Abstract
Aim
To report the long-term follow-up of a cohort of adult patients with LGG post-operatively irradiated in one institution, and to identify prognostic factors for progression free survival.
Background
There is little consensus about the optimal treatment for low-grade glioma (LGG), and the clinical management of LGG is one of the most controversial areas in neurooncology. Radiation therapy is one option for treatment of patients with LGG, whereas other options include postoperative observation.
Materials and methods
Between 1975 and 2005, 180 patients with LGG (WHO II) received postoperative irradiation after non radical (subtotal or partial) excision. Patients had to be 18 years of age or older, and have histologic proof of supratentorial fibrillary (FA), protoplasmic (PA) or gemistocytic astrocytoma (GA). Radiotherapy was given within 3–10 weeks after surgery. Treatment fields were localized and included the preoperative tumor volume, with a 1–2 cm margin, treated to a total dose of 50–60 Gy in 25–30 fractions over 5–6 weeks.
Results
Actuarial ten-year progression free survival (APFS) in the whole group was 19%. The worse prognosis was observed in patients with GA. Ten-year APFS rates for GA, PA and FA were 10%, 18% and 22%, respectively.
Conclusion
The findings from our long-term cohort of 180 patients with LGG confirmed by uni- and multivariate analysis demonstrated that only astrocytoma histology significantly determined the prognosis. The best survival was observed in patients with the fibrillary variant, and the worst for the gemistocytic one.
Keywords: Low-grade gliomas, Surgery, Radiotherapy
1. Background
Low-grade gliomas (LGGs), the World Health Organization (WHO) grade 2 tumors, account for about 11% of all primary brain tumors.1 The most common histologic subtypes of LGG include astrocytomas, oligodendrogliomas, and mixed oligostrocytomas.2 This terminology is helpful in differentiating LGG from lower or higher grade gliomas that have a significantly different prognosis. Patients with LGG may survive for relatively long periods, but often (80%) progress to higher-grade tumors which are invariably fatal to the patient. Unfortunately, there is little consensus about the optimal treatment for LGG, and the clinical management of LGG is one of the most controversial areas in neurooncology.3
Radiation therapy is one option for treatment of patients with LGG, whereas other options include postoperative observation. The role of chemotherapy remains undefined.4 In general, treatment is reserved for patients with symptomatic residual disease despite optimal surgical resection or for patients who are suspected to have high risk features. The current hypothesis is that patients older than forty, with residual disease, should receive earlier intervention. The basis for these recommendations is three prospective studies done over the past ten years.5–7
2. Aim
To report the long-term follow-up of a cohort of adult patients with LGG post-operatively irradiated in one institution, and to identify prognostic factors for progression free survival.
3. Materials and methods
The study population was derived from neurosurgical centers which referred patients to the Maria Skłodowska-Curie Memorial Center in Kraków for radiation therapy. Patients had to be 18 years of age or older, and have histologic proof of a supratentorial fibrillary (FA), protoplasmic (PA) and gemistocytic astrocytoma (GA). Oligodendrogliomas, mixed oligoastrocytomas and other variants were excluded. Central pathology review was performed at the Department of Neuropathology of the Jagiellonian University.
Between January 1, 1975 and May 31, 2005, 180 patients with LGG received postoperative irradiation. The oldest patient was 64, the youngest 19 years old (median 47 years).
The distribution of patho-clinical characteristics in our series is given in Table 1.
Table 1.
Patho-clinical characteristics of 180 adult patients with LGG.
| Characteristics | N | % |
|---|---|---|
| Age (years) | ||
| 40 and less | 79 | 44 |
| More than 40 | 101 | 56 |
| Gender | ||
| Male | 95 | 53 |
| Female | 85 | 47 |
| KPSa | ||
| 60–70 | 83 | 46 |
| More than 70 | 97 | 54 |
| Seizures | ||
| Yes | 103 | 57 |
| No | 77 | 43 |
| TCMb | ||
| Yes | 61 | 34 |
| No | 119 | 66 |
| Extent of surgery | ||
| Subtotal | 126 | 70 |
| Partial | 54 | 30 |
| Histology | ||
| Fibrillary astrocytoma | 104 | 58 |
| Protoplasmic astrocytoma | 36 | 20 |
| Gemistocytic astrocytoma | 40 | 22 |
| Total dose | ||
| 50 Gy | 72 | 40 |
| More than 50 Gy | 108 | 60 |
Karnofsky's performance status.
TCM – tumor crossing midline.
Radiotherapy was given within 3–10 weeks after surgery. The 2D technique was used until 1995 when it was replaced by the 3D-Conformal technique. The treatment fields were localized and included the preoperative tumor bed with a 1–2 cm margin treated to a total dose of 50–60 Gy (median 56 Gy) in 25–30 fractions over 5–6 weeks.
After the completion of therapy (every 4 months for 2 years, every 6 months for 3 years, and yearly thereafter until year 15), patients had a physical examination that included a neurologic examination and CT or MRI scan, or when clinically indicated.
Actuarial 10-year progression free survival (APFS) rates were calculated from date of surgery, and estimated using the Kaplan–Meier method and compared using one- or two sided log rank tests.8,9 Only variables that were identified as significantly (p ≤ 0.05) associated with the end point (APFS) were considered valid prognostic parameters. The Cox proportional hazards model was used to assess the strengths of association of APFS with various histo-clinical characteristics.10
4. Results
The treatment was generally well tolerated, signs and symptoms of increased intracranial pressure occurred in 21 patients (12%), and resolved with steroid administration.
APFS for all 180 patients is presented in Fig. 1. The 10-year APFS was of 19%.
Fig. 1.
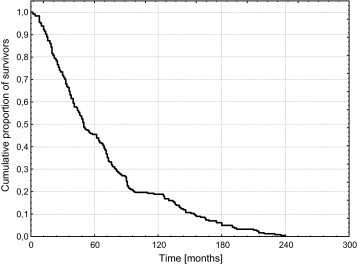
Actuarial progression-free survival (APFS) in the whole group of 180 patients with low-grade gliomas (LGGs).
Kaplan–Meier estimates of survival by histology are given in Fig. 2. The 10-year APFS for FA, PA and GA was of 25%, 18% and 9%, respectively. The results of uni- and multivariate analysis are shown in Tables 2 and 3.
Fig. 2.
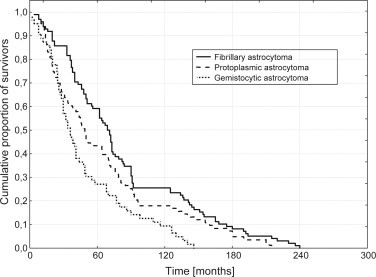
Actuarial progression-free survival (APFS) by histology.
Table 2.
Univariate analysis.
| Characteristics | 10-Year APFS (%) | p |
|---|---|---|
| Age (years) | ||
| 40 and less | 22 | |
| More than 40 | 16 | NSc |
| Gender | ||
| Male | 19 | |
| Female | 19 | NS |
| KPSa | ||
| 60–70 | 17 | |
| More than 70 | 23 | NS |
| Seizures | ||
| Yes | 20 | |
| No | 16 | NS |
| TCMb | ||
| Yes | 12 | |
| No | 22 | NS |
| Extent of surgery | ||
| Subtotal | 25 | |
| Partial | 16 | NS |
| Histology | ||
| Fibrillary astrocytoma | 22 | |
| Protoplasmic astrocytoma | 18 | 0.1387 |
| Gemistocytic astrocytoma | 10 | 0.0312 |
| Total dose | ||
| 50 Gy | 20 | |
| More than 50 Gy | 16 | NS |
Karnofsky's performance status.
TCM – tumor crossing midline.
NS – not significant.
Table 3.
Multivariate analysis (Cox's model).
| Characteristics | Relative risk | p |
|---|---|---|
| Histology | ||
| Fibrillary astrocytoma | 1 | 0.52 |
| Protoplasmic astrocytoma | 1.16 | 0.020 |
| Gemistocytic astrocytoma | 2.92 | |
5. Discussion
LGG is a vexing problem. Some patients present with readily controllable seizures will enjoy years of freedom from tumor progression without intervention, whereas others progress rapidly with neurologic decompensation and death.11–14 Although these tumors are grouped together under the category of “low-grade glioma”, they are actually an extremely heterogeneous group with a median survival time ranging from 5 to 10 years.15–18 The best treatment policy for these tumors is still unclear. Some physicians advocate early and extensive surgery, while others tend to postpone treatment until functional deficits are present. Radiotherapy is prescribed for most patients with LGG, only the timing of treatment is debated.19–23
Our series of 180 patients with LGG is one of the largest groups described in the literature, coming from one center. The patho-clinical features of the present material are similar to those reported by other authors.5–7,14,24,25 More than 50% of patients had seizures at presentation, the majority of our population were in their fifth and sixth decades of age. The tumor was located predominantly in the frontal lobe in 41% of patients, in the temporal lobe in 39%, in the parietal lobe in 17%, and in the occipital lobe in 3%. Thirty percent of patients had bulky residual tumor after surgery, in 54%, Karnofsky performance status was more than seventy.
Our results with a 10-year APFS of 19% are close to or slightly worse than results achieved by other authors (Table 4). It must be emphasized, however, that the literature data presented in Table 4 refer to patients after both radical and partial surgery, and that they include as well grade 2 astrocytomas as mixed forms of gliomas and oligodendrogliomas. We restricted our review only to adult patients with grade 2 astrocytoma, according to the WHO classification. This may pose certain interpretative difficulties in direct comparison of our results with those of other authors, including grade 1 glioma and mixed astro-oligodendroglioma variants. The notorious difficulties with respect to the histologic diagnosis of gliomas may also be an important cause for differences between study results. Interobserver variability in the diagnosis and grading of gliomas has been well documented. Coons et al. observed that even with well-defined criteria, expert observers and formal training, the maximal concordance rate achieved was 86%. Distinguishing between oligodendrogliomas and diffuse astrocytomas is particularly problematic, as is distinguishing between pure oligodendroglioma and mixed oligoastrocytoma. Entities that can confound low-grade classification include pilocytic astrocytoma, pleomorphic xanthoastrocytoma, ganglioglioma, and gliosarcoma.33
Table 4.
Results of postoperative irradiation of patients with LGG.
Several studies have attempted to identify prognostic factors in LGG. A number of patient and tumor characteristics, such as age at diagnosis, performance status, histology subtype, presence of seizures at diagnosis and extent of resection, have been proposed as prognostic factors.
In our group, only the histologic subtype was most consistently and significantly associated with survival in uni- and multivariate analysis. In our study, the outcome of patients with gemistocytic astrocytomas was slightly worse than that of those with protoplasmic and fibrillary variants, with ten year APFS rates of 10%, 18% and 22%, respectively. Hazard ratio for gemistocytic vs. fibrillary variant being of 2.92 means that death rate of patients presenting with GA was about three times higher in comparison with fibrillary variant. Better prognosis for fibrillary astrocytoma was confirmed by Ducray, Durmaz and Piepmeier.13,17,20 Gemistocytic astrocytomas and a high MIB labeling index have also been related to poor prognosis in LGG.29,32
We found that TCM has not significantly influenced the prognosis. Ten year APFS was 12% and 22% for TCM (+) and TCM (−) patients, respectively. Our findings do not consist with the results of the EORTC 22845 trial, in which a median survival time for TCM (+) and TCM (−) characteristics was 3.6 years and 7.9 years, respectively, with a hazard ratio of 1.43.14
In the univariate analysis, the extent of surgery in our material reached the borderline of significance, with a p-value of 0.0579. There was no statistical difference in survival between patients who had been treated with subtotal resection and those who had undergone a partial one. The 10-year APFS for the former group was of 25% compared to 16% for the latter. The role of surgical resection in management of patients with LGG has remained controversial. The theoretical goals of surgical resection in LGG are to improve neurologic deficits and to minimize the risk of recurrence, or malignant transformation. The literature is replete with retrospective series analyzing the impact of surgical resection on patient outcome.3,18,19,26 Keles et al. identified 30 articles on LGG published between 1970 and 2000 that incorporated statistical analyses and addressed the issue of resection. In order to reduce known biases, they eliminated studies that included pediatric patients, contained WHO grade 1 astrocytoma, or evaluated small numbers of patients (less than 75). They were left with only five articles that they deemed as valid studies, all of which demonstrated that extent of resection was a statistically significant variable in univariate analysis, and in four out of the five studies it was a significant factor in multivariate analysis.23 Pignatti et al. argued that good prognosis of LGG patients having undergone an extensive resection may not be due to the resection itself but to the limited size and superficial site of the tumor (thus being accessible to more extensive surgery). Based on our own experience, we think that the impact of extent of surgery is difficult to ascertain due to the inadequate terminology used in surgical reports, which vary from surgeon to surgeon.
Age is a well-established prognostic factor for survival in LGG, the prognosis being worse for older patients.7,13,18,25 The present series has failed to show any significant survival benefits for younger patients. A cut-off point at 40 years was chosen, but in clinical practice, this should not be interpreted as an absolute cut-off value.
A number of studies concerning LGG found some association between prognosis and signs and symptoms at presentation. One series observed a favorable outcome in patients presenting with seizures, other found the performance status to be of prognostic significance.7,17,18,25,30 The present study did not reveal a significant association between the described parameters and patient survival.
Radiotherapy has been a mainstay of LGG therapy for decades, but much controversy has surrounded the radiation dose which should be delivered.27 The EORTC 22844 trial randomly assigned 379 patients with histologically confirmed LGG to receive irradiation postoperatively (or post biopsy) with either 45 Gy in 5 weeks or 59.4 Gy in 6.6 weeks. The minimum length of follow-up was 54 months. The conclusion of this study was that there was no significant difference between the low-dose and high-dose radiation groups as their 5-year progression-free survival (PFS) values were 47% and 50%.5 In a similar trial, the North Central Cancer Treatment Group (NCCTG), the Radiation Therapy Oncology Group (RTOG), and the Eastern Cooperative Oncology Group (ECOG) randomly assigned 211 patients to two groups. One hundred and eight patients received 50.5 Gy in 28 fractions and one hundred and three patients received 64.8 in 36 fractions. There was no difference in survival or PFS between the two groups. The 2- and 5-year survival in the low-dose radiation group was 94% and 72%, respectively. The 2- and 5-year survival in the high-dose radiation group was 85% and 64%. Likewise, the PFS at 5 years was 55% and 52%, respectively.7 In our study, the total dose was not a prognostic factor for the 10-year APFS, like in the described trials.
6. Conclusion
The findings from our study confirmed by uni- and multivariate analysis, demonstrated that only astrocytoma histology is an important, statistically significant prognostic factor for progression free survival. The best prognosis is for patients with the fibrillary variant, and the worst for the gemistocytic one.
Conflict of interest
None declared.
References
- 1.Central Brain Tumor Registry of the United States. Statistical report: primary brain tumors in the United States, 1992–1997. Chicago; 2000. p. 2–31.
- 2.Louis D.N., Ohgaki H., Wiestler O.D. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang F.F., Gilbert M.R. Diffusely infiltrative low-grade gliomas in adult. J Clin Oncol. 2006;24:1236–1245. doi: 10.1200/JCO.2005.05.2399. [DOI] [PubMed] [Google Scholar]
- 4.Prados M.D., Haas-Kogan D. Low-grade glioma. Potential new markers and strategies. Neuro-Oncology. 2009;12:19–21. [Google Scholar]
- 5.Karim A.B.M.N.F., Maat B., Hatlevol R. A randomized trial on dose–response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 228444. Int J Radiat Oncol Biol Phys. 1996;36:549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 6.Karim A.B.M.F., Afra D., Cornu P. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council Study BR04: an interim analysis. Int J Radiat Oncol Biol Phys. 2002;52:316–324. doi: 10.1016/s0360-3016(01)02692-x. [DOI] [PubMed] [Google Scholar]
- 7.Shaw E., Arusell B., Scheithauer B. Prospective randomized trial of low- versus high dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan M.E., Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–483. [Google Scholar]
- 9.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1996;20:163–170. [PubMed] [Google Scholar]
- 10.Cox D.R. Regression models and life tables. J R Stat Soc B. 1971;34:187–220. [Google Scholar]
- 11.Schiff D., Brown P.D., Giannini C. Outcome in adult low-grade glioma: the impact of prognostic factors and treatment. Neurology. 2007;69:1366–1373. doi: 10.1212/01.wnl.0000277271.47601.a1. [DOI] [PubMed] [Google Scholar]
- 12.Brown P.D. Low-grade gliomas: the debate continues. Curr Oncol Rep. 2006;8:71–77. doi: 10.1007/s11912-006-0012-x. [DOI] [PubMed] [Google Scholar]
- 13.Ducray F., Dutertre G., Ricard E. Actualités dans la biologie, l’imagerie et le traitement des gliomes de l’adulte. Bull Cancer. 2010;97:17–36. doi: 10.1684/bdc.2009.1019. [DOI] [PubMed] [Google Scholar]
- 14.Pignatti F., vandenBent M., Curran D. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 15.Henderson K.H., Shaw E.G. Randomized trials of radiation therapy in adult low-grade gliomas. Semin Radiat Oncol. 2001;11:145–151. doi: 10.1053/srao.2001.21424. [DOI] [PubMed] [Google Scholar]
- 16.Chang E.F., Clark A., Jensen R.L. Multiinstitutional validation of the University of California at San Francisco low-grade glioma prognostic scoring system. Clinical article. J Neurosurg. 2009;111(2):203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 17.Durmaz R., Vural M., Isildi E. Efficacy of prognostic factors on survival in patients with low-grade glioma. Turk Neurosurg. 2008;18:336–344. [PubMed] [Google Scholar]
- 18.Leighton C., Fisher B., Bauman G. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 19.Berger M.S., Deliganis A.V., Dobbins J. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Piepmeier J., Crostopher S., Spencer D. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996;38:872–879. doi: 10.1097/00006123-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Recht L.D., Lew R., Smith T.W. Suspected low-grade gliomas: is deferring treatment safe? Ann Neurol. 1992;31:431–436. doi: 10.1002/ana.410310413. [DOI] [PubMed] [Google Scholar]
- 22.Papagikos M.A., Shaw E.G., Stieber V.W. Lessons learned from randomized clinical trials in adult low grade glioma. Lancet Oncol. 2005;6:240–244. doi: 10.1016/S1470-2045(05)70095-4. [DOI] [PubMed] [Google Scholar]
- 23.Keles G.E., Lamborn K.M., Berger M.S. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735–745. doi: 10.3171/jns.2001.95.5.0735. [DOI] [PubMed] [Google Scholar]
- 24.Yeh S.A., Ho J.T., Lui C.C. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005;78:230–235. doi: 10.1259/bjr/28534346. [DOI] [PubMed] [Google Scholar]
- 25.Lote K., Egeland T., Hager B. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;17:3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 26.North C.A., North R.B., Epstein J.A. Low-grade cerebral astrocytomas: survival and quality of life after radiation therapy. Cancer. 1990;66:6–14. doi: 10.1002/1097-0142(19900701)66:1<6::aid-cncr2820660103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Shaw E. The low-grade glioma debate: evidence defending the position of early radiation therapy. Clin Neurosurg. 1995;42:488–494. [PubMed] [Google Scholar]
- 28.Janny P., Cure H., Mohr M. Prognostic parameters in benign astrocytomas. Cancer. 1994;73:1937–1945. doi: 10.1002/1097-0142(19940401)73:7<1937::aid-cncr2820730727>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Scerrati M., Roselli M., Iacoangeli M. Prognostic factors in low-grade gliomas of the cerebral hemispheres. J Neurol Neurosurg Psychiatry. 1996;61:291–296. doi: 10.1136/jnnp.61.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauman G., Fisher B., Watling C. Adult supratentorial low-grade glioma: long-term experience at a single institution. Int J Radiat Oncol Biol Phys. 2009;5:1401–1409. doi: 10.1016/j.ijrobp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Schomas D.A., Laack N.N., Brown P.D. Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer. 2009;17:3969–3978. doi: 10.1002/cncr.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffer D., Cavalla P., Chio A. Proliferative activity and prognosis of low-grade astrocytomas. J Neurooncol. 1997;34:31–35. doi: 10.1023/a:1005755119526. [DOI] [PubMed] [Google Scholar]
- 33.Coons S.W., Johnson P.C., Scheithauer B.W. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]


