Abstract
All radiation devices in use nowadays are subject to cause serious incidents and accidents, with potential risks in exposed population groups. These risks may have immediate or long term health implications. The detection of radioactive incidents is a procedure that should be systematized in economically developed societies. International organizations may provide support to other states in the event of a radioactive incident. Prevention, mitigation and treatment of the radiation effects are done by anticipating the moment of exposure and by establishing new efforts for investigation of radioprotective products.
In this article we will analyze the causes of radiological incidents, the means to detect them, and the current preventive and therapeutic procedures available, with special emphasis on new biodosimetry methods for triage and investigational radioprotective drugs. Finally, we will explore the most efficient measures, for future prevention.
Keywords: Radioactive accident, Radiological incident, Biodosimeter, Radioprotection, Nuclear prevention
1. Introduction
The response to emergency events, such as chemical, biological, radiological or nuclear accidents, requires the existence of a general health organization. These events are usually classified together in order to share common protocols.1 However, each of these risks has its own idiosyncrasy. An accident refers to an undesired event which may cause death, an established illness, injuries or other damages. An incident is the event which, without causing such harmful damages, has the potential to cause an accident. Risk assessment of an incident is of great interest, for it may reveal unknown or underestimated risks, therefore controlling processes before damages may occur.
Undeniably, the prevention of radioactive incidents requires the identification of the environments in which they may occur. These were classically classified into two clearly different scenarios: Civilian or accidents in time of peace (radiation and health industry)2 and War time or terrorist attacks.3 Newer risk environments such as the exposure to cosmic rays by pilots or astronauts were later added.4 In this sense, the increasing use of air transportation may extend the use of preventive procedures to non-professional population in these areas. Recently, the limits between civilian and war time accidents and their consequences have become less clear. International organizations concerning atomic energy are developing rules and procedures to prevent the uncontrolled use of ionizing radiations in the sanitary and industrial fields as well as to minimize the effects of a possible massive use for military purposes.
The experience gained from incidents occurring during peace time with ionizing radiation should be used to reassess catastrophe protocols.5 Drills should include actions not only in terms of radioactive damage but also in that of traumatisms, burn wounds, inhalation injuries and psychological impact. The current world scenario is more prone to terrorist-derived “dirty bomb” type incidents,6 and in these cases, economic, political and mass media response should be optimized.
1.1. Response to a radiological accident or radiological terrorist attack
If a radioactive accident is expected, it is important to have a strategic plan. The following steps should be taken in order to systemize the procedures:
-
(a)
Determining the radioactive incident: This report should normally be obtained through a statement of those implied in the incident. Should this not be the case, a series of environmental dose detector stations should be established, with reinforcement in those areas at a higher risk. Along with this information, other interfering data, such as weather conditions, should be gathered.
-
(b)
Establishing initial estimations using environmental dosimetry. This can be obtained from detection stations or by sending moving field detectors, or using aircraft, to obtain dose distributions.
-
(c)
Population assessment. Immediate assessment of the affected area's demographics in relation to the total dose received, therefore establishing the radiation dose received by different population groups.
-
(d)
Decontamination. Decontamination teams should be sent to affected areas or victims brought to previously built decontamination stations.
-
(e)
Sample gathering: This should take place once the decontamination process has concluded.
-
(f)
Preliminary assessment. Once enough information is gathered about each patient, the risk of each patient should be established individually. In severe individual cases, patients should be carried to reference hospitals for further treatment. In case of mass affection, protocols should be properly adapted for optimization of all available means.
-
(g)
Rapid analysis of biological dosimetry, in hours or days depending on the procedure used. Biological injury will be related to the clinical presentation of the radiation syndrome.
-
(h)
Establishing the most appropriate medical treatment to deal with the deterministic effects of radiation. Depending on the complexity of the treatment required, patients that have received lethal doses of radiation will be sent to low level medical centres to receive palliative measures. Patients that receive near-lethal doses will be sent to maximum level centres for highly sophisticated treatments such as bone marrow transplants. Those with sub-lethal doses will be sent to medium-level equipped centres for supportive treatment. Finally, patients who have received a minimum dose of radiation, without deterministic effects, will be sent to primary care units for thorough symptom registration for future follow-up of long term or stochastic effects.
-
(i)
Deferred analysis of stochastic effects, by means of periodical medical follow-up and examinations. Stochastic effects of radiation are the appearance of cancer or congenital disorders in the patient or descendants.7 One of the most typical tumor is the papillar thyroid cancer. This could be due to either chance, or to a certain genetic susceptibility that would favour the appearance of these diseases in certain families.8 (Fig. 1).
Fig. 1.
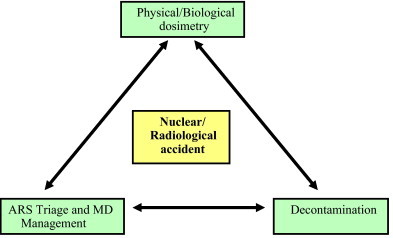
Strategic plan for radiation accidents.
2. New methods of detection of radiation effects
Biological dosimetry refers to procedures that quantify radioactive damage. They consist of laboratory tests that correlate the genetic modifications that take place with the amount of dose received. These biological dosimeters quantify biological signals and establish a relationship with the amount of the radiation exposure dose.
Presently, the most suitable biodosimetry methods for epidemiologic studies are: Dicentric chromosome assay, fluorescence in situ hybridization (FISH) of peripheral blood lymphocytes and Electron paramagnetic resonance (EPR) measurements made on tooth enamel. These types of measurements, however, are usually invasive and require difficult to obtain biological samples. Moreover, doses derived from these methods are not always directly relevant to the tissues of interest.
2.1. Dicentric chromosome assay (DCA)
This is currently the tool of choice. Because the assay is labor-intensive and time-consuming, strategies are needed to increase throughput for use in radiation mass casualty incidents. One of the strategies to overcome its limitations is to truncate metaphase spread analysis for triage, as reported in Room's study.9 The triage dose is estimated by scoring 50 or fewer metaphases, compared to a routine analysis of 500–1000 metaphases, and to increase throughput using a large group of scorers in a biodosimetry network. These results demonstrate that for rapid triage of acute radiation syndrome (ARS), a network of cytogenetic biodosimetry laboratories can accurately assess doses even with a lower number of scored metaphases.
2.2. Fluorescent in situ hybridization
The fluorescent in situ hybridization (FISH) of cytogenetic samples is more selective for the detection of chromosomal translocations. These procedures are highly laborious and require a long processing technique. This is why they are of little use in the event of a medical emergency. However, they can be used in medical check-ups in workers with radiation exposure. A recent study examined aberrations in FISH painted chromosomes in vitro irradiated blood samples, and the results suggested that translocations can be used as a test to identify individuals with a potentially elevated radiosensitivity.10
2.3. Electron paramagnetic resonance (EPR) in vivo dosimetry
It is feasible to perform tooth dosimetry measurements in situ, greatly expanding the potential for using this approach for immediate screening after radiation exposures. EPR tooth dosimetry, have an immediate and permanent signature that reflects the dose. The current cycle time of 5 min enables this method for high-throughput primary screening of hundreds of patients per hour at a response center when using multiple systems11,12. Other physical techniques (e.g. EPR based on nails) may have a gradual fall-off in measurable intensity, and this needs to be taken into account in assessing when such measurements are appropriate.
The characteristics that would make a biodosimetry method ideal from the point-of-view of assisting long-term health risk studies are numerous and while each attribute might have varying importance for different studies, methods should include some or all of the following attributes:
-
•
Register the actual absorbed energy regardless of type of ionizing radiation to which it is exposed.
-
•
Have a radiation-induced signal that is stable over long periods of time (tens of years at minimum).
-
•
Be specific to ionizing radiation.
-
•
Has a well-characterized dose–response.
-
•
Have low inter-individual variation.
-
•
Have a low minimum detectable dose (on the order of a few tens of mGy) or at least, be able to measure doses that are as low as those received by a substantial fraction of the subjects of the epidemiologic study.
-
•
Have moderately good precision (on the order of ±30%) at two-times the minimum detectable dose (and possibly better at higher doses)
-
•
Have good accuarcy(low bias).
-
•
Be field-friendly.
-
•
Depend on minimally invasive sampling.
-
•
Produce a measurement that directly reflect the absorbed energy in a single identifiable tissue.
-
•
Produce measurements that can be interpreted to reflect doses in other organs besides the tissue assayed.
-
•
Have low per-sample cost.
In practice, all of those conditions are not met by any current biodosimetry technique. In particular, the characteristics of low invasiveness and low cost have not been met. Of these numerous characteristics, all are not equally important to all long-term health risk studies though the combination of attributes available in a particular biodosimetry method would determine its usefulness for any particular study.13
Other biososimetry methods that are under investigation, and can be potentially applied in large scale incidents, are:
2.4. Blood protein immunoassay
Cytokines are a class of proteins and glycoproteins involved in intercellular signaling. Most act through autocrine and paracrine cellular communication but can be found in the circulation. They include growth factors, angiogenesis and angiostatic proteins, interleukins, adhesion molecules and chemokines. Subsequently, the impact of TGFβ1 on pulmonary radiation effects has now been studied extensively in humans. The bulk of current evidence suggests that if TGFβ1 is elevated for a long period of time before, during, or after irradiation, the risk of pulmonary toxicity increases. The most promising macromolecular markers are cytokines: TGFβ, IL-1, IL-6, and TNFα being lead molecules in this class as both markers and targets for therapy.14
In one study, human cell lines were screened by enzyme-linked immunosorbent assay (ELISA) for the expression of a dozen secreted cytokines that have been reported to have changes in protein or mRNA levels. They identified four cytokine molecules that had altered levels after radiation exposure, one of which, Interleukin (IL) 6, was consistently elevated after irradiation in vitro and in vivo.15
2.5. Rapid automated biodosimetry tool (RABIT)
This is a new method of biologically based biodosimetry developed at Columbia University, designed to be a completely automated, ultra-high throughput robotically based biodosimetry workstation.15 It analyzes fingerstick-derived blood samples, designed to score either micronuclei or phosphorylation of the histone H2AX. The rapid phosphorylation of histone H2AX at serine 139 (γH2AX) serves as a sensitive marker for DNA double-strand breaks induced by ionizing radiation or other genotoxic agents. Parallel handling of multiple samples through the use of dedicated, purpose-built, robotics and high speed imaging allows analysis of up to 30,000 samples per day.16
2.6. Gene expression microarray
It is possible to specifically detect radioactive damaged DNA fragments using gene expression microarrays. There is a report17 on the development of a biodosimetry device that comprises a set of 14 genes selected on the basis of their abundance and differential expression level in response to radiation. Gene expression is analyzed through direct signal amplification using a quantitative Nuclease Protection Assay (qNPA). The assay can be performed with volumes as small as 30 μL of whole blood, which is compatible with collection from a fingerstick. The results revealed statistically significant discrimination between irradiated and non-irradiated samples. These results indicate that it is a valid test to measure biological radiation exposure based on a small number of genes. The scalability characteristics of the assay make it appropriate for population triage.18
Gene expression signatures are looking increasingly attractive as potential biodosimeters for radiation exposure. However, further validation in terms of in vivo responses in cancer patients and animal models (including non-human primates), inter-individual variability and radiation specificity of the signatures is still needed. Other future areas for investigation also include the study of gene expression responses to partial body irradiation, responses to internal emitters, and the effects of age, co-morbidities, combined injuries, low dose-rate exposures, and different radiation qualities.
2.7. Metabolomics
This method is the profiling of induced small-molecule metabolites. Irradiation in vivo triggers the expression of many genes involved in intercellular signaling, whose proteins can have wide-ranging effects on cellular metabolism. These changes are reflected in alterations in the spectrum of small-molecule metabolites in blood, urine, and saliva. Such metabolomic analyses offer several key advantages, particularly simple, non invasive collection, and thus the potential for very high-throughput radiation biodosimeter screening. Distinct metabolomic changes have been detected within hours of exposure to doses as low as 0.5 Gy, and some alterations persist for at least 30 days. Studies in this field to date have focused on higher radiation doses, but it has great potential also for low-dose studies. The high-throughput and non-invasive nature of sample collection may even make it feasible for epidemiological studies.19,20 (Fig. 2).
Fig. 2.
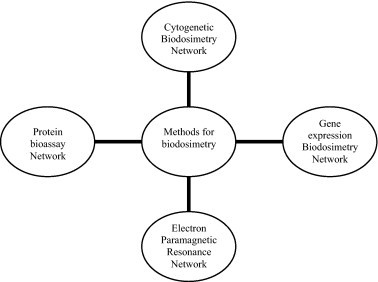
Networks of expert reference laboratory for dose assessment.
3. Radioprotective drugs
The molecular mechanisms activated in an irradiated tissue, involve pathways that communicate not only between cell types within the tissue but also between the vasculature, its coagulation system, and the bone marrow-derived inflammatory and immune systems. Late normal tissue damage reflects a failure to regenerate functional tissue, whether through a lack of stem cells or radiation-induced dysregulation of the normal healing process.
A common alternative pathway of tissue restoration is by fibrogenesis, which is often invoked to maintain structural integrity. After irradiation, there are recurring patterns of expression of pro- and antiinflammatory cytokines, molecules that promote and diminish cell adhesion, proteases and antiproteases, oxidants, and antioxidants. This interactive network of factors is critical to tissue regeneration and healing after irradiation, and it changes with time and space to move the healing processes forward in a controlled way. The severity of injury, extent of cell death, nature and extent of disease, and adjunctive therapies will all impact the balance of forces within the network.
The radiation-induced bystander responses are the occurrence of biological changes in unirradiated cells in the proximity of or sharing medium with cells that have been traversed by ionizing radiation. An important characteristic of bystander effects is that the responses occur at low radiation doses (<0.05 Gy), increase rapidly with dose, and then reach a plateau, usually by 0.1–0.3 Gy. When normal human fibroblasts are irradiated with 0.1–0.5 Gy X rays, bystander cells exhibit increased DNA damage in the form of c-H2AX foci or micronuclei and increased reactive oxygen species (ROS) generation. With all three radiation types, the bystander responses are decreased by addition of catalase, SOD or c-PTIO to the shared medium, indicating roles for hydrogen peroxide, superoxide and/or NO in the signaling. These data suggest that the increase of damage in the irradiated population at low doses is a result of bystander signaling rather than a direct effect of radiation on traversed cells. The bystander signaling depends on cell type, radiation quality and end point. At low-dose of radiation exposures presented there may be an epigenetic modification of DNA. The epigenome refers to the sum of heritable alterations in DNA that do not involve base sequence changes and includes histone modifications, DNA methylation and chromatin conformation changes.21
Clearly, there are multiple possible targets for intervention aimed at improving the outcome, although minimizing the effect of any one molecule may not have the same effect in all tissues, at all intervention times, and at all levels of damage.22
Radioprotection refers to the reduction of cytotoxic damage induced by ionizing radiation to normal tissues by chemical or physical agents. Radioprotective drugs can minimize the organic impact of radiation in comparison to the natural history of a patient without these drugs. Many of these drugs were developed during the Cold War to prepare the army and the general population in case of nuclear attacks. Initially, they were to be used before an attack took place, though they may also be used immediately afterwards. Some of these agents have been later used in the clinic to protect normal tissues during radiotherapy, thus the experience with them has been broadened. Great amounts of drugs have been researched, though few conclusions have been reached as to which substances should be part of a strategic plan in the event of a radioactive incident or accident.
There is a challenge for Renin-angiotensin system (RAS) suppression as a modulator of late radiation injury because the mechanism is currently unknown; it may be less of a challenge for agents aimed at Chronic oxidative stress because their development is more mechanistically driven. Irradiating late responding normal tissues leads to chronic increases in reactive oxygen species (ROS) that serve as intracellular signaling species to alter cell function/phenotype, resulting in chronic inflammation, organ dysfunction, and ultimate fibrosis and/or necrosis.23
There are three fundamental approaches to pharmacologic intervention. Protection or prophylaxis would refer to therapies that must begin before the time of irradiation; a classic example is the use of a free radical scavenger such as amifostine. Mitigation would refer to therapies that could begin after irradiation but before there was overt evidence of clinical disease; examples include the use of angiotensin-converting enzyme (ACE) inhibitors (e.g. Captopril) to mitigate radiation-induced pulmonary, renal or central nervous system (CNS) injuries. Treatment would refer to therapies that could be effective after overt clinical disease has developed; examples include the use of pentoxifylline and tocopherol to treat radiation-induced fibrosis24 (Fig. 3).
Fig. 3.

Pharmacologic intervention.
3.1. Amifostine
Amifostine (WR-2721) is an organic thiophosphate that was selected from over 4400 compounds that were screened by the US Army as the best radioprotectant compound. Its subsequent declassification made the drug available for medical experimentation. It is dephosphorylated by alkaline phosphatase at the tissue site to its active metabolite WR-1065. Differential alkaline phosphatase activity and intracellular pH have been shown to result in differential uptake of WR-1065. Once inside cells, WR-1065 acts as a scavenger of free radicals that are induced by ionizing radiation.
The cytoprotective mechanism of amifostine is complicated, involving free radical scavenging, DNA protection and repair acceleration, and induction of cellular hypoxia. Amifostine is a broad-spectrum cytoprotective agent, with activity directed to all normal tissues; the only exception is the central nervous system because the blood–brain barrier prevents the accumulation of clinically relevant concentrations of amifostine metabolites in neural and glial cells.
The drug is administered intravenously, although a subcutaneous administration is also feasible, with a bioavailability of 72%. Because amifostine is inactivated by gastric acidity, oral administration is not possible. In oncology practice, amifostine was approved by the FDA for radiotherapy-induced dryness of mouth in head and neck cancer patients, and for prevention of renal damage from cisplatin.25 Additionally, diminished dysphagia, hematologic toxicity and mucositis have been reported.
Important experimental evidence has been also provided on the protective role of amifostine against leukemogenesis and carcinogenesis. Amifostine prevents the induction of mutations of the hypoxanthine-guanin phosphoribosyl transferase gene induced by radiation. Results of in vivo experiments in mice showed a significant reduction in the rate of development of leg fibrosarcomas when amisfotine 400 mg/kg was administered before a single dose of 35–57 Gy gamma-irradiation. As the development of malignancies after irradiation can be quite high, amifostine may also prove of value in the prevention of stochastic effects of radiation, especially for long-term survivors.
Although well tolerated in general, intravenous administration can result in hypotension, nausea and emesis. The main caveats pertaining the use of this drug are that is expensive, it is not self-administrable and it is not suitable for prolonged use. Also its lack of brain radioprotection can limit its use in some patients suffering of acute radiation syndrome.
3.2. Keratinocyte growth factor
Keratinocyte growth factor (KGF, Palifermin), a member of the fibroblast growth factor family, is an important stimulator of epithelial cell growth, regeneration and repair. The potential therapeutic use of KGF has been evaluated in disease models associated with damage to epithelial cells of the skin, digestive tract, and bladder. KGF has shown beneficial effects in models of dermal injury, in irradiation-induced oral and gastrointestinal mucositis, and in preventing lung injury caused by radiation.26
KGF is produced by mesenchymal cells located adjacent to the epithelium, pancreas, liver, lung, urothelium, prostate epithelium and other tissues. It is produced by dermal fibroblasts within the skin and by lamina propria cells of the intestines. The widespread expression of KGF in normal tissues indicates a role in homeostasis. The level of KGF messenger mRNA within dermal wounds is 160-fold greater than the level found in intact skin, and it is also elevated in the intestines of patients with inflammatory bowel disease. These observations indicate that increased production of KGF is a normal response to epithelial injury and that it may represent a component of damage repair.
Palifermin is being developed to reduce the severity and duration of oral mucositis and related clinical sequelae and improve patient functioning after radiation therapy to the head and neck area, and after total body irradiation in patients with haematological malignancies.27 A significant reduction on the incidence of severe oral mucositis was found in patients treated with chemoradiation for head and neck cancer receiving weekly intravenous palifermin, at a dose of 120 μg/kg, in a double-blind, randomized trial.28
The rational for the use of palifermin in the treatment of acute radiation syndrome is early stimulation of oral and gastrointestinal mucosa regeneration, ameliorating the enteritis that would follow accidental whole body irradiation.
3.3. Melatonine
Melatonin (N-acetyl-5-mehoxytryptamine) is a substance produced by the pineal gland which is also known to have robust radioprotective features. Both human and experimental animal studies have clearly shown that it is a unique antioxidant and a DNA and chromosome protector against a variety of harmful agents including ionizing radiation. In the human body, melatonin is not exclusively produced by pineal gland; every single cell with DNA may produce melatonin in small amounts basically to protect themselves from the harmful effects of free radicals.29 Ionizing radiation generates free radicals an also directly hit DNA.
Melatonin is an amphipathic molecule and can readily enter all cells. There is no known biological barrier for melatonin, including the blood–brain barrier. After oral administration, melatonin rapidly passes into blood stream as well as into the cerebrospinal fluid, bile, seminal, amniotic and ovarian follicular fluid. Melatonin has been administered in both physiological and pharmacological amounts to humans and animals, and there is widespread agreement that it is a non-toxic and non-teratogenic molecule. It has been recently documented that melatonin is an excellent candidate as a countermeasure against radiation exposure.30 It reduces the oxidative injuries due to ionizing radiation, decreasing the risk of cancer and several other health problems which can be seen in decades after a nuclear accident. It would be indicated for people not heavily irradiated.
3.4. Captopril
The angiotensin-conveting-enzyme (ACE) inhibitor captopril is an effective mitigator of pulmonary dysfunction caused by survivable doses of radiation. At doses (on a mg/m2/day basis) approved by the FDA for use in humans, captopril improves vascular, functional and structural derangements that develop in the rat lung by 8 weeks after a single dose of radiation. Also the initiation of captopril therapy after a delay of 1 week following injury also has mitigating properties.31
ACE inhibitors and antagonists (e.g. Captopril) of angiotensin type 1 receptors (e.g. Losartan), are able to mitigate radiation nephropathy,32 radiation injury to the central nervous system and skin in rats.33 All these advantages make it imperative to continue investigations on a promising drug that can reduce morbidity after exposure to radiation.
Inhibition of ACE is considered to be cardioprotective in part by suppressing the breakdown of bradykinin by ACE. The roles of renin-angiotensin-system (RAS) and bradykinin in cardiac radiation injury need further investigation.34
3.5. Antioxidants
Superoxide dismutase agents have shown promising results in the reduction of acute and long-term radiation-induced injuries. Endogenous superoxide dismutases (SODs) are natural enzymes that catalyze the conversion of single-electron reduced species of molecular oxygen to hydrogen peroxide and oxygen. Free copper/zinc (Cu/Zn)-SOD (bovine) was developed in the 1980s as a new drug with a very short serum half-life for treatment of severe inflammatory diseases.35 Several studies have described a radioprotective effect when SOD is given during irradiation. Free Cu/Zn-SOD (orgotein) was administered during pelvic irradiation to reduce acute effects, such as cystitis and proctitis, in double-blind randomized trials, but the results are controversial.
There is some evidence that therapies aimed at reducing oxidative stress are effective in reducing radiation-induced normal tissue injury. In experimental models, it has been shown that preirradiation introduction of manganese superoxide dismutase (MnSOD) plasmid/liposomes provides protection against a wide range of normal tissue injuries.36,37 However, because the MnSOD plasmid/liposomes therapy is started before irradiation, the efficacy could be caused by classic radioprotection.
3.6. Steroids
Corticosteroids have long been used extensively to reduce edema caused by radiation. They have been used to attenuate late radiation induced effects since the 1950s. In vitro, steroids inhibit polynuclear cell and macrophage recruitment, collagen synthesis, prostaglandins, and leukotrienes. In vivo, dexamethasone has been used to treat radiation pneumonitis, nephropathy, and liver injury in rats and appears to delay development of organ dysfunction.38 In the radiation pneumonitis treatment, according to retrospective studies, steroids are used: prednisone 1 mg/kg/d for several weeks followed by a slow decrease in the dose.39
Dexametasone has proved effectiveness in the treatment of radiation-induced vomiting. Also, corticosteroid therapy (methylprednisolone) may improved clinical and radiological/endoscopy features after several weeks, in patients with radiation enteritis and proctitis secondary to radiotherapy.40
3.7. Pentoxyfilline and tocopherol
It has been suggested that the combination of pentoxifylline and tocopherol may abrogate radiation-induced fibrosis through antioxidant effects. The possible utility of pentoxyfilin-vitamin E (PE), is being investigated to decrease lung toxicity, improving parameters as the diffusion capacity and the lung perfusion, and is useful in the prevention and treatment of fibrotic injuries.41
Recent in vitro studies using PE have shown an antioxidant effect in irradiated human fibroblasts or endothelial cells, by reducing immediate and late reactive oxygen species (ROS) production, and interference with DNA repair processes. Long-term tolerance of combined PTX-vitamin E is excellent for its use for long periods of time, and the PE combination is an efficient and safe treatment of moderately severe cases of established radiation induced fibrosis.42
Animal experimentation has proved that haematopoietic growth factors treated with this product in blood transfusions show a survival increase, as these organisms are better prepared to fight against the sepsis that usually takes place in animals subject to lethal radiation.43
3.8. Sulfasalazine
Sulfasalazine (SAZ) and its metabolite, 5-aminosalicylic acid (5-ASA) is an aminosalicylate that is an established agent for the treatment of inflammatory bowel disease through a reduction of prostaglandin production and has a free radical scavenging activity.44 Sulfasalazine 500 mg orally twice daily can help to reduce the incidence and severity of radiation-induced enteropathy in patients receiving external beam radiotherapy to the pelvis. Several small trials of 5-ASA, orally or enemas for radiation proctitis have produced mixed results.40
Some of these products, like melatonin and antioxidants, can be taken for long periods of time since they lack of secondary effects, and, therefore, can be indicated for people exposed during days or weeks to small amounts of irradiation, not enough for causing acute radiation syndrome, but certainly enough to cause an increase in the incidence of cancer in the future.
4. Future actions
Future actions in the prevention of radioactive incidents should focus on economical areas, politics, international diplomacy, central intelligence services, and of course, investigation. Medical services should be instructed in the management of the effects of total body irradiation, with a need to identify treatment experts for these patients. In this sense, specialists with professional competence in this field are haematologists, radiation oncologists, nuclear medicine and health physicits.45
In terms of detecting genetic damage, certain individuals’ genetic status must be taken into account depending on the biodosimetrical system used, as the most commonly laboratory procedures usually quantify DNA alterations. A genetic bank would be useful to compare biological situations before and after radioactive impact. The current data suggest that late normal tissue injury is the result of the interaction of multiple genes, each making a relatively minor contribution to the phenotype, making it difficult to identify each gene. Layered on top of this genetic complexity is the likelihood that at least some of these genes interact with environmental factors, which currently are completely undefined.46
There is certain progress in the investigation of pharmacological presentations that help radioprotective drugs reach the inside of tissue cells. Gene therapy can play an important role in this area, as with the use of manganese superoxide dismutase plasmid liposome.47 The transfer of a radioprotective gene into normal tissue cells would provide the opportunity to reduce the risks associated with haematopoietic or intestinal toxicity after irradiation. Several potentially radioprotective genes like multidrug resistance 1 (MDR1), snail homolog 2 (SNAI2), and superoxide dismutases have been evaluated in preclinical models for their radioprotective potential in the last years. For gene transfer and ectopic expression, adenoviral, adeno-associated virus (AAV) or retroviral vectors were used.48Gene therapy of attenuated Salmonella typhimurium Ty21a-recombined KGF gene (TPK strain) ameliorated radiation-induced pulmonary injury in an experimental rats model at acute inflammatory pneumonitis phase. Administration of TPK strain could decrease radiation-induced reactive oxygen species (ROS) and TGF-β expression in lung tissues, and increase SP-A and SP-C levels against radiation damage. This study suggested that this gene therapy is a promising potential treatment for radiation-induced pulmonary injury. Further studies are needed to assess the effects of TPK strain on the pulmonary fibrosis phase.49
Post-radiation inflammatory reaction leads to an irreversible pulmonary fibrosis which may cause lethal respiratory insufficiency. Pathological inflammatory and fibrotic changes might be attenuated by inhibiting tumour necrosis factor (TNF)-α activity using TNF-α soluble receptors. In a study,50 an experimental antifibrotic gene therapy with the plasmid vector encoding a mouse soluble receptor I for TNF-α (psTNFR-I) was assessed. The ability of psTNFR-I expressing vector to transfect cells, and its biological activity in vitro and in vivo were examined by PCR, RT-PCR, MTT assay and ELISA. Sequential administration of soluble TNFR-I by a nonviral, intramuscular gene transduction in the early and late post-radiation inflammatory phase prolonged survival of irradiated mice and attenuated the symptoms of lung fibrosis. They conclude that psTNFR-I gene transduction may provide a safe and simple method to partially neutralise TNF-α activity and prevent radiation-induced lung injury.Conclusion
Future actions in the prevention of radioactive incidents should focus on the implementation of emergency plans and establishing the most appropriate medical treatment in case of a nuclear accident occurs.
The actual use of pharmacologic approaches for modulation of radiation injuries after a mass-casualty event would be severely limited. Most of these agents are still experimental. Getting agents approved and labelled for such use, as would be required in a mass-casualty event, will be challenging.
Radioprotective gene therapy represents a very promising method for reducing radiotherapy-related cytotoxicity of normal tissue cells and thus may improve therapy success and the patient's quality of life.
References
- 1.Bland S.A. Chemical biological and radiation casualties: critical care considerations. JR Army Med Corps. 2009;155(2):160–171. doi: 10.1136/jramc-155-02-17. [DOI] [PubMed] [Google Scholar]
- 2.Zielinski J.M., Garner M.J., Band P.R. Health outcomes of low-dose ionizing radiation exposure among medical workers: a cohort study of the Canadian national dose registry of radiation workers. Int J Occup Med Environ Health. 2009;22(2):149–156. doi: 10.2478/v10001-009-0010-y. [DOI] [PubMed] [Google Scholar]
- 3.Poston J.W., Sr., Ford J.R. How do we combine science and regulations for decision making following a terrorist incident involving radioactive materials? Health Phys. 2009;97(5):537–541. doi: 10.1097/HP.0b013e3181b0c06d. [DOI] [PubMed] [Google Scholar]
- 4.Butler G.C., Nicholas J., Lackland D.T. Perspectives of those impacted: airline pilot's perspective. Health Phys. 2000;79(5):602–607. doi: 10.1097/00004032-200011000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Akashi M., Kumagaya K., Kondo H. Concerns of disaster medical assistance team (DMAT) members about troubles at the nuclear power plant: experience from the Niigata Chuetsu-Oki earthquake. Jpn Health Phys. 2010;98(July (6)):804–809. doi: 10.1097/HP.0b013e3181ac924a. [DOI] [PubMed] [Google Scholar]
- 6.Wolbarst A.B., Wiley A.L., Jr., Nemhauser J.B. Medical response to a major radiologic emergency: a primer for medical and public health practitioners. Radiology. 2010;254(3):660–677. doi: 10.1148/radiol.09090330. [DOI] [PubMed] [Google Scholar]
- 7.Little M.P. Heterogeneity of variation of relative risk by age at exposure in the Japanese atomic bomb survivors. Radiat Environ Biophys. 2009;48(3):253–262. doi: 10.1007/s00411-009-0228-x. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson A.L., Rosenthal A. Thyroid carcinoma secondary to radiation cloud exposure from the chernobyl incident of 1986: a case study. Case Rep Oncol. 2010;3(1):83–87. doi: 10.1159/000308358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romm H., Wilkins R.C., Coleman C.N. Biological dosimetry by the triage dicentric chromosome assay: potential implications for treatment of acute radiation syndrome in radiological mass casualties. Radiat Res. 2011;175(March (3)):397–404. doi: 10.1667/RR2321.1. [DOI] [PubMed] [Google Scholar]
- 10.Huber R., Braselmann H., Geinitz H. Chromosomal radiosensitivity and acute radiation side effects after radiotherapy in tumour patients – a follow-up study. Radiat Oncol. 2011;6:32. doi: 10.1186/1748-717X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gougelet R.M., Rea M.E., Nicolalde R.J. The view from the trenches: part 1 – emergency medical response plans and the need for EPR screening. Health Phys. 2010;98(February (2)):118–127. doi: 10.1097/HP.0b013e3181a6de7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B.B., Dong R., Kmiec M. Development of in vivo tooth EPR for individual radiation dose estimation and screening. Health Phys. 2010;98(February (2)):327. doi: 10.1097/HP.0b013e3181a6de5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon S.L., Bouville A., Kleinerman R. Current use and future needs of biodosimetry in studies of long-term health risk following radiation exposure. Health Phys. 2010;98(February (2)):109–117. doi: 10.1097/HP.0b013e3181a86628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okunieff P., Chen Y., Maguire D.J. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27(September (3)):363–374. doi: 10.1007/s10555-008-9138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partridge M.A., Chai Y., Zhou H. High-throughput antibody-based assays to identify and quantify radiation-responsive protein biomarkers. Int J Radiat Biol. 2010;86(4):321–328. doi: 10.3109/09553000903564034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salerno A., Zhang J., Bhatla A. Design considerations for a minimally invasive high-throughput automation system for radiation biodosimetry. IEEE Int Conf Autom Sci Eng, 2007 CASE. 2007:846–852. [Google Scholar]
- 17.Garty G., Chen Y., Salerno A. The rabit: a rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98(February (2)):209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brengues M., Paap B., Bittner M. Biodosimetry on small blood volume using gene expression assay. Health Phys. 2010;98(2):179–185. doi: 10.1097/01.HP.0000346706.44253.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul S., Barrer C.A., Turner H.C. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175(March (3)):257–265. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson A.D., Lanz C., Gonzalez F.J. The role of mass spectrometry-based metabolomics in medical countermeasures against radiation. Mass Spectrom Rev. 2010;29(May-June (3)):503–521. doi: 10.1002/mas.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radiation Research Society Meeting report radiation exposures in medicine: biological and public health significance American statistical association conference on radiation and health Annapolis, Maryland. Radiat Res. 2011;175(June):131–142. [Google Scholar]
- 22.Brush J., Lipnick S.L., Phillips T. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W., Diz I., Robbins M.E. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 24.Moulder J.E., Cohen E.P. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman T.H., Brizel D.M., Henke M. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head and neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63(4):985–990. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 26.Rubin J.S., Bottaro D.B., Chedid M. Keratinocyte growth factor. Cell Biol Int. 1995;19:399–411. doi: 10.1006/cbir.1995.1085. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger R., Stiff P., Bensinger W. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351(25):2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 28.Henke M., Alfonsi P., Foa Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer. J Clin Oncol. 2011;13(June) doi: 10.1200/JCO.2010.32.4103. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Vijayalaxmi, Reiter R.J., Tan D.X., Herman T.S., Thomas C.R., Jr. Melatonin as a radioprotective agent: A review. Int J Radiat Oncol Biol Phys. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Korkmaz A., Tan D.X., Riter R.J. Melatonine: an established radioprotective agent against Japan nuclear disaster. TAF Prev Med Bull. 2011;10.(2) [Google Scholar]
- 31.Ghosh S.N., Zhang R., Fish B.L. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(December (5)):1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen E.P., Robbins M.E.C. Radiation nephropathy. Semin Nephrol. 2003;23:486–499. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.H., Brown S.L., Kolozsvary A. Modification of radiation injury by ramipril, inhibitor of angiotensin converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137–142. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- 34.Boerma M., Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2011 doi: 10.4061/2011/858262. Article ID 858262, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michelson A. Harwood Academic Publishers; United Kingdom: 1987. Medical aspects of superoxide dismutase. Life chemistry reports. p. 1-142. [Google Scholar]
- 36.Carpenter M., Epperly M.W., Agarwal A. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–693. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 37.Greenberger J.S., Epperly M.W., Gretton J. Radioprotective gene therapy. Curr Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 38.Delanian S., Lefaix J.L. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 39.López M., Cerezo L. Toxicity associated to radiotherapy treatment in lung cancer patients. Clin Translat Oncol. 2007;9:506–512. doi: 10.1007/s12094-007-0094-4. [DOI] [PubMed] [Google Scholar]
- 40.López M., Martín M., Cerezo L. Gastrointestinal toxicity associated to radiation therapy. Clin Translat Oncol. 2010;12:554–561. doi: 10.1007/s12094-010-0553-1. [DOI] [PubMed] [Google Scholar]
- 41.Laurent C., Pouget J.-P., Voisin P. Modulation of DNA damage by pentoxifylline and alpha-tocopherol in skin fibroblasts exposed to gammarays. Radiat Res. 2005;164:63–72. doi: 10.1667/rr3383. [DOI] [PubMed] [Google Scholar]
- 42.Laurent C., Voisin P., Pouget J.P. DNA damage in cultured skin microvascular endothelial cells exposed to gamma rays and treated by the combination pentoxifylline and alpha-tocopherol. Int J Radiat Biol. 2006;82:309–321. doi: 10.1080/09553000600733150. [DOI] [PubMed] [Google Scholar]
- 43.Singh V.K., Brown D.S., Kao T.C., Seed T.M. Preclinical development of a bridging therapy for radiation casualties. Exp Hematol. 2010;38(1):61–70. doi: 10.1016/j.exphem.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Joshi R., Kumar S., Unnikrishnan M. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res. 2005;39(November 11):1163–1172. doi: 10.1080/10715760500177880. [DOI] [PubMed] [Google Scholar]
- 45.Hotz M.E., Fliedner T.M., Meineke V. Radiation accident preparedness: a European approach to train physicians to manage mass radiation casualties. Health Phys. 2010;98:894–897. doi: 10.1097/HP.0b013e3181ab3e71. [DOI] [PubMed] [Google Scholar]
- 46.Travis E. Genetic susceptibility to late normal tissue injury. Semin Radiat Oncol. 2007;17:149–155. doi: 10.1016/j.semradonc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Epperly M.W., Smith T., Zhang X. Modulation of in utero total body irradiation induced newborn mouse growth retardation by maternal manganese superoxide dismutase-plasmid liposome (MnSOD-PL) gene therapy. Gene Ther. 2011;18(June 6):579–583. doi: 10.1038/gt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier P., Veldwijk M.R., Wenz F. Radioprotective gene therapy. Expert Opinion on Biological Therapy. 2011 doi: 10.1517/14712598.2011.580271. Posted online May 2. [DOI] [PubMed] [Google Scholar]
- 49.Chun-Jie L.I.U., Xiao-Qin H.A., Jun-Jun JIAN.G. Keratinocyte growth factor (KGF) gene therapy mediated by an attenuated form of Salmonella typhimurium ameliorates radiation induced pulmonary injury in rats. J Radiat Res. 2011;52:176–184. doi: 10.1269/jrr.10148. [DOI] [PubMed] [Google Scholar]
- 50.Przybyszewska M. Soluble TNF-α receptor I encoded on plasmid vector and its application in experimental gene therapy of radiation-induced lung fibrosis. Arch Immunol Ther Exp (Warsz) 2011;59(August 4):315–326. doi: 10.1007/s00005-011-0133-2. [DOI] [PubMed] [Google Scholar]


