Abstract
This paper evaluates the reasons behind the rise in the use of proton beam for prostate cancer, the economics drivers behind it, and the evidence that exists to support it. It concludes that clinical outcome data underlying the notion that this is a superior treatment remains sparse and discusses what is needed to fill in the gaps.
Keywords: Prostate cancer, Proton beam
1. Introduction and rationale
The properties of the proton beam have been long recognized as having therapeutic potential. In particular, their singular property of slowing rapidly in tissue, depositing energy at depth, and without any dose beyond the target, were envisioned to have great clinical advantage. This was first recognized in the 1940s and has been the basis of its use ever since.1 Early therapeutic facilities were merely physics laboratories at universities cleverly adapted to allow patient treatment. The beam energies were generally low and so treatment was limited to tumors at relatively shallow depth such as the eye, the spine, or the base of skull. In the realm of pediatrics where low dose, or indeed any dose, radiation to normal tissues can have disastrous consequences proton beam was used with enthusiasm. Luckily the lower energy beams then available had sufficient penetration to reach most pediatric sarcomas or CNS tumors and indeed tumors almost anywhere in a baby.
In the 1990s patient-dedicated proton facilities were developed and since then their use and establishment has greatly accelerated. Dozens of facilities now exist globally, ten of which are fully functional in the Unites States. Contracts have been signed on many more facilities and construction is underway. It is the wave of the future and, at current costs, a very expensive wave. The enthusiasm, based upon simple dosimetric studies, has actually preceded the results of prospective clinical studies designed to assess the outcomes of this therapy and, in some cases, has actually replaced it. Data is starting to emerge demonstrating clear advantages in terms of organ function and a reduction in second malignancies among the pediatric population.2–4 This has retrospectively justified its use in an area where it was long presumed to be advantageous. The problem is that, in the USA at least, 80% of patients treated with proton beam have prostate cancer.5 The reasons are simple. Between 3 and 5 prostate patients can be treated in the time it takes to treat a single complex pediatric case. The installation of a proton facility is so expensive that, at current rates of US reimbursement, a hospital can only begin to cover its debt with a high throughput of prostate cancer cases. This has lead to a dangerous distortion of patterns of care. Many centers that should be treating children treat prostate cancer cases, and many elderly patients with prostate cancer who should be having no treatment at all receive it unnecessarily. An “arms race” has begun with many centers taking a huge financial and moral risk investing in proton therapy to keep up with their competitors.6 As prostate cancer is the US economic engine for proton therapy it is worth spending some time assessing the evidence supporting its use.
2. Uncertainties in the physics and biology
The Bragg Peak has a tremendous therapeutic appeal but in vivo the distribution of the proton beam is subject to many perturbations and uncertainties. The deeper the beam in tissue the less certain one is of its stopping point. This “end of range uncertainty” means that the beam has to be planned to overshoot the target to guarantee good coverage. In addition, at greater depths there is considerable lateral scattering resulting in a significant penumbra making the lateral margins of the beam less sharp.7 Proton beam, like other forms of particle therapy, is highly subject to tissue inhomogeneities. Trofimov et al. have demonstrated the difference that small movements of the hips can make to a lateral beam delivered to the prostate.8
The Radio-Biologic Effect of the proton beam has been measured at 1.1 relative to X-rays yet this number may not be so precise. Just beyond the Bragg Peak it may be a little higher. Furthermore the RBE may differ slightly for different tissues. While this would not matter at lower doses when one is giving close to 80 Gy to a prostate, for example, small differences in RBE can be critical. A passively scattered proton beam, and this remains the prevalent delivery technique in 2013, also generates neutrons in the collimator with a very high RBE and a completely unknown contribution to the effect on normal tissues.
Many of these issues will either be clarified by future experimentation or modeling (RBE) or by moving to spot scanned beam techniques (neutrons) but, for now they may or they may not contribute to the morbidity of proton therapy or, at least, dilute the benefits.9,10
3. The clinical evidence
The management of localized prostate cancer has been controversial for decades. It has been difficult to decide whether or not any treatment is better than simple observation and, if so, which of the curative therapies is superior: surgery, external radiation, or brachytherapy. Among the radiation options new technologies have been readily adopted although there is remarkably little evidence of the benefit they bring. 3-D external radiation was shown to reduce rates of radiation proctitis over simple 2-D therapy.11 In the early 2000s IMRT became extremely popular and is now almost exclusively the external beam strategy of choice in the US. A recent analysis of RTOG data suggests that the advantage of IMRT over 3-D in terms of morbidity and quality of life may be remarkably small.12 It is impossible to turn back the clock but if this data had been available over a decade ago it is possible that the big switch to IMRT may have been slowed or, had there been no financial drivers, stopped altogether. New techniques of image-guidance may ultimately prove to be more significant than either the planning or beam delivery technique.
Convincing data now exists in the form of five randomized trials demonstrating that higher radiation doses are more likely to reduce the risk of prostate cancer recurrence and, in the case of high-risk tumors, the rate of metastases.13,14 This now drives the use of high-doses in practice with the presumption that only IMRT or proton beam are adequate to safely them. High quality evidence demonstrating that that is the case is indeed rare. At the end of the day the highest radiation doses are delivered by brachytherapy, a low cost and thus high-value alternative. Quality of life studies have failed to show that patients receiving brachytherapy fare any worse than those receiving any form of high-tech external beam treatment.15
One randomized trial has compared proton beam with conventional radiation and showed no difference in any outcome. Interpretation of this study is, however, greatly limited by the fact that it treated advanced cases who would have been better served by the addition of hormonal therapy and because it took place in the pre-PSA era when many patients would have had occult metastatic disease at the time of presentation.16
The work of the Massachusetts General Hospital has tested a number of hypotheses:
Does proton beam produce superior dose distributions over IMRT for prostate cancer? The answer is mixed. There is undoubtedly less of a “dose bath” to the anterior and posterior tissues but more radiation passes through the femoral heads and, because of beam uncertainty, the high-dose volume is actually a little larger with protons than IMRT17 Fig. 1. In addition, two regions associated with morbidities (the prostatic urethra and peri-prostatic nerve bundles) are treated equally with the two techniques. The volume of rectum treated likely depends more on image guidance, choice of margins, and the use or not of a rectal balloon than it does the delivery technique.
Fig. 1.
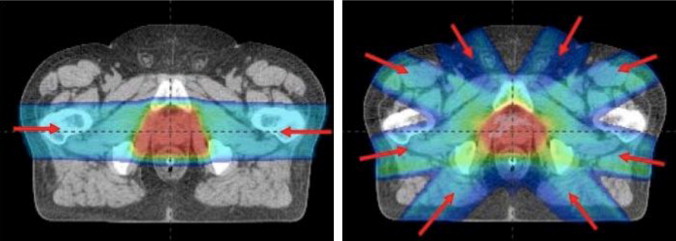
Comparative planning study showing the difference in dose distribution between opposed lateral, passively scattered proton beams and an intensity modulated photon plan.
Does proton beam cure patients with prostate cancer? Indeed it does, as do all forms of radiation delivered in high dose. A prospective phase III study clearly shows that doses of 79 Gy can be delivered safely with protons and that over 90% of low-risk patients may be cured this way. The problem is that 79 Gy can also easily be delivered by IMRT and probably by 3-D therapy also.18 A case-controlled study has also shown that the cure rates at 10 years from proton beam and brachytherapy appear to be identical19 Fig. 2.
Fig. 2.
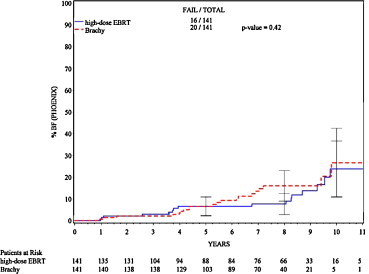
Case-controlled study from the Massachusetts General Hospital comparing patients treated with either high-dose proton beam radiation or low dose-rate brachytherapy. Figure shows cumulative biochemical recurrence rates.19
Can proton beam be used to dose escalate further? This has been tested in a phase II study of 90 patients. 82 Gy was delivered but unacceptable levels of rectal toxicity were reached implying that, for whole gland treatment using current techniques at least, 79 Gy is close to the practical limit.20
Can proton beam be used to escalate the dose further to part of the prostate gland? It is certainly appealing to imagine the entire gland being treated to one dose and a dominant nodule being treated to a higher dose. This could represent intelligent dose escalation without morbidity escalation and is theoretically possible using scanned beam techniques. The problem is that our imaging, although improving, rapidly, is currently insufficient to pick out a target within the prostate with great reliability. In addition our techniques of image-guidance and prostate immobilization may not yet be sufficiently reliable to allow us to sharp shoot in this manner.
Does proton beam reduce the morbidity of prostate treatment? This is, perhaps, the most important question of all. If it does then the size of the effect can be measured against the cost of the therapy and policy decisions about its value be made. Talcott et al. have performed some prospective and other cross-sectional quality of life studies on prostate cancer patients receiving proton beam therapy as well as IMRT and 3-D. The first item of note is that there is indeed significant sexual, bladder, and rectal dysfunction among patients after proton beam. While any treatment that covers the nerves, prostatic urethra, bladder neck, and anterior rectum would be expected to have these consequences somehow the “internet hype” around proton beam suggests that this is never the case. When the late effects (5–10 years) are measured against 3-D and IMRT few differences can be seen.21 A recent study by Efstathiou et al. suggests that there may be a small reduction in proctitis during the first post-treatment year but this is a single study and the effect was small and temporary.22 New data emerging from other US proton centers certainly show excellent (though non-comparative) quality of life-outcomes.23 Whether this is the result of the proton beam or the techniques of image-guidance adopted in parallel remains unclear.
A highly controversial study was recently published which used the SEER database to assess the late consequences of different types of external radiation therapy measuring the outcomes using billing codes for interventions such as cystoscopy, colonoscopy, or argon plasma coagulation.24 The data appeared to show that, for the time interval studied, the rectal morbidity of proton beam was actually higher than photons. There are many weaknesses in this kind of study, one that does not use information gathered directly from patients and which preceded the era of image-guidance, but it certainly does make the case that the superiority of proton beam cannot be presumed in every situation. It is possible that some of the current physical and biological uncertainties are indeed limiting.
4. Cost considerations
It is the cost of proton beam therapy that makes its use in prostate cancer, when there are many alternatives, so controversial. In the USA the price charged and the reimbursement delivered vary greatly from one insurer to another and from one state to the next and so the reimbursement for almost all forms of treatment fluctuate considerably. It is, however, generally agreed that the cost of proton treatment is greater than that of IMRT, which is, in turn, greater than brachytherapy, surgery, or active surveillance. Brachytherapy and active surveillance are generally agreed to be the “best bargains”. Many modeling studies have been performed which attempt to determine the value of proton therapy but most make assumptions about benefit which are, as was seen in the previous sections, somewhat shaky. One particular modeling study made the assumption that an additional 10 Gy could be safely delivered by proton beam pushing the delivered dose to 91 Gy and that this would lead to a small but detectable survival advantage for intermediate-risk men.25 Even then the cost per life saved (quality adjusted life years) was prohibitively high. As has been shown both these assumptions were incorrect anyhow.
The cost of proton beam remains very fluid especially in an era of cost control and health care reform and no-one is yet clear what direction prices are heading. There are, however, two changes on the horizon that will likely change the cost equation in some fashion. The first is that hypo-fractionated radiation is emerging as a convenient lower-cost method of administering external beam with high-level phase III support. It is possible that the homogeneity of the proton beam is better suited to large doses per fraction than the less homogeneous IMRT. Even so, other photon platforms for delivering these fractions with high accuracy now exist. If hypo-fractionation becomes the norm then proton beam will also become more competitive in terms of price.
A second trend is the development of smaller single-gantry proton facilties. Johnstone et al. have calculated that the debt incurred is easier to recuperate with a smaller proportion of prostate cases.5 With further technological advances it is likely that the cost differential between protons and photons will, in time, diminish.
5. Proton treatment for prostate cancer in the future
Prostate cancer treatments have, to date, largely employed opposed lateral beams because the end-of-range uncertainty has made overshooting of the target necessary. Any beam angled toward the anterior would thus enter the rectum in an undesirable way. Unfortunately, lateral beams have to take the longest route to the prostate possible and must pass through the inhomogeneous and mobile hips. It has been said that in this scenario we are taking an advanced technology and, frankly, getting the least out of it. It is clear that the technology will not stand still and the development of scanned proton beams with their ability to be modulated should further increase the conformality of the proton beam in the near future allowing more creative beam angles and target shaping. The advantage of IMRT in that it can scoop out a hollowed volume on the rectal side of the prostate will be mimicked and perhaps exceeded, by intensity modulated protons. Partial prostate boosting may become more of a technical reality with scanned beams if imaging can improve correspondingly.
Much of the controversy over prostate cancer comes from its high price tag and much would evaporate if the cost came down substantially. This is likely to happen but the installation costs of even a single gantry facility will be greater than linear accelerators for the forseeable future and treatment costs will always greater than simple brachytherapy or active surveillance because of the high staffing and maintenance levels required. Thus, the debate will continue to turn upon the advantage patients (or payers) are receiving for the additional cost. As I have shown the evidence so far does not suggest that this advantage is great but it may still be present at a level that has been difficult for recent studies to detect. For many years the call has been heard for the establishment of a randomized trial. The strong arguments against come from those who feel that the do simetry speaks for itself, that protons cannot be inferior, and thus an RCT is ethically unjustifiable. It also comes from those who feel that RCTs are simply not the way to solve technology questions. An RCT for every new technology would slow down the engine of technological innovation, indeed bring it to a halt. Most technologies are simply sharper knives and where would one draw the line of RCT necessity? Others feel that the RCT should be used for technology but rarely and selectively.26 This will be for: new technologies that are vastly expensive and potentially disrupt the flow of health care dollars elsewhere; for technologies that potentially introduce new biology and thus may have unpredictable outcomes; and for technologies that require a great deal of retraining and recertification. Proton beam fits the bill. No-one would argue for a trial in every clinical scenario. For example, most would be very uncomfortable randomizing children to proton beam or IMRT when the IMRT dose carries such risk. Equally there would be little value randomizing skin cancers where no benefit could be anticipated, or perhaps diseases such as localized lymphomas or plasmacytomas where the radiation doses are relatively low. Prostate cancer fits into a gray zone of high use and high cost but unpredictable outcomes. If nothing else an RCT would quantify the benefit, if there is any, which could be used to inform the debate, and drive rational policies. The same is also true of proton beam in lung cancer where the unpredictable behavior of proton beam across air cavities makes the ultimate outcome uncertain.
Despite the media and internet hyperbole patients do appear willing to participate in such a trial for prostate cancer and several major US proton centers led by the Massachusetts General Hospital and University of Pennsylvania have recently launched a phase III study comparing proton beam against IMRT for localized low and low-intermediate risk prostate cancer (http://clinicaltrials.gov/ct2/show/NCT01617161), with patient-reported quality of life outcomes, as well as other clinical, physical, biological, and economic end-points. It is hoped that the results of this trial will come before the landscape of prostate cancer treatment has been changed in a dramatic and, perhaps needless, fashion.
Financial disclosure statement
None declared.
Conflict of interest statement
None declared.
References
- 1.Wilson R.R. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Miralbell R., Lomax A., Cella L. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald S.M., Safai S., Trofimov A. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71:979–986. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Childs S.K., Kozak K.R., Friedmann A.M. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82:635–642. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone P.A., Kerstiens J., Helsper R. Proton facility economics: the importance of “simple” treatments. J Am Coll Radiol. 2012;9:560–656. doi: 10.1016/j.jacr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Elhahal S.M., Kerstiens J., Helsper R.S. Proton beam therapy and accountable care: the challenges ahead. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Goitein M. Magical protons? Int J Radiat Oncol Biol Phys. 2008;70:654–656. doi: 10.1016/j.ijrobp.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 8.Trofimov A., Nguyen P.L., Efstathiou J.A. Interfractional variations in the setup of pelvic bony anatomy and soft tissue, and their implications on the delivery of proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:928–937. doi: 10.1016/j.ijrobp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner D.J., Elliston C.D., Hall E.J. Reduction of the secondary neutron dose in passively scattered proton radiotherapy, using an optimized pre-collimator/collimator. Phys Med Biol. 2009;54:6065–6078. doi: 10.1088/0031-9155/54/20/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang S., Both S., Bentefour H. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys. 2012;83:408–418. doi: 10.1016/j.ijrobp.2011.06.1974. [DOI] [PubMed] [Google Scholar]
- 11.Dearnaley D.P., Khoo V.S., Norman A.R. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353:267–272. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 12.Michalski J.M., Yan Y., Watkins-Bruner D. Preliminary analysis of 3D-CRT vs. IMRT on the high dose arm of the rtog 0126 prostate cancer trial: toxicity report. Int J Radiat Oncol Biol Phys. 2011;81:S1–S2. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zietman A.L., Bae K., Slater J.D. A randomized trial comparing conventional dose conformal radiation therapy with high-dose in early stage adenocarcinoma of the prostate. Long-term results from Proton Radiation Oncology Group (PROG)/American College of Radiology (ACR) 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuban D.A., Levy L.B., Cheung M.R. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer: who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–1317. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Sanda M.G., Dunn R.L., Michalski J. Quality of life and satisfaction with outcome among prostatecancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 16.Shipley W., Verhey L., Munzenrider J. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;30:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 17.Trofimov A., Nguyen P.L., Coen J.J. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelefsky M.J., Fuks Z., Hunt M. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 19.Coen J.J., Zietman A.L., Rossi C.J. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int J Radiat Oncol Biol Phys. 2012;82:e25–e31. doi: 10.1016/j.ijrobp.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Coen J.J., Bae K., Zietman A.L. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology Phase II study 03-12. Int J Radiat Oncol Biol Phys. 2011;81:1005–1009. doi: 10.1016/j.ijrobp.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 21.Talcott J.A., Rossi C., Shipley W.U. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. J Am Med Assoc. 2010;303:1046–1053. doi: 10.1001/jama.2010.287. [DOI] [PubMed] [Google Scholar]
- 22.Gray P.J., Paly J.J., Yeap B.Y. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer (ePub) 2013 doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendenhall N.P., Li Z., Hoppe B.S. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–221. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Sheets N.C., Goldin G.H., Meyer A.M. Intensitymodulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. J Am Med Assoc. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konski A., Speier W., Hanlon A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 26.Zietman A., Tepper J., Goitein M. Technology evolution: is it survival of the fittest? J Clin Oncol. 2010;28:4275–4279. doi: 10.1200/JCO.2010.29.4645. [DOI] [PubMed] [Google Scholar]


