loaded with high-Z elements the physical concepts of photoelectric interaction has been employed for photon energy selection. In other words, it is physically obvious that the possibility of photoelectric interaction is raised when the photon energy is just above the k-edge of high-Z elements. Additionally, the release of photoelectrons and Auger electrons leads to a great energy deposition in the vicinity of nanoparticles. However, this pure physical concept was not completely realized in practice. Biston et al. used a combination of synchrotron irradiation and cis-diamminedichloroplatinum (II) on rat F98 glioma cells below and above the K-edge of platinum (78.4 keV). Surprisingly, the results were identical for both cases. The suggested reason for such a strange event was that for an incident photon with the energy of K-edge, all the energy is spent to eject K-electron and the photoelectron would not receive enough energy to result in excessive damage to surrounding material.
Another influencing factor which should be taken into account to explain the observed discrepancies is absorption differences between high-Z element and surrounding medium. In Fig. 1, the variation of relative mass energy absorption coefficient of gold to water is presented. The data was derived from National Institute of Standards and Technology (NIST). As can be seen, the maximum value locates around 40–50 keV, which means that the highest achievable enhancement factor with GNPs could be realized with the photon energy of 40–50 keV. The finding of Burn et al. was in accordance with the theoretical assumption on optimum energy of photons suggested for radiation therapy with GNPs. On the other hand, the application of low energy photons for radiation therapy is associated with the problem of high skin dose and rapid drop off of absorbed dose with depth. In the MeV energies used for the current radiation therapy, the ratio of absorption between gold and water becomes theoretically negligible, as can be seen in Fig. 1. However, as other studies have shown, the enhancement effect can be seen also with MeV range of photons. In higher energies, the photon interaction with matter produces Compton electrons with a spectrum of energy which have higher absorption coefficient with GNPs compared to the surrounding biological matter. It can be suggested that some refinement in photon energies and more studies on sensitizing effect of GNPs with higher energy photon may help to overcome the keV range photon problems in radiation therapy with GNPs. Another approach to avoid higher dose to skin with low energy photons is to use GNPs with newly developed miniature X-ray sources which can be inserted into the body and located beside or inside the tumor using laparoscopic and endoscopic techniques.4
Fig. 1.
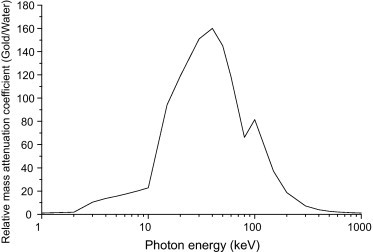
The variation of relative mass attenuation coefficient of gold to water with photon energy.
7. Conclusion
The concept of using GNPs for radiation therapy has been studied by several experimental and MC simulation investigations during last years. Although the enhancement of radiation dose in tumors loaded with high-Z materials have been attempted for several decades, the emergence of new gold nanoparticles with biocompatible characteristics has motivated scientists to investigate their applications in conjunction with radiation therapy. The results of all aforementioned studies are agreed in that GNPs can enhance the dose deposition phenomenon in GNP loaded tumors. But there are controversial results about the impact of photon energy and GNP size in recently published articles. To optimize the technique of GNP-based radiation therapy for clinical application, some studies should be carried out to address the effect of photon energy and GNP size separately. Also, more biological experiments on cell lines and animal models are required to clarify the observed differences in dose enhancement effect concerning the magnitude of enhancement effect and impact of cell type in GNP-based radiation therapy.
References
- 1.Praetorius N.P., Mandal T.K. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee P., Bhattacharya R., Bone N., Lee Y.K., Patra C.R., Wang S. Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): enhancing apoptosis. J Nanobiotechnol. 2007;5:4. doi: 10.1186/1477-3155-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porcel E., Liehn S., Remita H., Usami N., Kobayashi K., Furusawa Y. Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology. 2010;21:85–103. doi: 10.1088/0957-4484/21/8/085103. [DOI] [PubMed] [Google Scholar]
- 4.Brun E., Sanche L., Sicard-Roselli C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution. Colloids Surf B: Biointerfaces. 2009;72:128–134. doi: 10.1016/j.colsurfb.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Chang M.Y., Shiau A.L., Chen Y.H., Chang C.J., Chen H.H., Wu C.L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99:1479–1484. doi: 10.1111/j.1349-7006.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hainfeld J.F., Slatkin D.N., Smilowitz H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 7.Hainfeld J.F., Dilmanian F.A., Zhong Z., Slatkin D.N., Kalef-Ezra J.A., Smilowitz H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol. 2010;55:3045–3059. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Xing J.Z., Chen J., Ko L., Amanie J., Gulavita S. Enhanced radiation sensitivity in prostate cancer by gold-nanoparticles. Clin Invest Med. 2008;31:E160–E167. doi: 10.25011/cim.v31i3.3473. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y., Hunting D.J., Ayotte P., Sanche L. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat Res. 2008;169:19–27. doi: 10.1667/RR1080.1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S.X., Gao J., Buchholz T.A., Wang Z., Salehpour M.R., Drezek R.A. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomed Microdevices. 2009;11:925–933. doi: 10.1007/s10544-009-9309-5. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Coulter J, Butterworth K, Hounsell A, Currel F, Price K et al. Gold nanoparticles as sensitizers for radiation therapy at clinically relevant megavoltage X-ray energies; 2010, http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=73&abstractID=30431.
- 12.Zhang X.D., Guo M.L., Wu H.Y., Sun Y.M., Ding Y.Q., Feng X. Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int J Nanomed. 2009;4:165–173. doi: 10.2147/ijn.s6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol. 2005;50:N163–N173. doi: 10.1088/0031-9155/50/15/N01. [DOI] [PubMed] [Google Scholar]
- 14.Cho S.H., Jones B.L., Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/X-ray sources. Phys Med Biol. 2009;54:4889–4905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chithrani B.D., Ghazani A.A., Chan W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 16.Kong T., Zeng J., Wang X., Yang X., Yang J., McQuarrie S. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small. 2008;4:1537–1543. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]


