Abstract
Background
The impact of neoadjuvant chemotherapy (CT) and radiotherapy (RT) on overall survival (OS) has been controversial. Some studies have pointed to an improvement in OS and disease-free survival (DFS) in patients with pathologic complete response (pCR).
Aim
To evaluate the therapeutic response and impact on survival of preoperative RT, alone or combined with CT, in patients with locally advanced rectal cancer (LARC).
Materials and methods
A set of 132 patients with LARC were treated preoperatively. GROUP 1: RT alone, 19 patients. GROUP 2: RT and concomitant oral CT (Capecitabine or UFT + leucovorin), 68 patients. GROUP 3: RT and concomitant CT with 5-FU in continuous infusion, 45 patients. 58.2% of patients were submitted to adjuvant CT.
Results
GROUP 1: no pCR, tumoral downstaging was 26.7%. GROUP 2: pCR in 16.9%; tumoral downstaging was 47.7%. GROUP 3: pCR in 11.9%; tumor downstaging was 52.4%. The loco-regional control (LRC) was 95%. The 5-year OS (p = 0.038) and DFS (p = 0.05) were significantly superior in patients treated with CT + RT. Patients with pCR had a significant increase on DFS (p = 0.019). Patients cT3–4 that had a tumoral downstaging to ypT0–2, showed an increase on DFS, OS and LRC.
Conclusions
CT combined with RT has increased tumoral response and survival rate. Nodal downstaging and pCR were higher in the GROUP 2. The 5-year OS and DFS were significantly superior in CT + RT arms. Patients with pathologic response showed a better DFS. Adjuvant CT had no impact on LRC, DFS nor on OS.
Keywords: Rectal cancer, Neoadjuvant chemoradiotherapy, Pathologic complete response, Adjuvant chemotherapy
1. Introduction
Rectal adenocarcinoma has a very high rate of local relapse with surgery alone. Some studies have demonstrated that adjuvant chemotherapy (CT) and radiotherapy (RT) reduce the rate of local relapse and prolong survival in patients whose tumors extend into the perirectal fat (T3) or who have involvement of mesorectal or pelvic lymph nodes (N1–3).1
Preoperative chemoradiotherapy (CT + RT) offers some theoretical advantages over adjuvant therapy for patients with a tumor of the middle to lower rectum2: (i) micrometastases are treated early in the course of the disease; (ii) the risk of tumor seeding during surgery is reduced; (iii) RT toxicity is also reduced; (iv) the efficacy of CT and RT is higher in a tumor with an intact vasculature; (v) if the tumor shrinks, a sphincter preserving procedure can be performed. Nevertheless, this treatment also has some drawbacks: (i) definitive therapy is delayed, which may allow the growth and dissemination of the tumor; (ii) as preoperative staging is not very precise, patients on early stages (T1–2N0) of the disease, who do not need this therapy because of their very low risk of relapse, would be overtreated.
After the randomised trial CAO/ARO/AIO,3 neoadjuvant CT + RT became the standard treatment, since the 5-year local recurrence rate is reduced, adherence is better and it has fewer acute and long-term toxic effects than postoperative CT + RT. Neoadjuvant use of CT and RT allow a higher rate of resectability associated to a tumor and nodal downstaging.4
Concomitant neoadjuvant 5-FU CT + RT provides a pCR in 8–27% of the cases and is associated to an increased local control.2–14 The single randomized trial that compared preoperative vs. postoperative CT + RT, concluded that there was a higher tumoral downstaging (6% vs. 13%, p = 0.006) and a decrease on local recurrence with preoperative CT + RT.3 Theoretically, oral fluoropyrimidines are suitable to replace protracted infusion of 5-FU and avoid complicated procedures.
The impact of neoadjuvant CT + RT on survival has been controversial. Some studies have pointed to an improvement in overall survival (OS) and disease-free survival (DFS) in patients with pathological response after neoadjuvant therapy.9,15,16
Since the standard schedule of preoperative CT + RT for rectal cancer remains to be established, and due to the convenience of oral prodrugs of 5-FU, we have evaluated the therapeutic response to UFT and leucovorin (LV), capecitabine and 5-FU combined with preoperative RT in patients with stage II–III rectal cancer. Toxicity and survival were also analysed for those groups of patients, as well as the relationship between pathologic response and survival.
2. Methods
2.1. Patients
We analysed prospectively 132 patients with locally advanced rectal cancer (LARC) treated with neoadjuvant RT from November 2002 to March 2008. Patients were divided into 3 groups. GROUP 1: consisting of 19 patients who were treated with RT alone; most of these patients were treated in the beginning of the study, before well defined criteria for using CT + RT were established, whereas others were submitted to this therapy because of associated co-morbidities and advanced age. GROUP 2: consisting of 68 patients who were treated with RT and concomitant oral CT. GROUP 3: consisting of 45 patients, submitted to RT and concomitant CT with continuous infusion of 5-FU. Patients’ characteristics as represented by particular groups are described in Table 1.
Table 1.
Patients’ characteristics.
| Patients’ characteristics | GROUP 1 (n = 19) RT alone | GROUP 2 (n = 68) RT + oral CT | GROUP 3 (n = 45) RT + infusional CT | |
|---|---|---|---|---|
| Age (years) | Min–Max | 51–83 | 35–81 | 20–77 |
| Median | 74 | 64 | 61 | |
| Sex | Male | 13 (68.4%) | 46 (67.6%) | 25 (55.6%) |
| Female | 6 (31.6%) | 22 (32.4%) | 20 (44.4%) | |
| Karnofsky | 100% | 1 (5.3%) | 40 (58.8%) | 30 (66.7%) |
| 90% | 8 (42.1%) | 24 (35.3%) | 14 (31.1%) | |
| 80% | 10 (52.6%) | 4 (5.9%) | 1 (2.2%) | |
| Distance to anal margin | 0–5 cm | 12 (63.2%) | 38 (55.9%) | 22 (48.9%) |
| 6–11 cm | 7 (36.8%) | 30 (44.1%) | 23 (51.1%) | |
| Imaging staging (CT/MRI) | 52.6%/42.1% | 19.1%/79.4% | 0%/100% | |
| Clinical staging | cT2 | – | 7 (10.3%) | 5 (11.1%) |
| cT3 | 16 (84.2%) | 57 (83.8%) | 28 (62.2%) | |
| cT4 | 3 (15.8%) | 4 (5.9%) | 12 (26.7%) | |
| cN0 | 4 (21.1%) | 9 (13.2%) | – | |
| cN+ | 15 (78.9%) | 59 (86.8%) | 45 (100%) | |
2.2. Neoadjuvant radiotherapy
The patient prone position was recommended, and a belly board immobilization device was used. A pelvic CT in the treatment position was performed on all patients, from L5–S1 to 2 cm distal to the anus. All patients underwent three-dimensional treatment planning. CT was used to define gross tumor volume (GTV). Clinical target volume (CTV) included the GTV + 2 cm in all directions, perirectal, internal iliac and presacral nodes up to the promontory; for T4 (seminal vesicles, prostate, vagina or uterus involvement) external iliac nodes were also included; the inguinal areas were irradiated in those patients who had invasion of the anal canal.17,18
The planning target volume (PTV) was defined as CTV + 1 cm margin. The treatment was delivered through three to four fields via the isocenter technique, shaped with multileaf collimator, and high-energy photons of 18 MV. The total dose administered was 50.4 Gy with conventional fraccionation of 1.8 Gy/d, five times per week. The prescribed dose was specified at the International Commission on Radiation Units and Measurements point and isodose distribution to the PTV (95–107%).
2.3. Neoadjuvant chemotherapy
GROUP 2 was treated with oral CT concomitant to RT, including Capecitabine or UFT. The Capecitabine group received an oral 825 mg/m2 dose twice daily for the duration of RT (49 patients). The UFT group received a dose of 300 mg/m2/d of UFT together with LV 90 mg/d (19 patients), in three fractions/d, 5 days/week (Monday to Friday, with the weekend as a rest period).
GROUP 3 was treated with RT concomitant to infusional CT and 5-FU was administered at a dose of 225 mg/m2/d in a continuous infusion, 5 days/week, from Monday to Friday.
2.4. Surgery
Patients were scheduled for surgery between the sixth and eighth week following the conclusion of the neoadjuvant therapy and were treated with total mesorectum excision.
2.5. Toxicity assessment
Toxicity was evaluated weekly in each patient using Common Terminology Criteria for Adverse Events vs. 3.0 (CTCAE).19 A complete blood count and biochemical tests were obtained weekly.
2.6. Definition of response
Evaluation of response to preoperative treatment was defined pathologically. Resected tumors were classified pathologically according to the TNM staging system, version 6.20 Tumor downstaging was defined as postoperative ypT stage lower than preradiotherapy clinical cT stage. Nodal downstaging was defined as postoperative ypN stage lower than preradiotherapy clinical cN stage. Loco-regional response was defined as a downstaging from cTN to pTN. A pathologic complete response (pCR) was considered to occur when there were no malignant cells observed.
2.7. Adjuvant treatment
After surgery, adjuvant CT was given to patients who were considered by the treating physician to potentially benefit from the postoperative therapy. The protocols of adjuvant CT used were FOLFOX (28.2%), CAPOX (11.3%), De Gramont (16.9%), Capecitabine (28.2%), UFT (11.3%) or others (4.1%).
2.8. Follow-up
Following the conclusion of treatment, patients had outpatient clinic appointments every 3 months for the first 2 years, and then every 6 months.
2.9. Patterns-of-failure analysis and survival
Loco-regional failure was defined as a relapse in the pelvis (tumor bed, pelvic nodes, anastomosis, or perineal scar). Failure at distance was defined as relapse in any other site. OS, DFS, and loco-regional control (LRC) were calculated from the date of beginning of treatment. For the calculation of OS, only deaths related to the disease were scored as events.
2.10. Statistical considerations
Statistical analyses were performed using the SPSS 16.0 statistical package. The p-value was calculated by the chi-square test to compare variables. OS, DFS and LRC probabilities were calculated by the Kaplan–Meier method, and differences were evaluated by the log-rank test. A two-sided p-value of <0.05 was considered statistically significant.
3. Results
3.1. Toxicity and treatment adherence
3.1.1. Preoperative treatment
Overall, preoperative therapies were well tolerated and the most commonly reported events are shown in Table 2. In the RT arm, the rate of acute toxicity was 68.4%, but there were no grade 3–4 acute side effects. Group 2 presented acute toxicity in 80.9% of patients, of whom only 7 (10.3%) were grade 3–4. Group 3 showed an acute toxicity in 65.9% of patients, of whom 5 (11.4%) were grade 3–4. One patient had not completed treatment due to allergic reaction to 5-FU and was excluded.
Table 2.
Incidence of acute toxicity. CTCAE (v. 3.0).
| Acute toxicity (%) | GROUP 1 | GROUP 2 |
GROUP 3 |
|||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | |
| Diarrhea | 31.6 | 25.0 | 7.4 | 1.5 | 24.4 | 6.7 |
| Vomiting | 5.9 | 4.4 | 2.2 | |||
| Radiodermitis | 36.8 | 50.0 | 4.4 | 53.3 | ||
| Hand-Foot Synd. | 7.4 | 4.4 | ||||
| Anemia | 15.8 | 7.4 | 2.2 | 2.2 | ||
| Leukopenia | 4.4 | |||||
| Thrombocytopenia | 2.9 | |||||
| Elevated transaminases | 4.4 | |||||
| Elevated creatinine | 2.2 | |||||
| Nausea | 1.5 | |||||
| Urinary | 1.5 | |||||
There were no significant differences (p = 0.245) on toxicity of neoadjuvant therapy among the 3 groups.
3.1.2. At surgery
The median time interval between the end of RT and surgery was 6–7 weeks. A complete resection was done in most patients undergoing CT + RT (GROUP 2: 85.3%; GROUP 3: 88.9%), whereas in the RT group the rate was 63.1%. It should be noted that the group treated with RT alone was the one which had a higher percentage of unresectable disease (21.1%). One patient was not operated due to disease progression and another because of intercurrent illness (Table 3). The main postoperative complications were surgical wound infections, fistula, suture dehiscence and sub-occlusive disease (Table 4). These complications did not increase with the addition of CT to RT (p = 1.0). Two patients died postoperatively due to pulmonary thromboembolism.
Table 3.
Median time surgery-RT and surgical status.
| GROUP 1 (N = 19) RT alone | GROUP 2 (N = 68) RT + oral CT | GROUP 3 (N = 44) RT + infusional CT | |
|---|---|---|---|
| Timing to surgery (median) | 7 weeks | 6 weeks | 6.5 weeks |
| Surgical resection | |||
| R0 | 12 (63.1%) | 58 (85.3%) | 40 (90.9%) |
| R1 | 3 (15.8%) | 7 (10.3%) | 2 (4.5%) |
| Unresectable | 4 (21.1%) | 2 (2.9%) | 1 (2.2%) |
| Non-operated | – | 1 (1.5%) | 1 (2.2%) |
Table 4.
Incidence of postoperative complications.
| Postoperative complications | GROUP 1 (N = 15) RT alone | GROUP 2 (N = 65) RT + oral CT | GROUP 3 (N = 42) RT + infusional CT |
|---|---|---|---|
| Suture dehiscence | 1 (5.9%) | 7 (10.8%) | 2 (4.8%) |
| Sub-occlusion/occlusion | 3 (20%) | 5 (7.7%) | 1 (2.4%) |
| Fistula | – | 4 (6.1%) | 4 (9.5%) |
| Infection | 2 (11.7%) | 11 (16.2%) | 3 (7.1%) |
3.1.3. Postoperative treatment
Of the 122 patients who were submitted to radical surgery and who were candidates for adjuvant CT, only 58.2% (71/122) received the proposed treatment. The respective acute toxicities (67.6% of patients) are described in Table 5.
Table 5.
Adjuvant CT acute toxicities incidence.
| CTCAE (v. 3.0) | Adjuvant CT toxicities |
|
|---|---|---|
| Grade 1–2 | Grade 3–4 | |
| Diarrhea | – | 3 (4.2%) |
| Vomiting | 2 (2.8%) | – |
| Anorexia | 1 (1.4%) | – |
| Hand–foot synd. | 2 (2.8%) | 5 (7%) |
| Anemia | 31 (43.7%) | – |
| Asthenia | 2 (2.8%) | – |
| Thrombocytopenia | 18 (25.3%) | – |
| Paresthesia | 19 (26.8%) | 1 (1.4%) |
| Weight Loss | 1 (1.4%) | – |
| Nausea | 4 (5.6%) | – |
| Neutropenia | 15 (21.1%) | 4 (5.6%) |
The majority of the 51 patients who did not receive adjuvant CT were affected with postoperative complications (52.9%). The reasons why the latter did not undergo adjuvant CT are described in Table 6.
Table 6.
Reasons for non-prescription of adjuvant CT.
| Reasons for non-prescription of adjuvant CT | Number of patients (%) |
|---|---|
| Postoperative complications | 27 (52.9%) |
| Complete pathologic response + ypT2N0M0 | 8 (15.7%) |
| Not sent to oncology department | 4 (7.8%) |
| Poor performance status (≥3) | 3 (5.9%) |
| Progression of the disease | 2 (3.9%) |
| Deceased | 2 (3.9%) |
| Other | 4 (8%) |
3.2. Treatment response
Although tumor downstaging was higher in patients treated with preoperative CT + RT, no statistical significance (p = 0.224) was found (Fig. 1). Nodal downstaging (p = 0.008) and loco-regional response (p = 0.009) were higher in patients treated with oral CT + RT (Figs. 2 and 3). There was no pCR in GROUP 1. In turn, in GROUP 2 the rate of pCR was 16.9% and 11.9% in GROUP 3 (p = 0.207) (Fig. 4).
Fig. 1.
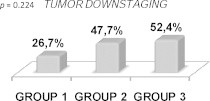
Tumor downstaging.
Fig. 2.

Nodal downstaging.
Fig. 3.
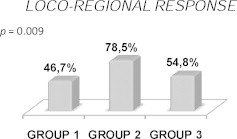
Loco-regional response to neoadjuvant therapy.
Fig. 4.

Pathologic complete response rate.
The median follow-up was 40 months (4–79 months) and one patient was lost in the follow-up.
The LRC was of 95% (115/121). In those patients who had loco-regional recurrence, 3 had undergone an R1 resection and 4 also had distant recurrence. The global 3- and 5-year DFS was 75% and 68%, respectively. The global 3- and 5-year OS was 88% and 80%, respectively.
Comparing the patients that underwent neoadjuvant CT + RT to those who were only treated with preoperative RT (Figs. 5 and 6), we verified that the former had better OS (p = 0.038) and DFS (p = 0.05).
Figs. 5 and 6.

OS and DFS of patients treated with neoadjuvant RT vs. neoadjuvant CT + RT.
Considering only the patients treated with neoadjuvant CT + RT, those with pCR had a better 5-year OS (100% vs. 81%, p = 0.111) and DFS (100% vs. 62%, p = 0.023) (Figs. 7 and 8).
Figs. 7 and 8.
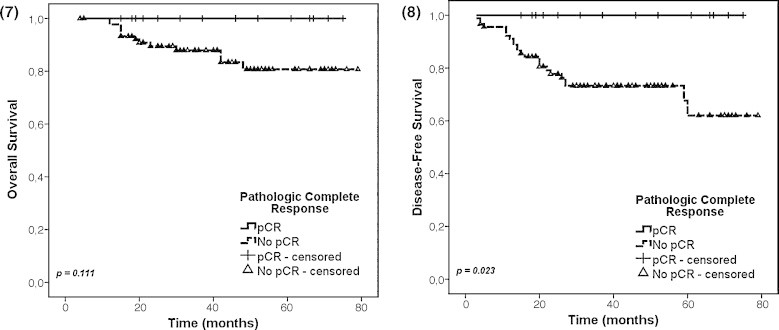
pCR impact on OS and DFS.
If we only consider the patients clinically staged as cT3–4 that had undergone neoadjuvant CT + RT, we noticed a significantly better 5-year LRC (100% vs. 89%, p = 0.027), DFS (88% vs. 43%, p = 0.003) and OS (89% vs. 77%, p = 0.048) in those patients who had downstaging to ypT0–2 (Figs. 9–11).
Figs. 9–11.

Impact of pathologic downstaging to ypT0–2 on LRC, DFS and OS.
The addition of adjuvant CT to the patients scheduled for neoadjuvant CT + RT did not bring a benefit on 5-year LRC (95% vs. 95%, p = 0.828), on 5-year DFS (60% vs. 78%, p = 0.654) (Fig. 12) nor on 5-year OS (75% vs. 84%, p = 0.932). For the patients who had pathologic downstaging to ypT0–2, the 5-year DFS was similar for those who were submitted or not to adjuvant CT (90% vs. 93%, respectively; p = 0.578). In those patients who had no pathologic downstaging (ypT3–4) and were/were not treated with adjuvant CT, there was also no evidence of benefit on the 5-year DFS (32% vs. 65%; p = 0.773).
Fig. 12.

Impact of adjuvant CT on DFS in patients submitted to neoadjuvant CT + RT.
4. Discussion and conclusions
Preoperative CT + RT can lead to tumor downstaging and improves ressectability in LARC.21,22 Continuous i.v. 5-FU infusion is superior to 5-FU bolus in terms of tumor response, and it is associated to a slight increase in OS and LRC in advanced colorectal cancer.23,24 Although continuous i.v. infusion has the biologic advantage of prolonging the exposure of cells to 5-FU and improving antitumor activity, its disadvantages include the requirement of a central venous access with potential complications, such as bleeding, thrombosis and pneumothorax.25 Oral CT mimics the pharmacokinetics of continuous 5-FU infusion while avoiding technical barriers of i.v. infusion and being more convenience. Oral fluoropyrimidines, UFT and Capecitabine, constitute an attractive alternative.
Neoadjuvant pelvic RT combined with CT should be regarded as standard treatment for stage II and III rectal cancer. Although there is an increase in acute toxicity, it did not alter the compliance to treatment.26
The most commonly reported early endpoint is the rate of pCR. It appears to be associated in some non-randomized studies with improvement in DFS.9,27 It has been shown in one randomized trial that time interval between RT and surgery influences the degree of downstaging, with 10% of patients operated within 2 weeks after RT experiencing pathological downstaging compared to 26% of patients operated in 6–8 weeks after RT (p = 0.005).28 Many studies have shown that neoadjuvant CT + RT significantly increases the rate of pCR, as well as nodal and tumor downstaging (Table 7).
Table 7.
Neoadjuvant chemoradiotherapy results (2–14). C: capecitabine; L: leucovorin.
| Authors | No. | RT | CT | Downstaging T (%) | Downstaging N (%) | pCR (%) |
|---|---|---|---|---|---|---|
| De Paoli (5) | 53 | 50.4 | C | 57 | 78 | 24 |
| Krishnan (6) | 54 | 52.5 | C | 51 | 52 | 18 |
| Kim (7) | 95 | 50.4 | C | 57 | 69 | 12 |
| De la Torre (8) | 77 | 45–50.4 | 5-FU | 43.3 | 25 | 13.2 |
| 78 | UFT,L | 59.2 | 23.7 | 13.2 | ||
| Feliu (2) | 41 | 50.4 | UFT,L | 61 | – | 15 |
| Janjan (9) | 117 | 45 | 5-FU | 62 | – | 27 |
| NSABP R-03 (10) | 58 | 50.4 | 5-FU,L | – | – | 8 |
| Sauer (3) | 421 | 50.4 | 5-FU | – | – | 8 |
| Kim (11) | 145 | 50.4 | 5-FU,L | – | – | 11.3 |
| 133 | C | – | – | 16.1 | ||
| Crane (12) | 207 | 45 | 5-FU | 62 | – | 23 |
| 196 | – | 42 | – | 5 | ||
| Gérard FFCD 9203 (13) | 375 | 45 | 5-FU,L | – | – | 11.4 |
| 367 | – | – | – | 3.6 | ||
| Bosset EORTC 22921 (4) | 473 | 45 | 5-FU,L | – | – | 13.7 |
| 476 | – | – | – | 5.3 | ||
| Fernandez-Martos (14) | 94 | 45 | UFT | 54 | – | 15 |
In our study we verified that the combination of CT to RT significantly increased the tumor response, especially the nodal downstaging, at the expense of a higher but manageable toxicity. It also allowed a higher complete surgical resection without increasing postoperative complications rate.
Tumor downstaging was superior in patients treated with CT + RT (GROUP 2: 47.7%; GROUP 3: 52.4%) than in patients of the RT arm (26.7%) (p = 0.224). Nodal downstaging was significantly better (p = 0.008) in GROUP 2 (82.1% vs. 54.5% and 54.8%). The neoadjuvant CT + RT groups had better pCR (GROUP 2: 16.9%; GROUP 3: 11.9%) compared to the RT arm (0%) (p = 0.207). All these results are comparable to those described in several other studies.2–14
We registered a LRC of 95%. The global 3- and 5-year DFS was 75% and 68%, respectively. The global 3- and 5-year OS was 88% and 80%, respectively.
Both 5-year OS (84% vs. 59%, p = 0.038) and DFS (69% vs. 55%, p = 0.05) were significantly higher in patients treated with neoadjuvant CT + RT than in the RT group.
A recent randomized phase III study compared preoperative RT with CT + RT in non-resectable rectal cancer and verified that the patients treated with CT + RT had better pCR (16% vs. 7%, p = 0.04), local control (82% vs. 67%, p = 0.03), time to treatment failure (63% vs. 44%, p = 0.03), cancer-specific survival (72% vs. 55%, p = 0.02) and OS (66% vs. 53%, p = 0.09).29
Comparing our results with the single randomized phase III trial8 that compared 5-FU vs. oral fluoropyrimidine, we noticed that 3-year OS (87% vs. 74%) and LRC (92.5% vs. 91.1%) were similar to those of the CT + RT group in our study (90% and 77%, respectively). Sauer et al. conducted a randomized trial comparing preoperative vs. postoperative CT + RT and in the preoperative group 5-year OS was 76% and LRC 94%.3
Carlos Fernandez-Martos et al. studied preoperative CT + RT with UFT and the actuarial rate of 3-year DFS was 72% and OS was 75%. DFS was 92% for downstaging patients and 51% for patients who had not responded (p < 0.00001). OS was significantly higher (p = 0.002) for patients with downstaging following preoperative treatment than for patients who had not responded.14 Another study15 also verified that pCR or intermediate response was related to an improved DFS after CT + RT. Julio Garcia-Aguilar et al. analysed 168 patients treated with CT + RT and had a 5-year LRC of 95%, OS of 68% and a DFS of 95.2% in patients who had pCR and 55.4% in patients without pCR. Their study suggested that a pCR to CT + RT is a favorable prognostic factor in patients with LARC.16
The Gastro-Intestinal Working Group of the Italian Association of Radiation Oncology analysed retrospectively 566 patients with LARC achieving pCR after neoadjuvant therapy and they verified that this favorable group of patients had a very low rate of local recurrence (1.2%) and a favorable clinical outcome independent of the neoadjuvant CT schedule used, achieving a 5-year DFS of 84.7% and 5-year OS of 91.6%. In such a group of patients, the use of postoperative CT could be very debatable. Conversely, the subset of patients older than 60 years, with cStage III and treated with a radiation dose of 45 Gy or less experienced a relatively worse prognosis, even after achieving ypCR. The prognosis of the high-risk group of patients compares with the outcome of a non-selected population.30
In our study, considering only the patients treated with neoadjuvant CT + RT, those with pCR also had a better 5-year DFS (100% vs. 62%, p = 0.023) and OS (100% vs. 81%, p = 0.111). When we consider only the patients clinically staged as cT3–4 who underwent neoadjuvant CT + RT, we also verified a significantly better 5-year LRC (p = 0.027), DFS (p = 0.003) and OS (p = 0.048) in those patients who had downstaging to ypT0–2.
On the other hand, Pucciarelli et al. have not found statistically significant differences for DFS and OS on comparing the actuarial survival curves of patients with different tumor responses to preoperative treatment, whether evaluated as tumor regression grade or as pTNM stage.31
There are still insufficient data on adjuvant postoperative chemotherapy after preoperative treatment with chemoradiation to come to any conclusions about its use.32,33 A recent study showed that adjuvant CT was still of borderline significance (worse for adjuvant CT).30 In the EORTC 22921 trial postoperative chemotherapy had a non-significant influence on local relapse and relapse free and overall survival. Exploratory subgroup analyses suggest that only good-prognosis patients with downstaging of cT3–4 to ypT0–2 benefit from adjuvant CT, with better DFS and OS.34 They concluded it was not because tumor downstaging was achieved that those patients also benefited from further CT, but rather that the same patients who achieved downstaging had a disease which was responsive to both the preoperative and the adjuvant treatment. These data support those shown in other trials, as the QUASAR trial, that had a significant benefit on survival of 3–6%.35
In our study the addition of adjuvant CT to the patients scheduled for neoadjuvant CT + RT did not bring a benefit on 5-year LRC (95% vs. 95%, p = 0.828), on 5-year DFS (p = 0.654) nor on 5-year OS (p = 0.932). However, in the subgroup analyses our results disagree from those observed in the EORTC trial. If we only consider those patients who had pathologic downstaging to ypT0–2, the 5-year DFS was identical for those who were submitted to adjuvant CT and those were not (p = 0.578). For the patients who had no pathologic downstaging (ypT3–4), the administration of adjuvant CT did not have any benefit on 5-year DFS (p = 0.773).
Although the results of our study are very promising, we need to take into account that this study is not a randomized trial and it might have some bias in the patients’ treatment modality distribution that might influence some of the results. We hope soon to increase the total number of patients so we could be able to confirm the impact of the pathologic response and adjuvant CT on OS and DFS.
References
- 1.National Institutes of Health Consensus Conference Adjuvant therapy for patients with colon and rectal cancer. J Am Med Assoc. 1990;264:1444–1450. [Google Scholar]
- 2.Feliu J., Calvillo J., Escribano A. Neoadjuvant therapy of rectal carcinoma with UFT-leucovorin plus radiotherapy. Ann Oncol. 2002;13:730–736. doi: 10.1093/annonc/mdf116. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R., Becker H., Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Eng J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Bosset J.F., Calais G., Mineur L. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results of EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 5.De Paoli A., Chiara S., Luppi G. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol. 2006;17:246–251. doi: 10.1093/annonc/mdj041. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan S., Janjan N.A., Skibber J.M. Phase II study on capecitabine (xeloda®) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Phys. 2006;66:762–771. doi: 10.1016/j.ijrobp.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.C., Kim T.W., Kim J.H. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:346–353. doi: 10.1016/j.ijrobp.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 8.De la Torre A., Garcia-Berrocal M.I., Arias F. Preoperative chemoradiatiotherapy fr rectal cancer: randomized trial comparing oral uracil and tegafur and oral leucovorin vs. intravenous 5-fluorouracil and leucovorin. Int J Radiat Oncol Phys. 2008;70:102–110. doi: 10.1016/j.ijrobp.2007.05.068. [DOI] [PubMed] [Google Scholar]
- 9.Janjan N.A., Crane C., Feig B.W. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Roh M.S., Petrelli N., Wieand L. Phase III randomized trial of preoperative versus postoperative multimodality therapy in patients with carcinoma of the rectum (NSABP R-03) Proc Am Soc Clin Oncol. 2001;20:123. [Abstr 490] [Google Scholar]
- 11.Kim D.Y., Jung K.H., Kim T.H. Comparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67:378–384. doi: 10.1016/j.ijrobp.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 12.Crane C.H., Skibber J.M., Birnbaum E.H. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Phys. 2003;57:84–89. doi: 10.1016/s0360-3016(03)00532-7. [DOI] [PubMed] [Google Scholar]
- 13.Gerard J.P., Conroy T., Bonnetain F. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Martos C., Aparicio J., Bosch C. Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase II multicenter study with 3 years’ follow-up. J Clin Oncol. 2004;22:3016–3022. doi: 10.1200/JCO.2004.11.124. [DOI] [PubMed] [Google Scholar]
- 15.Rodel C., Martus P., Papadoupolos T. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 16.García-Aguilar J., de Anda E.H., Sirivongs P. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 17.Myerson R.J., Garofalo M.C., Naqa I.E. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roels S., Duthoy W., Haustermans K. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129–1142. doi: 10.1016/j.ijrobp.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Cancer Therapy Evaluation Program . DCTD/NCI/NIH/DHHS; 2006. Common terminology criteria for adverse events, version 3.0. [Google Scholar]
- 20.6th ed. Springer; 2001. AJCC cancer staging handbook. [Google Scholar]
- 21.Janjan N.A., Khoo V.S., Abbruzzese J. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M.D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027–1038. doi: 10.1016/s0360-3016(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 22.Minsky B.D., Cohen A.M., Enker W.E. Preoperative 5-FU, low-dose leucovorin, and radiation therapy for locally advanced and unresectable rectal cancer. Int J Radiat Oncol Biol Phys. 1997;37:289–295. doi: 10.1016/s0360-3016(96)00487-7. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell M.J., Martenson J.A., Wieand H.S. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 24.Meta-analysis Group in Cancer: efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 25.Grem J.L. Systemic treatment options in advanced colorectal cancer: perspectives on combination 5-fluorouracil plus leucovorin. Semin Oncol. 1997;24(Suppl. 18):8–18. [PubMed] [Google Scholar]
- 26.Bosset J.F., Calais G., Daban A. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance: report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur J Cancer. 2004;40:219–224. doi: 10.1016/j.ejca.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Valentini V., Coco C., Picciocchi A. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 28.Francois Y., Nemoz C., Baulieux J. Influence of the interval between radiation therapy and surgery on downstaging and rate of sphincter sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 29.Brændengen M., Tveit K.M., Berglund A. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–3694. doi: 10.1200/JCO.2007.15.3858. [DOI] [PubMed] [Google Scholar]
- 30.Capirci C., Valentini V., Cionini L. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72(1):99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Pucciarelli S., Toppan P., Friso M.L. Complet pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum. 2004;47:1798–1807. doi: 10.1007/s10350-004-0681-1. [DOI] [PubMed] [Google Scholar]
- 32.Valentini V., Beets-Tan R., Borras J.M. Evidence and research in rectal câncer. Radiother Oncol. 2008;87:449–474. doi: 10.1016/j.radonc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Valentini V., Aristei C., Glimelius B. Multidisciplinary rectal cancer management: 2nd Europena Rectal Cancer Consensus Conference (EURECA-CC2) Radiother Oncol. 2009;92:148–163. doi: 10.1016/j.radonc.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Collette L., Bosset J.F., Den Dulk M. Patients with curative resection of cT3–4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organization for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379–4386. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]
- 35.QUASAR. Collaborative Group Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomized study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]


