Abstract
Aim
The aim of this work is to evaluate rectal and bladder dose for the patients treated for gynecological cancers.
Background
The GZP6 high dose rate brachytherapy system has been recently introduced to a number of radiation therapy departments in Iran, for treatment of various tumor sites such as cervix and vagina.
Materials and methods
Our analysis was based on dose measurements for 40 insertions in 28 patients, treated by a GZP6 unit between June 2009 and November 2010. Treatments consisted of combined teletherapy and intracavitary brachytherapy. In vivo dosimetry was performed with TLD-400 chips and TLD-100 microcubes in the rectum and bladder.
Results
The average of maximum rectal and bladder dose values were found to be 7.62 Gy (range 1.72–18.55 Gy) and 5.17 Gy (range 0.72–15.85 Gy), respectively. It has been recommended by the ICRU that the maximum dose to the rectum and bladder in intracavitary treatment of vaginal or cervical cancer should be lower than 80% of the prescribed dose to point A in the Manchester system. In this study, of the total number of 40 insertions, maximum rectal dose in 29 insertions (72.5% of treatment sessions) and maximum bladder dose in 18 insertions (45% of treatments sessions) were higher than 80% of the prescribed dose to the point of dose prescription.
Conclusion
In vivo dosimetry for patients undergoing treatment by GZP6 brachytherapy system can be used for evaluation of the quality of brachytherapy treatments by this system. This information could be used as a base for developing the strategy for treatment of patients treated with GZP6 system.
Keywords: Brachytherapy, Rectal dose, Bladder dose, GZP6 afterloading unit
1. Background
Recently a GZP6 high dose rate afterloading unit, with 60Co sources, manufactured by the Nuclear Power Institute of China (NPIC),1 has been introduced to the radiotherapy centers of Iran. The GZP6 brachytherapy unit uses six 60Co source braids in six channels, as described in one of our previous publications.2 These units are utilized for treatment of cancers of the cervix, rectum, esophagus and nasopharynx.2 Unlike 192Ir sources, the 60Co has a relatively higher half life and lower specific activity, which makes it unfavorable as an high dose rate (HDR) source. However, higher exposure rate constant of 60Co (13.2 Rcm2/mCi h versus 4.69 Rcm2/mCi h) and larger source size, multiple source versus a single source, compensate some of the deficiencies.3 Moreover, a longer half life source reduces the need for frequently changing the source, which is an advantage for some countries such as Iran. This isotope has advantage in those countries where various sources are not commercially and widely available. Besides, 60Co sources have higher air kerma rate constant than other brachytherapy sources.
The rectum and bladder are two radiosensitive organs adjacent to the target volume in patients who are treated for vaginal or cervical cancers.4 It is recommended that the dose received by these organs during therapeutic sessions should be identified.5 The International Commission of Radiation Units and Measurements (ICRU) in its report number 38 recommended clear definition of the rectum and bladder reference points in the implemental procedures. The rectum and bladder dose in reference to point A dose should be limited to 80% of prescribed point A dose based on the recommendations of the report.5
There are various studies on TG-43 dosimetric evaluation of the parameters, as well as tabulated dose rate distributions of GZP6 sources.6–9 Mesbahi et al. have evaluated radial dose function for three GZP6 brachytherapy 60Co sources. They have compared the Monte Carlo calculated radial dose function values with those obtained from GZP6 treatment planning system and their results have shown that there is a good agreement between the Monte Carlo and treatment planning system values, except for points close to the source.6 Mesbahi and Naseri have calibrated three GZP6 sources through an in-air measurement of air kerma rate. They have compared air kerma rate values obtained from measurement with those provided by the GZP6 manufacturer. The manufacturer provided air kerma rate values at 1 m distance were 6.195, 6.78 and 5.44 cGy/s for sources 1, 2 and 3, respectively. The corresponding measured air kerma rate values were 6.18, 6.95, 5.58 cGy/s. The measured data for all the three sources were in agreement with the data provided by the GZP6 manufacturer.7 Bahreyni et al. have determined air kerma strength of a GZP6 source through Monte Carlo simulation and measurement and GZP6 treatment planning system. When considering the large uncertainty incorporated with the GZP6 treatment planning value, the air kerma strength values determined by the three methods were in agreement.2 Naseri and Mesbahi have verified dose distribution of GZP6 treatment planning system through simulation of three GZP6 sources. In their study, the dose distributions calculated by the GZP6 treatment planning system were validated, but at the points near the sources and beyond the tip of the sources the agreement between the Monte Carlo and GZP6 treatment planning results was not good.8 Hariri Tabrizi et al. have derived TG-43 parameters for GZP6 sources in a comprehensive Monte Carlo study of the sources. Their results can be used for validation of the GZP6 treatment planning system and also for introduction of a complementary software for this system.9 However, the GZP6 treatment planning system still performs the treatment planning based on Sievert integral and there are several prefixed treatment plans with predesigned dwell positions and dwell times in tandem and ovoids for gynecological implants. Therefore, a clinical application of these units requires in vivo dosimetry for a more accurate evaluation of dose received by normal organs at risk, for patients who are being treated by this system.
Despite a detailed dosimetric evaluation of the GZP6 system with the most recently available recommendations by the American Association of Physicists in Medicine (AAPM) task group No. 43 (TG-43) report,10 there had been no clinical available on this system at the time that this study began. Particularly, for the pre-fixed plans, knowing the bladder and rectal dose arising from intracavitary treatments of cervical cancers by this unit would be useful for clinical evaluation of the unit.
2. AIM
The goal of the present investigation is to present the retrospective evaluation of the measured bladder and rectal dose in 40 intracavitary brachytherapy procedures in 28 patients with cervical or vaginal cancer who underwent treatment by a GZP6 60Co afterloading unit, between June 2009 and November 2010. This report will provide a guideline for our future procedures.
3. Materials and methods
3.1. Patients
These investigations include a total number of 40 intracavitary brachytherapy procedures that were performed on 28 patients with cervical or vaginal cancers. These patients had undergone a combined external beam radiotherapy and HDR brachytherapy with a GZP6 afterloading unit at the Reza Radiotherapy and Oncology Center, in the period between June 2009 and November 2010. The measurements of rectal and bladder doses on these patients were performed using TLD. All the intracavitary HDR treatments were performed under general or partial anesthesia depending on the patients’ situations. In accordance with the guidelines of our institutional ethical committee, written consent of patients were obtained before measurements were carried out. In these investigations, coordinates of dose prescription point for each patient were based on the anatomy of the patient and extent of the disease. Fig. 1 shows the schematic diagram of the applicator, prescription points, and other points of interest in cases of cylindrical applicator, pair ovoids and a tandem plus two ovoids. The origin of the coordinate system for each applicator combination is also evident in this figure.
Fig. 1.
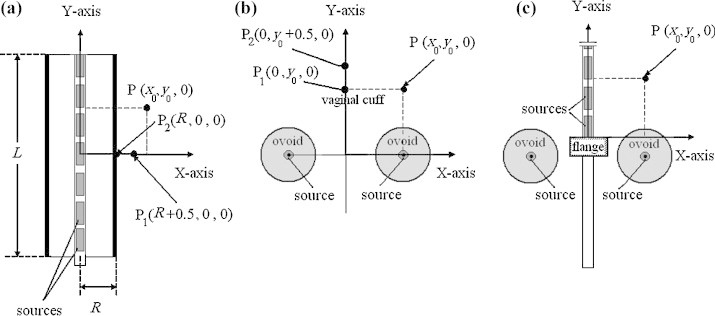
A diagram illustrating prescription point (P) which was the point of dose prescription for the cases of: (a) cylinder, (b) pair ovoids and (c) tandem plus two ovoids in this study. There can be a number of other points of interest (P1, P2) in each case.
3.2. TLD calibrations
All TLDs (TLD-400 chips used for the rectum and TLD-100 microcubes used for the bladder dosimetry) were calibrated in 10 × 10 cm2 fields of a Theratron Phoenix cobalt teletherapy machine. The TLDs were positioned at 0.5 cm depth in a cubic 20 cm × 20 cm × 10 cm Perspex phantom in the calibration process. The calibration was performed for dose of 1 Gy and in the next step dose response of the TLDs was evaluated for 1–20 Gy and 1–11 Gy dose ranges, respectively for TLD-400 chips and TLD-100 microcubes. The output of the cobalt unit was regularly calibrated by a 0.6 cc farmer type ionization chamber (model NE 2581). Calibration procedure consisted of three stages to determine ECC (element correction coefficient) and RCF (reader correction factor).
The TLDs were read by a Harshaw TLD reader (model 3500) 24 h after irradiation. Standard time temperature profile (TTP) defined for reading of TLD-100 and TLD-400 chips were applied. The annealing process of TLDs was performed at 400 °C for 1 h followed by a 2 h heating at 100 °C.
3.3. In vivo measurements
A pilot study for a number of patients with respect to measurement of rectum dose by TLD-100 chips showed that there are cases in which the rectum dose measured by the TLDs are higher than 10 Gy value which is the upper dose limit for this type of TLDs. However, TLD-400 (CaF2:Mn) has a linear response and can be used for a wider dose range (0.1 μGy–100 Gy) to respond to low dose as well as high dose values. Hence, TLD-400 (Harshaw/Bicron, Solon, OH) with 3.2 mm × 3.2 mm × 0.9 mm dimensions was used for rectum dose measurements. According to our pilot study, the bladder dose was not high and therefore microcubes of TLD-100 (LiF:Mg, Ti, Harshaw/Bicron, Solon, OH) sized 1 mm × 1 mm × 1 mm were employed for the purpose of bladder dose measurements.
For the rectum dosimetry, 10 TLD-400 chips were inserted in a hollow sterile plastic tube designed to cover an overall length of the rectum equal to 12 cm. 0.9 mm long plastic spacers were utilized to maintain the spacing between the ten chips. The total length of the rectum tube was about 17 cm, including a 12 cm length containing 10 TLDs and an interval of 5 cm including a plastic spacer without any TLD.
To measure bladder dose, three TLD-100 microcubes were used. The microcubes were inserted in a hollow sterile plastic tube designed for this purpose. The outer diameter of the tube was 3.2 mm. The total length of the bladder tube was 8 cm. This length was divided into two parts: the first 5.5 cm space was filled with a plastic spacer and the second part included three TLDs. Intervals of 1 cm were maintained by inserting plastic spacers between the TLDs.
The tubes designed for the rectum and bladder were inserted into the patients’ rectum and bladder before insertion of the sources, when the applicators were being inserted. The tubes were fixed by attaching to the application set up.
4. Results
4.1. Dose–response of the TLDs
The dose–response curves for the TLDs (type 400 and type 100) are shown in Fig. 2. As evident from this figure, the dose given to the TLDs was plotted versus measured dose over a dose range of 1–20 Gy for the type 400 and 1–11 Gy for type 100 TLDs.
Fig. 2.
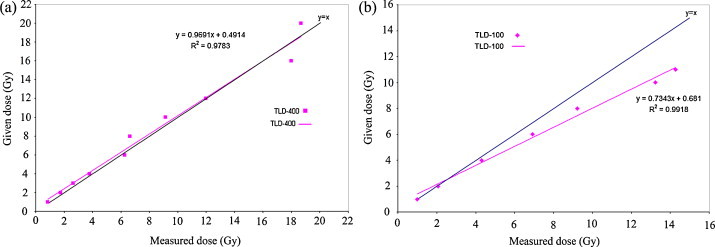
Dose–response (given dose (Gy) versus measured dose (Gy)). (a) For the TLD-400 chips and (b) for the TLD-100 microcubes used in this study.
As evident from Fig. 1(a) for the TLD-400 chips, the slope of the fitted line to the data points (0.9691) is very close to unity. So in our further measurements of rectum dose, the measured dose by the TLD-400 chips, in the dose range of 1–20 Gy, could be considered equal to the actual dose received by the TLDs. For the TLD-100 microcubes, the following equation was fitted to the dose response data (Fig. 1(b)):
| (1) |
Since the slope of the above linear function is different from unity, in our further measurement of bladder dose, we have utilized this equation to convert the measured dose by the TLDs to the actual dose received by them. Reported bladder doses in this work are converted dose values derived from Eq. (1).
4.2. In vivo measurements
The results of in vivo dose measurements during 40 sessions of insertions are listed in Table 1. The table also includes the treatment details for each session.
Table 1.
The measured maximum rectum and bladder dose values (in Gy). The dose values are also presented relative to the prescribed dose. All of the distances are presented in cm.
| Type of applicators | Insertion number | Diameter of cylinder (cm) | Stepping length (cm) | Prescribed dose (Gy) | Prescription point (x, y, z) | Rectum dose (Gy) |
Bladder dose (Gy) |
||
|---|---|---|---|---|---|---|---|---|---|
| Maximum | Relative | Maximum | Relative | ||||||
| Cylinder | 1 | 3 | 5.5 | 5 | 2, 3, 0 | 3.95 | 79.02 | 3.70 | 74.06 |
| 2 | 2.5 | 5 | 5 | 1.75, 2.5, 0 | 3.16 | 63.20 | 7.86 | 157.26 | |
| Type of applicators | Insertion number | Size of ovoids | Prescribed dose (Gy) | Prescription point (x, y, z) | Rectum dose (Gy) |
Bladder dose (Gy) |
||
|---|---|---|---|---|---|---|---|---|
| Maximum | Relative | Maximum | Relative | |||||
| Pair ovoids | 3 | Small | 6 | 0.5, 0, 0 | 2.20 | 36.67 | 0.72 | 11.99 |
| 4 | Small | 6 | 0.5, 0, 0 | 1.72 | 28.68 | 1.19 | 19.87 | |
| 5 | Small | 5 | 2, 2, 0 | 3.42 | 68.48 | 9.64 | 192.89 | |
| 6 | Small | 5 | 2, 2, 0 | 10.87 | 217.47 | 7.95 | 159.00 | |
| 7 | Small | 5 | 1, 1, 0 | 7.54 | 150.82 | 2.35 | 46.94 | |
| 8 | Small | 5 | 1, 1, 0 | 7.02 | 140.44 | 2.08 | 41.53 | |
| 9 | Medium | 6 | 2, 2, 0 | 9.91 | 165.09 | 10.40 | 173.34 | |
| 10 | Medium | 6 | 1, 1, 0 | 2.03 | 33.75 | 3.17 | 52.84 | |
| 11 | Small | 6 | 2, 2, 0 | 6.52 | 108.68 | 8.17 | 136.14 | |
| 12 | Medium | 5 | 2, 2, 0 | 18.55 | 371.10 | 3.79 | 75.75 | |
| 13 | Half | 5 | 2, 2, 0 | 4.55 | 91.07 | 8.04 | 160.73 | |
| 14 | Half | 5 | 2, 2, 0 | 9.22 | 184.42 | 10.97 | 219.50 | |
| 15 | Medium | 6 | 0.5, 0.5, 0 | 5.70 | 95.01 | 3.12 | 52.04 | |
| 16 | Small | 6 | 2, 2, 0 | 14.00 | 233.35 | 7.90 | 131.72 | |
| 17 | Small | 6 | 2, 2, 0 | 14.26 | 237.59 | 8.70 | 144.92 | |
| 18 | Small | 5 | 2, 2, 0 | 17.75 | 354.99 | 7.32 | 146.49 | |
| 19 | Medium | 5 | 0.5, 0.5, 0 | 2.47 | 49.43 | 3.56 | 71.10 | |
| 20 | Small | 4 | 0.5, 0.5, 0 | 2.16 | 54.02 | 2.75 | 68.81 | |
| 21 | Small | 5 | 0.5, 0.5, 0 | 1.85 | 37.08 | 15.85 | 317.05 | |
| 22 | Small | 6 | 2, 2, 0 | 13.81 | 230.21 | 5.75 | 95.84 | |
| 23 | Medium | 6 | 0.5, 0.5, 0 | 7.02 | 117.06 | 2.35 | 39.25 | |
| 24 | Medium | 7 | 0.5, 0.5, 0 | 5.92 | 84.63 | 14.41 | 205.85 | |
| 25 | Medium | 6 | 0.5, 0.5, 0 | 5.93 | 98.80 | 1.41 | 23.52 | |
| 26 | Medium | 7 | 0.5, 0.5, 0 | 11.37 | 162.40 | 1.83 | 26.14 | |
| 27 | Medium | 5 | 2, 2, 0 | 16.92 | 338.43 | 6.02 | 120.46 | |
| 28 | Half | 5 | 2, 2, 0 | 8.91 | 178.19 | 4.74 | 94.73 | |
| 29 | Small | 5 | 1, 1, 0 | 6.00 | 120.00 | 3.48 | 69.59 | |
| 30 | Small | 5 | 1, 1, 0 | 11.36 | 227.20 | 1.91 | 38.11 | |
| Type of applicators | Insertion number | Size of ovoids | Stepping length (cm) | Prescribed dose (Gy) | Prescription point (x, y, z) | Rectum dose (Gy) |
Bladder dose (Gy) |
||
|---|---|---|---|---|---|---|---|---|---|
| Maximum | Relative | Maximum | Relative | ||||||
| Tandem and ovoids | 31 | Small | 4 | 6 | 2, 2, 0 | 4.42 | 73.69 | 3.42 | 57.02 |
| 32 | Small | 4 | 6 | 2, 2, 0 | 5.07 | 84.56 | 2.36 | 39.40 | |
| 33 | Small | 4 | 4 | 2, 2, 0 | 6.41 | 160.21 | 2.04 | 51.11 | |
| 34 | Small | 4 | 4 | 2, 2, 0 | 4.23 | 105.67 | 7.50 | 187.57 | |
| 35 | Small | 5 | 6 | 2, 2, 0 | 8.04 | 133.98 | 6.89 | 114.85 | |
| 36 | Small | 5 | 5 | 2, 2, 0 | 10.08 | 201.66 | 0.76 | 15.15 | |
| 37 | Small | 5 | 5 | 2, 2, 0 | 10.94 | 218.85 | 0.78 | 15.54 | |
| 38 | Medium | 4 | 5 | 2, 2, 0 | 2.80 | 56.07 | 4.57 | 91.50 | |
| 39 | Medium | 7 | 6 | 2, 2, 0 | 6.75 | 112.43 | 2.89 | 48.19 | |
| 40 | Medium | 7 | 6 | 2, 2, 0 | 9.90 | 165.01 | 4.26 | 71.00 | |
Percentage measured maximum rectal and bladder dose of patients normalized to the dose at the point of dose prescription was divided into different ranges. The results are presented in Table 2. As evident in this table, the relative maximum dose to the rectum in 29 insertions (72.5% of treatments sessions) and to bladder in 18 insertions out of 40 (45% of treatments sessions) are higher than the value of 80%.
Table 2.
Summary of measured rectum and bladder dose relative to the prescribed dose.
| Relative measured dose to the prescribed dose (%) | Number of cases |
|
|---|---|---|
| Rectum | Bladder | |
| 0–25% | 0 | 5 |
| 25–50% | 5 | 7 |
| 50–100% | 11 | 13 |
| 100–200% | 14 | 12 |
| 200–300% | 7 | 2 |
| 300–350% | 1 | 1 |
| 350–400% | 2 | 0 |
5. Discussion and conclusion
In this study, the dose to the rectum and bladder of cervical or vaginal patients treated by a GZP6 brachytherapy unit were measured. It has been recommended by the ICRU and the American Brachytherapy Society that the maximum dose to the rectum and bladder in intracavitary treatment of vaginal or cervical cancer should be lower than 80% of the prescribed dose to point A (point of dose prescription in the Manchester system).5,11 In summary, our results showed that out of the total number of 40 insertions, the maximum rectum dose in 29 insertions (72.5% of treatments sessions) and the maximum bladder dose in 18 insertions (45% of treatments sessions) were higher than the 80% of prescribed dose to the point of dose prescription. This may be related to either the experience of cardiologists or to GZP6 treatment planning system. A more detailed study in this field is required to illuminate the cause. In brachytherapy departments, dose to the rectum and bladder is determined during treatment planning procedures by radiography, but there are studies reporting discrepancies between radiography and in vivo measurement methods.12–15 Since in vivo dosimetry is a method that estimates dose to the rectum and bladder through measurements in real conditions, in vivo measurement of rectum and bladder doses of patients for all therapeutic sessions could be clinically useful. Performing patient dosimetry in each session, in which the dose to the rectum and/or bladder may be high, will provide an option to reduce the overall dose to the rectum and/or bladder which is the summation of doses over the sessions given to a particular patient. GZP6 treatment planning system has no option to define stepping sources for ovoid applicators. It is not possible either to apply time weighting for different dwell positions in the stepping sources which are used in tandem applicators in this system. However, there are other methods to reduce rectal and bladder dose: using ovoids with shields, changing the treatment plan, reducing the number of brachytherapy sessions, etc. Although in this study, the averaged measured maximum doses to the rectum and bladder were respectively 7.62 and 5.17 Gy, there are also other studies in which the identified doses to these organs were higher than 5 Gy,13,16–18 which is a common prescribed dose to point A in the Manchester system.
The present retrospective study was based on the evaluation of brachytherapy treatments performed by a GZP6 afterloading system during a limited period of time at the Reza Radiotherapy and Oncology Center. At the Reza Radiotherapy and Oncology Center, the rectum and bladder doses are not measured routinely but are calculated by radiographs. Our results will encourage more actions to reduce rectum and bladder doses in this center, as well as other centers in Iran which are using GZP6 unit. Following the publication of our results, the treatment team in the Reza Radiotherapy and Oncology Center and other radiotherapy centers in Iran which are using this system may have an evaluation on the treatments performed by the GZP6 unit. This evaluation will have an impact on their treatment strategies in the future brachytherapy practice involving the GZP6 afterloading system.
Conflict of interest
None declared.
Financial disclosure
This work which was part of a Ph.D. thesis, was funded by Mashhad University of Medical Sciences (MUMS).
Acknowledgments
The authors wish to thank Mr. Mohsen Layegh and Dr. Shokoohozaman Soleimani Fard for their help to irradiate the TLD chips. The authors also would like to thank the personnel of the Reza Radiotherapy and Oncology Center for their most appreciated help in patient dosimetry.
References
- 1.Nuclear Power Institute of China (NPIC); 2010. Available at: www.npic.ac.cn/ [accessed October 24].
- 2.Bahreyni Toossi M.T., Ghorbani M., Mowlavi A.A. Air kerma strength characterization of a GZP6 Cobalt-60 brachytherapy source. Rep Pract Oncol Radiother. 2010;15(6):190–194. doi: 10.1016/j.rpor.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suntharalingam N., Podgorsak E.B., Tolli H. Brachytherapy: physical and clinical aspects. In: Podgorsak E.B., editor. Radiation oncology physics: a handbook for teachers and students. International Atomic Energy Agency (IAEA); Vienna: 2005. pp. 456–464. [Google Scholar]
- 4.Sun L.M., Huang E.Y., Ko Sh.F. Computer tomography-assisted three-dimensional technique to assess rectal and bladder wall dose in intracavitary brachytherapy for uterine cervical cancer. Radiother Oncol. 2004;71(3):333–337. doi: 10.1016/j.radonc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Report 38 IVRU. Dose and volume specification for reporting intracavitary therapy in gynecology. Bethesta, MD: International Commission on Radiation Units and Measurements (ICRU); 1985.
- 6.Mesbahi A. Radial dose functions of GZP6 intracavitary brachytherapy 60Co sources: treatment planning system versus Monte Carlo calculations. Iran J Radiat Res. 2008;5(4):181–186. [Google Scholar]
- 7.Mesbahi A., Naseri A. In-air calibration of new high dose rate 60Co brachytherapy sources: results of measurements on a GZP6 brachytherapy afterloading unit. Rep Pract Oncol Radiother. 2008;13(2):69–73. [Google Scholar]
- 8.Naseri A., Mesbahi A. Application of Monte Carlo calculations for validation of a treatment planning system in high dose rate brachytherapy. Rep Pract Oncol Radiother. 2009;14(6):200–204. [Google Scholar]
- 9.Hariri Tabrizi S., Kamali Asl A., Azma Z. Monte Carlo derivation of AAPM TG-43 dosimetric parameters for GZP6 Co-60 HDR sources. Phys Med. 2012;28(2):153–160. doi: 10.1016/j.ejmp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Rivard M.J., Coursey B.M., DeWerd L.A. Update of AAPM Task Group No. 43 Report: a revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31(3):633–674. doi: 10.1118/1.1646040. [DOI] [PubMed] [Google Scholar]
- 11.Nag S., Erickson B., Thomadsen B., Orton C., Demanes J.D., Petereit D. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48(1):201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 12.Huh S.J., Lim D.H., Ahn Y.Ch. Comparison between in vivo dosimetry and barium contrast technique for prediction of rectal complications in high-dose-rate intracavitary radiotherapy in cervix cancer patients. Strahlenther Onkol. 2003;179(3):191–196. doi: 10.1007/s00066-003-1015-2. [DOI] [PubMed] [Google Scholar]
- 13.Waldhäusl C., Wambersie A., Pötter R., Georg D. In vivo dosimetry for gynaecological brachytherapy: physical and clinical considerations. Radiother Oncol. 2005;77(3):310–317. doi: 10.1016/j.radonc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Shin K.H., Huh S.J., Chie E.K. Analysis of correlation between rectal complications and rectal dose following high dose rate intracavitary radiotherapy in patients with uterine cervix cancer: in vivo dosimetric analysis. Radiat Med. 1999;17(4):289–293. [PubMed] [Google Scholar]
- 15.Kapp K.S., Stuecklschweiger G.F., Kapp D.S., Hackl A.G. Dosimetry of intracavitary placements for uterine and cervical carcinoma: results of orthogonal film, TLD, and CT-assisted techniques. Radiother Oncol. 1992;24(3):137–146. doi: 10.1016/0167-8140(92)90372-2. [DOI] [PubMed] [Google Scholar]
- 16.Baucal M., Babic J., Kuzmanovic Z. Rectal dosimetry in intracavitary applications of cervix carcinoma: comparison of two methods. Arch Oncol. 2002;10(4):253–259. [Google Scholar]
- 17.Onal C., Arslan G., Topkan E. Comparison of conventional and CT-based planning for intracavitary brachytherapy for cervical cancer: target volume coverage and organs at risk doses. J Exp Clin Cancer Res. 2009;28(1):95. doi: 10.1186/1756-9966-28-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamema S.V., Saju S., Mahantshetty U. Dosimetric evaluation of rectum and bladder using image-based CT planning and orthogonal radiographs with ICRU 38 recommendations in intracavitary brachytherapy. J Med Phys. 2008;33(1):3–8. doi: 10.4103/0971-6203.39417. [DOI] [PMC free article] [PubMed] [Google Scholar]


