Abstract
Aim
To evaluate the performance of volumetric arc modulation with RapidArc against conventional IMRT for head and neck cancers.
Background
RapidArc is a novel technique that has recently been made available for clinical use. Planning study was done for volumetric arc modulation with RapidArc against conventional IMRT for head and neck cancers.
Materials and methods
Ten patients with advanced tumors of the nasopharynx, oropharynx, and hypopharynx were selected for the planning comparison study. PTV was delineated for two different dose levels and planning was done by means of simultaneously integrated boost technique. A total dose of 70 Gy was delivered to the boost volume (PTV boost) and 57.7 Gy to the elective PTV (PTV elective) in 35 equal treatment fractions. PTV boost consisted of the gross tumor volume and lymph nodes containing visible macroscopic tumor or biopsy-proven positive lymph nodes, whereas the PTV elective consisted of elective nodal regions. Planning was done for IMRT using 9 fields and RapidArc with single arc, double arc. Beam was equally placed for IMRT plans. Single arc RapidArc plan utilizes full 360° gantry rotation and double arc consists of 2 co-planar arcs of 360° in clockwise and counter clockwise direction. Collimator was rotated from 35 to 45° to cover the entire tumor, which reduced the tongue and groove effect during gantry rotation. All plans were generated with 6 MV X-rays for CLINAC 2100 Linear Accelerator. Calculations were done in the Eclipse treatment planning system (version 8.6) using the AAA algorithm.
Results
Double arc plans show superior dose homogeneity in PTV compared to a single arc and IMRT 9 field technique. Target coverage was almost similar in all the techniques. The sparing of spinal cord in terms of the maximum dose was better in the double arc technique by 4.5% when compared to the IMRT 9 field and single arc techniques. For healthy tissue, no significant changes were observed between the plans in terms of the mean dose and integral dose. But RapidArc plans showed a reduction in the volume of the healthy tissue irradiated at V15 Gy (5.81% for single arc and 4.69% for double arc) and V20 Gy (7.55% for single arc and 5.89% for double arc) dose levels when compared to the 9-Field IMRT technique. For brain stem, maximum dose was similar in all the techniques. The average MU (±SD) needed to deliver the dose of 200 cGy per fraction was 474 ± 80 MU and 447 ± 45 MU for double arc and single arc as against 948 ± 162 MU for the 9-Field IMRT plan. A considerable reduction in maximum dose to the mandible by 6.05% was observed with double arc plan. Double arc shows a reduction in the parotid mean dose when compared with single arc and IMRT plans.
Conclusion
RapidArc using double arc provided a significant sparing of OARs and healthy tissue without compromising target coverage compared to IMRT. The main disadvantage with IMRT observed was higher monitor units and longer treatment time.
Keywords: RapidArc, IMRT, Planning study, Head and neck cancers
1. Background
Head and neck cancer arises from mucous lining of respiratory, digestive tracts, salivary glands, and lymph nodes. Head & neck cancer is histologically heterogeneous and organs at risk have less tolerance to radiation. Treatment planning for advanced head and neck cancer is a knotty problem due to the complex shape of target volumes and the need to spare critical organs like the mandible, parotid glands, brainstem, spinal cord, and normal structures. These organs often lie very close to the target volumes which commonly have an irregular concave shape. Due to the close proximity of tumor to critical structures, head and neck cancer presents a challenge for radiotherapy. Treatment with radiotherapy is curative for many patients with localized disease, but with current radiation techniques, dose is limited by both acute and late side effects and the anatomy of the head and neck region which is very complex, with bony structures, soft tissues and air cavities. The lack of tumor motion due to breathing makes patient set up easy and can be reproduced accurately.
The transition of radiotherapy for head and neck cancer from 3D conformal radiotherapy (3D-CRT) to intensity modulated radiation therapy (IMRT) made treatment of cancer easier and beneficial. The delivery of IMRT is accomplished with a set of fixed radiation beams, which are shaped using the projection of the target volume. In IMRT, treatment techniques are better tailored to an individual patient's situation based on the extent and location of disease and normal anatomy. IMRT can sculpt precise dose distribution in three dimensions. IMRT presents more conformity for irregular target volumes in the vicinity of critical organs and provides better tumor control and reduces dose to normal structures. The main disadvantage of IMRT, in spite of its efficiency in dose conformity to tumor, is increased treatment delivery time and increased monitor units (MUs).
2. Aim
To avoid the drawbacks offered by IMRT, a novel approach, volumetric modulated radiation therapy (VMAT), has been implemented.1 RapidArc offers highly conformal dose distribution, superior dosimetric accuracy, shrink in treatment time and enhanced sparing of critical structures. Recently, different planning studies have reported the efficiency of RapidArc over conventional IMRT.2–5 The aim of this study was to examine the potential clinical role of RapidArc (Varian Medical Systems, Palo Alto, CA) and compare them with conventional IMRT for head and neck cancers.
3. Materials and methods
Ten patients with advanced head and neck tumors (oropharynx, hypopharynx, and nasopharynx) were selected for the planning study. Plans were optimized with the aim to assess organs at risk and healthy tissue sparing while enforcing highly conformal target coverage. All patients underwent CT simulation in a supine position with the neck hyper extended using a head rest and custom aqua plastic masks. To reduce the dose to the mandible and tongue, both were separated by bite blocks, and to reduce dose to the shoulder it was brought down by a pull board. CT images were taken at 3 mm slice thickness by means of a devoted CT scanner.
PTV was delineated for two different dose levels (PTV boost and PTV elective) and planning was done by means of a simultaneously integrated boost technique (SIB technique). Studies have been reported by Vosmik et al. for simultaneous integrated boost and toxicity evaluation in IMRT for head and neck cancers with and without concurrent chemotherapy.6 In this study, PTV boost consisted of the gross tumor volume and lymph nodes containing visible macroscopic tumor or biopsy-proven positive lymph nodes, whereas the PTV elective consisted of elective nodal regions. The CTV was created by manual contouring which included the GTV with a margin of 10 mm for CTV and 3 mm for PTV, respectively, which involves microscopic spread and clinically uninvolved lymph nodes and retrophargeal nodes. For PTV elective, a margin of 3 mm was prescribed to account for the set up errors.7,8 The organs at risk contoured were the brainstem, spinal cord, parotid, and mandible. The healthy tissue was defined as the patient's volume covered by the body volume excluding the PTV.
3.1. Treatment planning objectives
A total dose of 70 Gy was delivered to the boost volume (PTV boost) and 57.7 Gy to the elective PTV (PTV elective), in 35 equal treatment fractions. The plans were normalized to 100% (70 Gy) dose which covered the PTV. The DVH for the PTV, parotid, spinal cord, mandible, and brain stem were calculated. For all plans (IMRT and RapidArc), planning objectives were optimized to achieve the following parameters.
-
•
For PTV, plans aimed to achieve the prescribed dose.
-
•
Maximum doses to the spinal cord, mandible, and brain stem were tried to limit within 45 Gy, 70 Gy, 50 Gy respectively.
-
•
Mean dose for both left & right parotid was aimed to restrict below 26 Gy.
-
•
A normalized total dose mean of 20 Gy was associated with a normal tissue complication rate of 13–14%, which was deemed acceptable.
-
•
For healthy tissue, various thresholds like V10, V15, and V20 Gy were scored.
3.2. Conventional IMRT planning
Planning was generated for the IMRT 9 field sliding window technique. Beams were equally placed for all IMRT plans. Nominal beam energy of 6 MV X-rays was used. The IMRT optimization engine computes optimal fluence maps from dose volume constraints derived from the general planning objective. Optimization was done by exploiting interaction window with different objectives and priorities. Optimal fluence maps are then converted by a leaf motion calculator into actual fluence maps which are deliverable using a multi-leaf collimator (MLC).
3.3. RapidArc planning
Two RapidArc plans were generated. RapidArc using single arc and double arc. Single arc RapidArc plan utilized full gantry rotation (gantry angles from 179 to 181°) and the double arc consisted of 2 co-planar arcs with the first arc in clockwise and the other arc in the counter clockwise direction (gantry angles from 181 to 179 and 179 to 181°, respectively). Collimator was rotated from 35 to 45° depending on the plan, to cover the entire tumor volume which reduced the tongue and groove effect during gantry rotation.
All plans were generated with 6 MV X-rays for CLINAC 2100 Linear accelerator for both IMRT and RapidArc. Optimization and calculations were done in the Eclipse planning system, version 8.6.15 (Varian Medical Systems, Palo Alto, CA), using the anisotropic analytical algorithm (AAA).9,10 The AAA is a 3D pencil beam convolution/superposition algorithm that uses separate Monte Carlo derived modeling for primary photons, scattered extra-focal photons and electrons scattered from the beam limiting devices. The dose deposition characteristics are modeled with six exponential curves. The functional shapes of the fundamental physical expressions in AAA enable analytical convolution, which significantly reduces the computational time. The AAA accounts for the tissue heterogeneity anisotropically in the entire three-dimensional neighborhood of an interaction site by using photon scatter kernels in multiple lateral directions. This will increase the accuracy of the scattered dose calculation. The final dose distribution is obtained by the superposition of the dose calculated with photon and electron convolutions. The grid size used for calculation is 2.5 mm for both IMRT and RapidArc. The dose objective was kept constantly the same as the IMRT plans for the planning target volumes (PTV) and organ at risk. The priority was changed to achieve the desired clinical outcome. The “normal tissue objective” features in IMRT and RA optimization were used to prevent the optimizer creating hot spots in parts of the body outside the PTV. Couch parameters were included while planning so as to reduce errors due to attenuation and scattering in both IMRT and RapidArc optimization as well as in dose calculations.
3.4. Parameters analyzed for planning
Dose volume histogram was used as a planning tool to estimate plans. The coverage of PTV was calculated as the ratio of target volume covered by 95% of isodose line divided by the volume of PTV. For PTV, D98% and D2% values were reported (dose received by 98% and 2% of the PTV volume) which represented the minimum and maximum doses.
Homogeneity index was evaluated as per ICRU 83, which is expressed in terms of (D2% − D98%)/D50% (ratio of difference between the dose covering 2% and 98% to the dose received by 50% of the PTV volume). The degree of conformity of the plans was measured with conformity index (CI). CI95% defined as the ratio between the patient volume receiving at least 95% of the prescribed dose and the volume of the PTV. Total MUs and the delivery time were accounted. Regarding the spinal cord, maximum dose was scored. For the parotid, mean dose and D50% were calculated. From healthy tissue, V10 Gy, V15 Gy and V20 Gy and the integral dose (equal to the mean dose times the volume of healthy tissue), were scored. The paired, two-tails Student's t-test was studied to evaluate the difference between the techniques.
4. Results and discussion
Clinically acceptable IMRT and RapidArc plans were achieved in all ten cases. PTV volumes for all the ten cases ranged from 294 cm3 to 510 cm.3 All the treatment plans were evaluated using dose volume histogram (DVH). Tables 1A, 1B and 2, provide an overview of the numerical findings from average DVH analysis on PTV and OARs are reported as mean values ± standard deviation (SD) to assess the relative inter-patient variability.
Table 1A.
Dosimetric results for the PTV-boost.
| Parameter | IMRT | Single arc RA plan | Double arc RA plan | P | P* | P** |
|---|---|---|---|---|---|---|
| D98% (Gy) | 69.89 ± 0.19 | 69.15 ± 1.04 | 70.12 ± 1.04 | 0.298 | 0.645 | 0.349 |
| D2% (Gy) | 78.05 ± 2.44 | 77.41 ± 0.61 | 77.26 ± 1.56 | 0.693 | 0.293 | 0.901 |
| HI | 0.1079 ± 0.027 | 0.1113 ± 0.008 | 0.0955 ± 0.011 | 0.830 | 0.430 | 0.643 |
| CI 95% | 1.06 ± 0.068 | 1.03 ± 0.051 | 1.01 ± 0.03 | 0.278 | 0.032 | 0.643 |
| MU | 947.5 ± 162.42 | 447.25 ± 45.30 | 474.25 ± 80.47 | 0.004 | 0.002 | 0.434 |
P – paired t-test analysis: IMRT vs. single arc RA; P* – paired t-test analysis: IMRT vs. double arc RA; P** – paired t-test analysis: single arc RA vs. double arc RA.
Table 1B.
Dosimetric results for the PTV-elective.
| Parameter | IMRT | Single arc RA plan | Double arc RA plan | P | P* | P** |
|---|---|---|---|---|---|---|
| D98% (Gy) | 59.43 ± 1.147 | 57.98 ± 0.914 | 58.93 ± 1.46 | 0.182 | 0.079 | 0.376 |
| D2% (Gy) | 64.56 ± 1.868 | 65.10 ± 0.966 | 64.38 ± 1.606 | 0.526 | 0.699 | 0.431 |
| HI | 0.0945 ± 0.010 | 0.1146 ± 0.014 | 0.0875 ± 0.005 | 0.006 | 0.024 | 0.011 |
P – paired t-test analysis: IMRT vs. single arc RA; P* – paired t-test analysis: IMRT vs. double arc RA; P** – paired t-test analysis: single arc RA vs. double arc RA.
Table 2.
Dosimetric results for the organ at risk and healthy tissue.
| Organ | Parameter | IMRT | Single arc RA plan | Double arc RA plan | P | P* | P** |
|---|---|---|---|---|---|---|---|
| Spinal cord | Max. dose (Gy) | 41.31 ± 2.65 | 40.78 ± 1.94 | 39.45 ± 1.94 | 0.054 | 0.011 | 0.030 |
| Brain stem | Max. dose | 44.30 ± 9.82 | 46.74 ± 9.32 | 44.86 ± 8.67 | 0.159 | 0.046 | 0.060 |
| L-parotid excluding PTV | D mean (Gy) | 20.28 ± 2.58 | 18.89 ± 2.39 | 18.86 ± 2.90 | 0.046 | 0.028 | 0.094 |
| R-parotid excluding PTV | D mean (Gy) | 20.24 ± 2.35 | 18.29 ± 1.81 | 15.95 ± 5.68 | 0.031 | 0.025 | 0.044 |
| Mandible | Max. dose (Gy) | 68.20 ± 4.03 | 65.15 ± 2.13 | 64.07 ± 2.44 | 0.140 | 0.017 | 0.386 |
| Healthy tissue | D mean (Gy) | 8.14 ± 1.24 | 8.21 ± 1.29 | 8.32 ± 1.28 | 0.376 | 0.376 | 0.531 |
| V10 Gy(%) | 22.92 ± 3.341 | 23.48 ± 3.43 | 23.74 ± 3.30 | 0.257 | 0.280 | 0.385 | |
| V15 Gy(%) | 20.47 ± 2.91 | 19.28 ± 2.78 | 19.51 ± 2.47 | 0.071 | 0.237 | 0.439 | |
| V20 Gy(%) | 17.02 ± 2.44 | 15.73 ± 2.83 | 16.01 ± 2.43 | 0.008 | 0.042 | 0.524 | |
| Int. dose (×104 Gy cm3) | 7.12 ± 1.43 | 7.18 ± 1.50 | 7.26 ± 1.32 | 0.362 | 0.272 | 0.625 |
P – paired t-test analysis: IMRT vs single arc RA; P* – paired t-test analysis: IMRT vs double arc RA; P** – paired t-test analysis: single arc RA vs double arc RA.
4.1. Planning target volume
Target coverage was almost similar in all the techniques. Dose conformity was described in terms of the CI. RA using double arc plans achieved the best conformity (CI95% = 1.01 ± 0.025) while RA using single arc (CI95% = 1.03 ± 0.051) was slightly inferior to RA with double arc but superior to IMRT plan (CI95% = 1.06 ± 0.068). The in-homogeneity for PTV boost was highest for single arc rapid plan with HI equal to 0.11127 ± 0.008 Gy and lowest for RA with double arc with 0.09554 ± 0.011 Gy and IMRT plan fell in between with HI 0.10793 ± 0.027 Gy. Also the homogeneity index for PTV elective was found to be 0.09453 ± 0.010, 0.11459 ± 0.014 and 0.08751 ± 0.005 for IMRT, single arc RapidArc and double arc RapidArc plans, respectively.
Fig. 1 shows the dose distribution of one of the patients under study for the axial, sagittal, and coronal view of all the techniques. Figs. 2 and 3 show the DVH for PTV and OARs comparing the three plans for that particular patient.
Fig. 1.
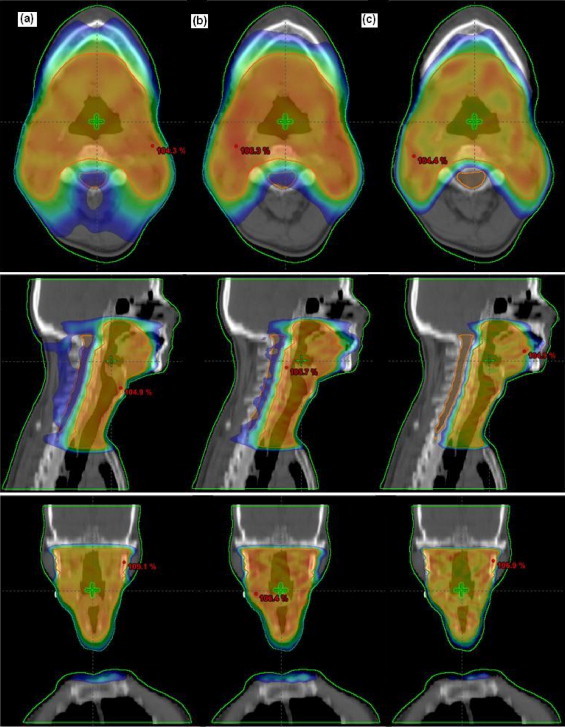
Dose distribution for one patient under study for axial, sagittal and coronal views. (a) IMRT, (b) RA_SA and (c) RA_DA.
Fig. 2.
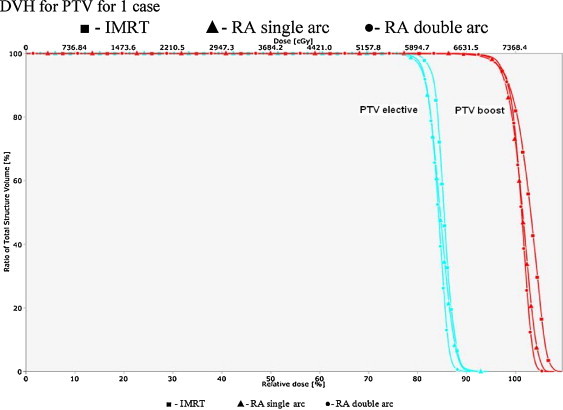
DVH for PTV for 1 case.
Fig. 3.
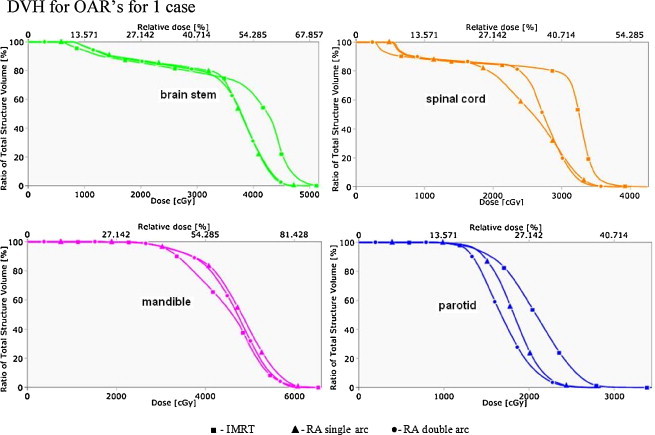
DVH for OAR's for 1 case.
The average MU (±SD) needed to deliver the dose of 200 cGy per fraction was 474 ± 80 MU and 447 ± 45 MU for double arc and single arc as against 948 ± 162 MU for the 9-Field IMRT plan. Calculated RapidArc “beam on” time (after patient setup) was 150–180 s for RA with double arc and 70–90 s for RA with single arc plans. Treatment times for a typical head and neck cancer treatment (2 Gy) with nine fields IMRT with a dose rate of 300 MU/min were in the range between 15 and 20 min.
4.2. Spinal cord
All plans respected the planning objective of 45 Gy as maximum dose to the spinal cord. The sparing of the spinal cord in terms of the maximum dose was better in the double arc technique by 4.5% when compared to IMRT and single arc techniques.
4.3. Mandible
All plans were able to achieve the objective of the mandible excluding PTV less than 70 Gy as maximum dose and RapidArc using double arc proved to be better in terms of sparing. A considerable reduction in maximum dose to the mandible by 6.05% was observed with double arc and 4.47% with single arc, respectively when compared to the IMRT technique. Mandible is not given high priority because of its late effect toxicity.
4.4. Brain stem
No significant difference was observed between the plans in terms of maximum dose. But maximum dose to brain stem was could be reduced well below 55 Gy. IMRT technique allowed enhanced sparing of the brain stem in terms of maximum dose of 44.30 Gy when compared to RapidArc using double arc with 44.74 Gy and single arc with 44.86 Gy, respectively. Priority in the optimization window for the brain stem was also kept low because of its late effects to irradiation. With the brain stem being a serial organ, maximum dose will be considered.
4.5. Parotid
The analysis was carried out for left and right parotids separately. Larger sparing was observed for the contra-lateral glands with all the techniques. Mean doses to the whole parotids could be to keep within 26 Gy for all the plans. The mean dose to the left parotid excluding PTV was reduced from 20.28 Gy (IMRT) to 18.89 Gy (single arc) and 18.86 Gy (double arc). The mean dose to the right parotid gland excluding PTV ranged from 20.24 Gy (IMRT) to 18.29 Gy (single arc) and 15.95 Gy (double arc). As the results suggest that the dose delivered to 1/3 or 2/3 of the gland volumes, RA using single arc and RA using double arc resulted in better sparing compared to IMRT with a more prominent efficiency with double arc compared to single arc. Also, mean doses to the parotid should be highly condensed to avoid xerostomia which needs more attention in head and neck cases.11
4.6. Healthy tissue
RapidArc and IMRT plans presented similar dose volume histogram shapes for healthy tissue and no significant changes were observed between the plans in terms of the mean dose and integral dose. The integral dose was computed as the mean times the volume of healthy tissue irradiated. But RapidArc plans showed a reduction in the volume of healthy tissue irradiated above V15Gy by 5.81% and 4.69% for single and double arc, respectively. Similarly, for V20Gy, reduction by 7.55% for single arc and 5.89% for double was observed.
Radiotherapy plays a vital role in the management of locally advanced head and neck cancer. The present planning study intends to scrutinize the potential clinical role of RapidArc in head and neck cancer. The main reason for selecting head and neck cases was that IMRT plans for head and neck cases are demanding and require a strong dose modulation because of large and irregular PTVs and more organs at risk. IMRT for head and neck cancer has been investigated both in planning and clinical levels. Studies on treatment outcome and major toxicity were published by Gregoire et al.,12 Lee et al.13 and Popovtzer and Eisbruch14 Also, a study related to late radiation toxicity in patients after radiotherapy for head and neck cancer was published by Golen et al.15
Different planning studies comparing the volumetric arc modulation technique with conventional IMRT in different sites have been published recently.2–5 The study published by Palma et al. shows RapidArc progenitor on prostate showing that variable dose rate volumetric arc modulation is beneficial compared to IMRT with constant dose rate. Cozzi et al.2 and Fogliata et al.16 appraised the behavior of RapidArc on cervix uteri cancer and on small benign brain tumors. In all those studies RapidArc proved to be at least equivalent to IMRT in terms of target coverage while showed benefit in organs at risk sparing.
When compared to IMRT, RapidArc using double arc proved to have superior dose homogeneity in PTV. Also, RapidArc plan showed improvements in organs at risk and healthy tissue sparing without compromising target coverage. Unlike in IMRT, optimization can be paused at each resolution level in RapidArc, which will ensure additional time for the optimization to attain saturation. This shows slight improvement in the final dose distribution. The main disadvantage of IMRT is the higher number of MUs and resulting longer treatment time. Such prolonged treatment fraction delivery times (greater than 15 min.) may have significant impact on IMRT treatment outcome, especially for tumors with a low α/β ratio and a short repair halftime.17 Also, the analysis performed by Cozzi et al. leads to the conclusion that RapidArc can reduce the peripheral dose from about 8% at 5 cm to about 30% at 15 cm from the PTV surface.
RapidArc treatment is delivered rapidly, which has the advantage of decreasing the risk of intra-fractional positional shifts of the patient.18 It was found that no dosimetric benefits for the target volume were noted with RA over IMRT treatment planning in terms of the CI. But when compared to IMRT RapidArc plans using double arc show a better homogeneity and, at the same time, presented a major reduction of irradiation to organs at risk. Even though the spinal cord dose is within the tolerance limits with IMRT, it spares less than RA plan. RapidArc requires only 40% of the number of MUs compared with the nine field sliding window IMRT techniques. RA plans were the fastest treatment option of modulated approaches in this comparison.
Dose to healthy organs not in the proximity of the PTV arises largely from collimator transmission and scatter radiation from the LINAC, and this dose is proportional to the number of MUs.19 Such scattered doses can increase the risk of secondary tumors.20 Such a risk is now largely reduced by the use of RapidArc without concessions to the dose distribution. Among RA plans, double arc RA plan, which involves two full gantry rotations, offers a greater freedom in dose modulation. The sum of two arcs reduces hot spots in the PTV where the second arc compensates for areas of suboptimal dose. A second possible explanation for the advantage of using two arcs is a physical limit to dose homogeneity for a single arc arising from limited leaf speed and the limited number of control points. Also, a second arc adds more freedom for possible leaf positions. Each RapidArc plan required only a single optimization session and the same number of optimization steps, independent of the amount of interactive change of the optimization objectives. It is obvious that sliding window IMRT can be planned in a much shorter period of time as compared to RapidArc, but RapidArc has the lowest estimated treatment delivery time. Also with respect to plan quality, RapidArc can meet the most dose–volume criteria.
5. Conclusion
The investigation for head and neck cancer using double arc RapidArc proved a significant sparing of OARs and healthy tissue without compromising target coverage compared to IMRT. The physical dose distribution was combined with a shorter delivery time, which can have an impact on the biological level. IMRT techniques result in higher monitor units and longer treatment time. RapidArc is a better treatment option in our comparison for head and neck cancers.
Conflict of interest statement
None declared.
References
- 1.Karl Otto Volumetric modulated arc therapy: IMRT in a single arc. Med Phy. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi L., Dinshaw K.A., Shrivastava S.K. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89:180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy. Comparison with intensity modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Clivio A., Fogliata A., Franzetti-Pellanda A. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: a treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:118–124. doi: 10.1016/j.radonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Wagner D., Christiansen H., Wolff H., Vorwerk H. Radiotherapy of malignant gliomas: comparison of volumetric single arc technique (RapidArc), dynamic intensity modulated technique and 3D conformal technique. Radiother Oncol. 2009;93:593–596. doi: 10.1016/j.radonc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Vosmik M., Kordac P., Paluska P. IMRT using simultaneous integrated boost (66 Gy in 6 weeks) with and without concurrent chemotherapy in head and neck cancer—toxicity evaluation. Rep Pract Oncol Radiother. 2008;13(2):86–95. [Google Scholar]
- 7.Gregoire V., Levendag P., Ang K.K. CT based delineation of lymph node levels and related CTVs in the node-negative neck: Dahanca, Eortc, Gortec, Ncic, RTOG consensus guidelines. Radiother Oncol. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Gregoire V., Eisbruch A., Hamoir M. Proposal for the delineation of the nodal CTV in the node-positive and post-operative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Van Esch A., Tillikainen L., Pyykkonen J. Testing of the analytical anisotropic algorithm for photon dose calculation. Med Phys. 2006;33:4130–4148. doi: 10.1118/1.2358333. [DOI] [PubMed] [Google Scholar]
- 10.Fogliata A., Nicolini G., Vanetti E. Dosimetric validation of the anisotropic analytical algorithm for photon dose calculation: fundamental characterization in water. Phys Med Biol. 2006;51:1421–1438. doi: 10.1088/0031-9155/51/6/004. [DOI] [PubMed] [Google Scholar]
- 11.Golen M., Skladowski K., Wygoda A. The influence of radiation technique on xerostomia in head and neck cancer patients—prospective study. Rep Pract Oncol Radiother. 2007;12(5):253–260. [Google Scholar]
- 12.Gregoire V., De neve W., Eisbruch A., Lee N. Intensity modulated radiation therapy for head and neck carcinoma. Oncologist. 2007;12:555–564. doi: 10.1634/theoncologist.12-5-555. [DOI] [PubMed] [Google Scholar]
- 13.Lee N., Puri D., Blanco A.I., Chao K.S. Intensity modulated radiation therapy in head and neck cancers: an update. Head Neck. 2007;29:387–400. doi: 10.1002/hed.20332. [DOI] [PubMed] [Google Scholar]
- 14.Popovtzer A., Eisbruch A. Advances in radiation therapy of head and neck cancer. Expert Rev Anticancer Ther. 2008;8:633–644. doi: 10.1586/14737140.8.4.633. [DOI] [PubMed] [Google Scholar]
- 15.Golen M., Skladowski K., Wygoda1 A. A comparison of two scoring systems for late radiation toxicity in patients after radiotherapy for head and neck cancer. Rep Pract Oncol Radiother. 2005;10(4):179–192. [Google Scholar]
- 16.Fogliata A., Clivio A., Nicolini G., Vanetti E., Cozzi L. Intensity modulation with photons for benign intracranial tumours. A planning comparison of volumetric single arc, helical arc and fixed gantry techniques. Radiother Oncol. 2008 doi: 10.1016/j.radonc.2008.07.021. [epub] [DOI] [PubMed] [Google Scholar]
- 17.Wang Jian Z., Allen Li, D'Souza Warren D., Stewart Robert D. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity modulated radiation therapy (IMRT) Int J Radiat Oncol Biol Phys. 2003;57:543–552. doi: 10.1016/s0360-3016(03)00499-1. [DOI] [PubMed] [Google Scholar]
- 18.Sukumar P., Padmanaban S., Jeevanandam P., Syam Kumar S.A., Nagarajan V. A study on dosimetric properties of electronic portal imaging device and its use as a quality assurance tool in volumetric modulated arc therapy. Rep Pract Oncol Radiother. 2011;16(6):248–255. doi: 10.1016/j.rpor.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ślosarek K., Szlag M., Bekman B., Grzadziel A. EPID in vivo dosimetry in RapidArc technique. Rep Pract Oncol Radiother. 2010;15(1):8–14. doi: 10.1016/j.rpor.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall E.J., Phil D. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Onc Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]


