Abstract
Breast cancer risk increases with age and about a third of female breast cancers are diagnosed in patients aged older than 70. Breast cancer in the elderly has, however, poorer outcome with lower survival rate compared to younger subjects. This may be partly explained by the delay in diagnosis and the ‘under-treatment’ of elderly breast cancer patients. In this review I try to provide recommendations for screening, surgery, radiotherapy, (neo)adjuvant hormone treatment and chemotherapy, and also the treatment of metastatic disease. Since large randomised trials usually exclude elderly patients with breast cancer, there is still an insufficient evidence for the treatment of such patients.
Keywords: Breast cancer, Cancer treatment, Elderly, Old age
1. Background
Breast cancer is one of the most common types of cancer affecting women, accounting for about 6500 new cases annually in the Czech Republic. Almost one-half of the newly diagnosed breast cancers occur in women older than 65 years. Age is the most important risk factor for breast cancer. As the median age of the general population in Western European countries steadily increases, occurrence of those diseases which manifest typically in elderly people (e.g. diabetes and rheumatic, cardiovascular and pulmonary disease), including cancer is also on the rise.
Although we could expect that the elderly will be treated with similarly intensive treatment as applied in younger age groups, the elderly patients with breast cancer are frequently undertreated, even after adjustment for confounding factors, such as comorbidities, need for social support, and functional status. Elderly women are less likely to undergo breast conserving therapy and axillary lymph node dissection; radiation therapy (RT) is more likely to be omitted after breast conserving surgery and elderly patients also less frequently receive systemic therapy, particularly chemotherapy. In contrast, the use of adjuvant hormonal therapy has been reported to be independent of patient age.
The reasons for these differences in approach to the care of older patients are probably multifactorial and may include a higher rate of patient comorbidities, poorer performance status, limited social support, difficulty with transportation, patient or family preference, concerns about quality of life, lower life expectancy. In addition, because so few research studies have included older women, the lack of available evidence may also lead to less aggressive care.
Studies that have examined the predictors of surgery and adjuvant radiation have found that chronologic age was associated with substandard therapy independently of performance status, or comorbidities, suggesting that physicians may be undertreating even otherwise “healthy” elderly women.
Elderly women with breast cancer are thus considered underdiagnosed and undertreated, and this adversely affects their overall survival. Most women who die of breast cancer are much more frequently older than 65 years of age. Despite this information, it has been confirmed by many studies that elderly patients do not receive a standard treatment given to their younger counterparts.1
2. Screening of breast cancer in elderly women
Screening is likewise a very controversial issue in the elderly population. There are very few retrospective and prospective studies looking specifically at cancer screening in the elderly. In the Czech Republic, screening mammography is recommended with or without clinical breast examination (CBE), every 2 years for women aged 45 and older. Despite this, mammography is not largely used for screening purposes in the elderly patients with breast cancer, but mostly for diagnosis of symptomatic patients. Screening mammography is in general not performed in women over 65–70 years.
Some elderly women delay reporting and underreport to their physicians suspicious symptoms and lesions and their breast cancer may then be more likely to be diagnosed at a more advanced stage with putatively reduced treatment options and inferior outcomes. Apparently, breast cancer awareness should be high especially in the elderly, population with the highest risk of developing breast cancer. Breast health promotion campaigns should include images of older women to make clear the relevance of this information to women in this age group.2
3. Biologic changes associated with aging
Just as aging in the general population alters the demographics of cancer, aging in the individual patient alters the biology of cancer. These biologic changes affect the risk of cancer, tumor activity, and the response to treatment.
4. Changes in tumorigenesis and host defenses
Increased risk of cancer in the aging population may be attributed mainly to two processes: slowly accruing damage to DNA and progressive decline in host defenses against tumor growth. Damage to DNA occurs as a result of cumulative exposure to carcinogenic chemicals, radiation, and viruses. Another source of damage are the cumulative effects of endogenous processes that lead to the formation of reactive oxygen species, which are highly damaging to cellular structures. In summary, carcinogenesis is a time-consuming process. Hence, the incidence of many types of cancer increases with age. The older the patient, the greater the potential for DNA damage to be accrued. The greater the cumulative damage to DNA, the greater the potential for malignancy.
Damage to DNA may take the form of abnormal linkages within and between nucleic acid strands, breaks in nucleic acid strands, or altered sequence of bases. General damage typically leads to cellular senescence (loss of mitotic ability) and apoptosis (programmed cell death). However, damage that involves certain specific genes may lead to cancer. The activation of oncogenes stimulates tumor growth, while the deactivation of tumor suppressor genes allows cellular growth to remain uncontrolled.
Aging is associated with decreases in the production and activity of protective enzymes and hormones with the capacity to repair or bypass damaged DNA, and in immune defenses against viruses and tumor growth. In addition, damage to mitochondrial DNA interferes with apoptosis, thus allowing DNA-damaged cells with malignant potential to persist.
5. Changes in pharmacokinetics
A number of specific age-related pharmacokinetic changes may result in an increase in the toxicity of chemotherapy in older patients. The volume of distribution (Vd) shrinks because of a decrease in total body water. For drugs that bind to erythrocytes (such as anthracyclines and epipodophyllotoxins), the Vd will also be affected by anemia, which is common in older patients with chronic disease. Similarly, hypoproteinemia (in most cases secondary to inadequate synthesis or excessive renal loss of albumin and other carrier proteins) can alter the Vd for numerous drugs that have significant binding to albumin and other serum proteins.
While changes in total body water, erythrocyte mass, and serum protein levels can affect the Vd of chemotherapeutic drugs, a decrease in the glomerular filtration rate (GFR) can cause decreased clearance of some drugs. The decrease in the GFR is due to a gradual loss of nephrons. A lower GFR is of particular concern in the case of drugs and drug metabolites whose clearance depends heavily on renal excretion (including such drugs as methotrexate, bleomycin, carboplatin and the daunorubicin metabolite, daunorubinicol).
In addition, renal excretion as a percentage of total excretion varies with each chemotherapeutic drug, and a decrease in renal excretion may be offset to some degree by an increase in hepatic excretion. On the other hand, hepatic metabolism also tends to decrease in older patients owing to reductions in hepatic blood flow and cytochrome P-450 enzyme system activity.
To summarize, age-related pharmacokinetic changes can increase the toxicity of chemotherapeutic drugs, but the effects tend to be highly variable.
6. Changes in pharmacodynamics
Age-related changes can also affect pharmacodynamics, often resulting in increased resistance to the anti-tumor activity of chemotherapeutic drugs.
Elderly people are more likely to express the multidrug resistance (MDR) gene, which causes tumor cells to extrude natural drugs such as antibiotics and plant derivatives, and this mechanism may account for the drug resistance that is often seen in older patients with acute myeloid leukemia (AML). In addition, chemotherapeutic drugs that depend on inducing apoptosis are less effective if a significant proportion of tumor cells have lost this capacity.
Finally, the tumoricidal effects of chemotherapy and radiotherapy are the best in well-oxygenated cells that are rapidly proliferating. Therefore, treatment in older patients may be less effective, because tumors in this age group are often relatively anoxic (owing to impaired circulation) and indolent (owing to a natural-selection process, as more-aggressive tumors typically cause death at an earlier age).
7. Changes in cancer activity
Aging often involves a decline in tumor aggressiveness and a corresponding decline in chemotherapy effectiveness. As stated above, tumor indolence in elderly patients may arise from a natural-selection process (as patients with more-aggressive tumors usually die at a younger age) and from compromised circulation (as poor oxygenation limits cell proliferation).
Aside from these general age-related changes, certain disease-specific changes related to aging may also affect tumor activity and the response to therapy.3
8. Management decisions in elderly patients with cancer
Breast Cancer Care recommends that breast multi-disciplinary teams (MDTs) consider using comprehensive geriatric assessment (CGA) tools in the treatment decision-making process for older women. CGA tools offer a useful way of making treatment decisions on the basis of functional status rather than chronological age. We are also concerned that many older women may not be having their information and support needs adequately assessed and addressed as part of their breast cancer treatment and care. CGA tools may also be useful in assessing the psycho-social needs of older women, for example mental health issues, or help needed with daily living activities.
It is also important that the treatment decision-making process adequately takes into account informed preferences of the patient and the impact of other significant factors in that person's life. Breast cancer is unlikely to be an older patient's only health concern. An older patient is also more likely to have a burden of caring responsibilities.
Just as age itself is not an automatic contraindication to standard cancer treatment, a diagnosis of cancer by itself is not an automatic indication for treatment. Factors that relate to both the disease and the patient must be considered in deciding whether and how to treat the cancer. For example, chemotherapy in intermediate-grade lymphoma is beneficial in virtually every circumstance, but the benefit of adjuvant chemotherapy in breast cancer varies with the likelihood that the patient will die of the disease. Statistically, chemotherapy becomes beneficial only when the risk of cancer-related death exceeds a certain threshold, and that threshold is lower in a 70-year-old woman than in an 80-year-old woman.4
9. Disease factors
The diagnosis and staging of cancer follow standard criteria, but the patient's age may affect these assessments. The tumor cell type indicates its proliferative rate and invasiveness, but these measures may vary in older patients, as in the previously cited example of breast cancer, which may be less aggressive but also less responsive to chemotherapy in older women.
Cancer in older patients is often diagnosed at a more advanced and therefore less treatable stage than in younger patients. A delayed diagnosis may be due in part to a clinical bias against aggressive diagnosis in the geriatric population, and in part to older patients’ reluctance to seek treatment.
10. Patient factors
The clinical workup and CGA should document potential risk factors that could affect the outcome of treatment. Age-related declines in major physiologic functions (especially cardiovascular function, renal and hepatic function, and hematopoietic reserve) may lessen the patient's ability to withstand surgery, radiation, and cytotoxic chemotherapy.
Comorbid diseases and functional impairment are clearly associated with a poorer prognosis.5 Similarly, problems with mental, emotional, or nutritional status, polypharmacy, and lack of family or social support can interfere with the effectiveness and tolerability of treatment, with negative implications for outcome and survival.6
Patient assessment is more subjective than tumor assessment, but no less important. The clinician must rely on estimates of the patient's life expectancy with and without cancer treatment, the probable impact of the disease and its treatment on the patient's quality of life, and all information obtained during the CGA. (Table 1).
Table 1.
Potential barriers to treatment of older patients with breast cancer.
| Ageism |
| Paternalism |
| Generalizations about homogeneity of age |
| Misunderstandings about life expectancy |
| Decreased access to care |
| Decreased screening |
| Delayed diagnosis |
| Incomplete staging and assessment |
| Comorbidity |
| Few clinical trials |
JNCI J Natl Cancer Inst (1993) 85 (3) 190–199.
Chronological age alone should not be a contraindication to potentially curative or life-prolonging treatment regimens. Elderly patients represent a heterogeneous population and differ substantially in age-related physiologic changes that can affect the safety and efficacy of various therapeutic interventions. The use of comprehensive geriatric assessment (CGA) tools benefits both clinician and patient by estimating life expectancy, screening for fitness for treatment, and assessing risks and benefits of treatment.
In older women, breast cancer generally tends to have a more favorable phenotype, with a lower proliferative rate, ER- and PR-positive status, and low rate of HER2 overexpression. Only a small part of those patients have triple-negative disease, a subtype that accounts for 15% of all types of breast cancer and occurs in a higher percentage of premenopausal women. Triple-negative breast cancer confers a poor prognosis and is a substantial treatment challenge.7
11. Role of surgery
Surgery is the primary component of curative therapy for early-stage breast cancer. Appropriately selected older women tolerate breast surgery well. Perioperative morbidity is low, and mortality rates range from 0% to 2%. These are related more to comorbidity than chronological age. Older women are just as concerned about body image and cosmesis as younger women and should be offered the option of breast conservation when medically appropriate. For those with significant comorbid illnesses, surgery under local anesthesia may be better tolerated than general anesthesia. Unlike younger patients, older individuals undergoing general anesthesia may experience short-term cognitive impairment.8
A Cochrane meta-analysis concluded that primary hormonal treatment with tamoxifen is inferior to surgery (with or without hormonal therapy) in terms of local control and progression-free survival in medically fit older women and does not result in significantly better overall survival.9
Despite growing research interest in management of breast cancer in women over the age of 65, no internationally agreed recommendations exist for this population. However, a task force from the International Society of Geriatric Oncology (SIOG) has provided evidence-based recommendations for diagnosis and treatment of breast cancer in elderly individuals.
The SIOG task force concluded that women older than 70 years of age should be offered the same surgical options as younger women. Breast-conservation therapy (BCT), lumpectomy, axillary lymph node sampling, and postoperative RT are recommended as the standard of care for patients of all ages with early breast cancer. Studies of elderly women have found that they also prefer BCT over mastectomy, and BCT is often associated with better quality of life.
The SIOG panel recommended sentinel lymph node biopsy for elderly patients with tumors of less than 2–3 cm and no clinical evidence of axillary involvement. In cases that involve high-risk tumors or clinical suspicion of axillary lymph node involvement, dissection is recommended to determine appropriate adjuvant treatment. According to recommendations from the International Society of Geriatric Oncology (SIOG), sentinel lymph node biopsy is preferable to axillary lymph node dissection, especially in older patients, who have low-risk tumors.10
12. Role of adjuvant chemotherapy and comprehensive geriatric assessment (CGA)
According to the SIOG recommendations, whether to use adjuvant chemotherapy should be based not on age but on a variety of factors, including estimated absolute benefit, life expectancy, treatment tolerance, and patient preference.
The National Comprehensive Cancer Network (NCCN) guidelines for breast cancer management recommend the use of a geriatric assessment tool when management of patients aged over 70 years is being planned.
Factors that must be considered when a chemotherapeutic treatment approach is being selected include function and performance status, comorbidities, geriatric syndromes, socioeconomic status, nutrition, and polypharmacy. While there is no standardized CGA, it is generally agreed that any tool should include the clinically relevant measures, such as those listed in Table 2.
Table 2.
Comprehensive geriatric assessment.
| Factor | Tools for assessment | Other assessments |
|---|---|---|
| Functional status | Activities of daily living Instrumental activities of daily living Performance status |
|
| Comorbidity | Charlson Comorbidity Index Cumulative Illness Rating Scale—Geriatrics |
|
| Socioeconomic issues | Living conditions Caregiver presence and competence Income Access to transportation |
|
| Nutritional status | Mini Nutritional Assessment | |
| Polypharmacy | Number of medications Drug–drug interactions |
|
| Geriatric syndromes | Geriatric Depression Scale Folstein Mini Mental Status |
Delirium Falls Osteoporosis Neglect and abuse Failure to thrive |
Ref. 11.
Adverse tumor-related prognostic factors include tumor histology and hormonal receptor expression. Other adverse factors include polypharmacy and >3 active comorbidities.
Elderly patients have decreased bone marrow reserves and are at a higher risk for chemotherapy-associated neutropenia and its complications (Table 3). Although guidelines from the European Organisation for Research and Treatment of Cancer (EORTC) and the American Society of Clinical Oncology (ASCO) specifically recommend a prophylactic use of colony-stimulating factors (CSFs) only in patients for whom the expected rate of febrile neutropenia is 20% or higher, age ≥65 years is itself a risk factor for febrile neutropenia. Although data are lacking to confirm a survival benefit with these agents in elderly patients with breast cancer, they do decrease the incidence of febrile neutropenia and hospitalization. Therefore, the NCCN recommends their use in older patients.
Table 3.
Physiologic changes of chemotherapy associated with aging in elderly patients with cancer.
| Physiologic change | Consequence of chemotherapy |
|---|---|
| Slower repair of DNA damage | Prolonged toxicity |
| Reduced stem-cell mass and hematopoiesis | Slow recovery of blood and mucosal cells |
| Reduced functional reserve of organ systems | Risk for organ failure with additional tissue loss |
| Reduced gastrointestinal absorptive surfaces, gastric motility, and gastric secretion | Reduced drug absorption |
| Reduced fat-free mass | Altered drug distribution |
| Greater anemia | Increased levels of circulating drugs |
| Decreased liver mass | Reduced drug metabolism |
| Decreased nephrons mass | Reduced drug excretion |
Ref. 12.
13. Role of chemotherapy
Anthracycline-containing regimens have been shown to improve overall survival and relapse rates among node-positive postmenopausal patients aged <70 years compared with no anthracyclines. However, the benefits of chemotherapy are less clear in older individuals.
A large retrospective review of 4 randomized trials that included patients with lymph node-positive breast cancer showed similar reductions in breast cancer mortality and recurrence among younger and older women who received chemotherapy, irrespective of hormone receptor activity. In a phase 3 trial that was conducted specifically in women ≥65 years of age, weekly epirubicin plus tamoxifen improved disease-free survival, but not overall survival, when compared with tamoxifen alone. According to the SIOG recommendations for women with hormone receptor-positive breast cancer, the benefit of adding chemotherapy to adjuvant hormone treatment is likely to be higher among those with tumors that are not clearly hormone-sensitive (e.g., they have low levels of hormone receptors, unknown receptor status, or high tumor grade).13
The SIOG guidelines recommend 4 courses of an anthracycline-containing regimen over cyclophosphamide/methotrexate/5-fluorouracil (CMF) in elderly patients with breast cancer. As in younger women, high-risk, fit elderly women may benefit from addition of a taxane. Among patients with increased cardiac risk, docetaxel/cyclophosphamide (TC) or CMF may replace anthracycline-based therapy.14
Treatment decisions regarding chemotherapy should be based not on chronological age but on individual patient factors, including life expectancy, treatment tolerance, estimated absolute benefit, and patient goals and preferences. In addition to a comprehensive geriatric assessment, such tools as Adjuvant Online (www.adjuvantonline.com) may help estimate the benefits of adjuvant treatment, although it has not been specifically validated in older patients (Fig. 1).15
Fig. 1.
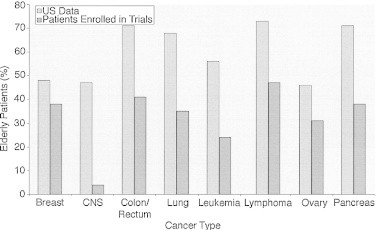
Proportion of elderly patients (≥65 years) enrolled onto registration trials compared with the proportion of elderly patients in the US cancer population. The differences between the two groups were significant for all cancer types except breast cancer (P < .001).
Ref. 17.
The importance of dose intensity in chemotherapy for breast cancer was established in the landmark study of Bonadonna et al., a 20-year follow-up of 386 patients who had been treated with surgery with or without adjuvant chemotherapy consisting of 12 cycles of CMF. The main findings are that survival was significantly increased in the patients who were given adjuvant chemotherapy but the benefit in older women who were given chemotherapy was less because they were given lower doses. The threshold for therapeutic effectiveness was the delivery of at least 85% of the planned chemotherapy dose. Subsequent research emphasizes the importance of not only total dose delivered but also dose intensity as a key determinant of outcome in breast cancer.
As previously discussed, the main obstacle to delivering an adequate total dose and maintaining adequate dose intensity is chemotherapy-induced myelo-suppression and the reliance on treatment delays and/or dosage modifications to allow hematopoietic recovery.16
14. Role of postoperative RT
Postoperative RT following breast-conserving surgery, combined with appropriate systemic adjuvant treatment, has been shown to significantly reduce both absolute risk for 5-year recurrence and 15-year absolute breast cancer mortality risk. Proportional reductions were found in all patients regardless of age, although absolute risk was lower in older patients.18
No significant increase in RT toxicity has been seen in older women. Therefore, among healthy older women, standard fractionation RT with a boost to the lumpectomy cavity is considered a standard component of breast-conserving therapy.
Studies in older women have found no important increase in RT toxicity. The SIOG panel recommends that RT after breast-conserving surgery and adjuvant systemic treatment should be considered in all older women with breast cancer. Factors that should be taken into account include life expectancy, patient health and functional status, mortality risk from comorbidities (especially cardiac or vascular), and risk for local recurrence.19
Since absolute risk for local recurrence is lower in older women, the benefits of RT may decline with age. In addition, the schedule and duration of conventional RT may be challenging for older women who have limited mobility or transportation. Alternatives that demonstrate promising early results include hypofractionated RT schedules, more rapid fractionation, and partial-breast rather than whole-breast irradiation.20
If adjuvant hormone therapy is planned after lumpectomy, it may be feasible to omit adjuvant RT in selected women >70 years who have small (<2 cm), ER-positive, clinically node-negative breast cancer.21
15. Adjuvant hormonal therapy
Adjuvant hormonal therapy has clear benefits in women with hormone receptor-positive early breast cancer, but it is underprescribed in elderly women.
In the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview analysis of data from women with ER-positive breast cancer, 5 years of adjuvant treatment with tamoxifen reduced the annual recurrence rate at 15 years by 34% in women aged 50–59, 45% in women aged 60–69, and 51% in women older than 70 years. Among patients in these 3 age groups, the annual death rate was reduced by 24%, 35%, and 37%, respectively. Relative risk reduction was similar for node-negative compared with node-positive subgroups, although the absolute magnitude of benefit was higher in women with node-positive disease.22
Compared with younger women, those over the age of 80 years are only half as likely to report having a discussion about tamoxifen with their physicians. Women aged 85–92 are 25% less likely to receive a tamoxifen prescription than those aged 80–84 years. This may be due to concerns regarding thromboembolic risk, other side effects, and treatment adherence.23,24
Until recently, tamoxifen was the only hormonal therapy option for postmenopausal women with breast cancer. Other data suggest that aromatase inhibitors (AIs), such as anastrozole, letrozole, or exemestane, are more effective than tamoxifen.
The enzyme aromatase converts testosterone to estradiol and converts androstenedione to estrone, which is the primary estrogen source in postmenopausal women. AIs have been shown to significantly prolong disease-free survival compared with tamoxifen in several studies. In the ATAC (Anastrozole Tamoxifen Alone and in Combination) trial, the AI anastrozole also significantly prolonged time to recurrence and reduced distant metastases and contralateral breast cancer compared with tamoxifen. In a subgroup analysis of the ATAC trial, women aged ≥65 years had a greater benefit from anastrozole than from tamoxifen (hazard ratio for recurrence, 1.19; 95% CI 1.04–1.36).
In addition, the ATAC trial showed that anastrozole was associated with a lower risk for ischemic cerebrovascular events, endometrial cancer, hot flashes, venous thromboembolic events, and vaginal bleeding than tamoxifen, although it was associated with a higher risk for bone fractures and musculoskeletal pain. The ATAC trial reported a higher rate of osteoporosis among women treated with an AI who had osteopenia at baseline. Therefore, tamoxifen may be the preferred option in women with preexisting osteoporosis, severe osteopenia, or a history of fractures. AIs may exacerbate musculoskeletal symptoms in women with severe arthritides or chronic pain syndromes.25
16. Managing AI-induced osteoporosis
Low BMD is associated with increased risk for fracture, which can result in limited mobility, depression, loss of independence, and chronic pain. Fractures of the hip and spine are also associated with an approximately 20% increased mortality rate. When AIs are being considered, BMD assessment is recommended before initiation of therapy in all women and especially in those with risk factors for osteopenia or osteoporosis. Risk factors include small and thin stature, older age, female gender, a diet low in calcium or vitamin D, hypothyroidism, smoking, excessive alcohol consumption, and immobility.
Studies have shown that attention to bone health decreases the risk for AI-associated osteopenia and osteoporosis. One study evaluated the impact of early use of bisphosphonates in 602 postmenopausal women receiving adjuvant AI treatment with letrozole. Patients were randomly assigned to zoledronic acid 4 mg every 6 months either up front or delayed until T-score decreased to less than −2.0 or a nontraumatic fracture occurred. At 1 year, BMD at the lumbosacral spine was 4.4% higher in the up-front treatment group.
Another potential benefit of bisphosphonate treatment in breast cancer is that it appears to have direct cytotoxic and antiangiogenic activity. A recent study found that premenopausal women with early-stage hormone-sensitive breast cancer derived a significant survival benefit when zoledronic acid was added to adjuvant therapy, even in patients without bone metastases. Treating physicians must also carefully consider the risks of bisphosphonate therapy, which include renal insufficiency and irreversible renal failure, microfractures, myalgias and arthralgias, osteonecrosis, hypocalcemia, and other electrolyte abnormalities.26
17. Treatment compliance
The Early Breast Cancer Trialists Collaborative Group meta-analysis reported improvements in the risk for recurrence and risk for death with the use of tamoxifen for early breast cancer, and the pivotal aromatase inhibitor (AI) trials showed further improvements in disease-free survival with the use of an AI over tamoxifen. Further, we see patients undergo chemotherapy or radiation, coping with such unpleasant side effects as nausea, skin rash, hair loss, infections, diarrhea, and oral mucositis, but there is no question of adherence because these treatments are directly observed. When our patients then shift to “easier” treatments such as hormonal therapy for breast cancer, an obvious assumption would be that if a patient can withstand chemotherapy and radiation, she surely will adhere to hormonal therapy. A review of the medical literature on this subject, however, indicates that a surprisingly high percentage of patients do not fully adhere to treatment as prescribed.
The pivotal AI trials demonstrated a range of hormonal treatment discontinuation because of toxicity that would fit the clinical experience of many medical oncologists (10–14%). These studies, however, were recording withdrawals based only on adverse events. A substantial additional number of patients stop these medications on their own for many reasons that are completely unrelated to tolerability.
A study from Kaiser Permanente of Northern California sought to establish adherence by examining prescription refill data on 8769 patients with breast cancer who filled prescriptions for tamoxifen only (43%), AI only (29%), or both (30%) within 1 year of a diagnosis of breast cancer.5 Patients were considered to be “adherent” if the number of pills dispensed in prescriptions from the date of the first prescription to the end of follow-up covered at least 80% of the days in the entire period. This statistic is called the medication possession ratio (MPR). Patients were considered to have discontinued therapy if a period of 180 days elapsed from the prior prescription without a refill. Over 4.5 years of follow-up, 32% of all patients discontinued medication. Of the remaining 68% who continued therapy, only 72% were fully adherent at the end of 4.5 years. Taken together, their data indicated that, overall, only 49% of patients were fully adherent (i.e., MPR > 80%) for the entire 4.5 years. Factors predicting poor adherence included age <40 or >75 years. Longer prescription refill intervals (i.e., 90 days) seemed to enhance full adherence to hormonal therapy.27
Results of the Patient's Anastrozole Compliance to Therapy (PACT) program were presented for the first time at the 2010 meeting of the American Society of Clinical Oncology. This study aimed to increase treatment adherence in postmenopausal women taking adjuvant anastrozole through the provision of a standardized information service. Patients were randomly assigned to either a group getting standard medical care or a group getting added education regarding medication use. The results presented at the meeting revealed an adherence rate of 88% in both arms, indicating no benefit to the intervention. Similar results are brought by Cariatide trial.
18. Palliative treatment
Although tumoricidal treatment may be given, a palliative approach to disease management will probably occur. Palliative and supportive care is an integral component of the management of the elderly breast cancer patients with advanced disease. Common problems include pain, cognitive impairment, depression, lymphoedema and ulcerating disease. End of life care and dignity therapy are also of great importance. Elderly patients with breast cancer are a unique cohort whose nuances with regard to palliative care issues rightly deserve special consideration.
Because metastatic disease carries an entirely different prognosis, the treatment goal is also different – palliation rather than cure. Cytotoxic chemotherapy is reserved for patients in whom primary endocrine therapy has failed.28
When treating older patients with nearly any disease process, it is essential for physicians to understand the specific patient's personal goals for medical care, his or her values and priorities for quality and quantity of life. This becomes especially critical as patients develop more comorbidities and have declining mental or physical function. When an elderly patient receives a diagnosis of cancer, the patient and family must understand the possibilities for cure, extension of life, and palliation. Treatment options, and their likelihood of success, must always be weighed against the risk of adverse effects and overall impact on quality of life.
Because of the complexity of cancer treatment and ongoing advances, it is important that every patient with a diagnosis of cancer be referred to an oncologist to discuss the treatment options in detail. Ideally, older patients with complex medical and social issues should be referred to a geriatric oncologist with experience in caring for elderly patients with cancer. In the case of a 50-year-old woman diagnosed with cancer with no comorbid medical problems, the direction for treatment may be obvious. However, for an 85-year-old with multiple medical problems, dementia, or physical limitations, the recommendations for care and treatment become more complex. These decisions must involve the patient and family members under the guidance of physicians who can understand and properly evaluate these complexities. Fortunately, dual-trained geriatric oncologists are becoming more common as these issues become clearer to the medical community. In addition, a shift to a multidisciplinary team approach that includes oncologists, geriatricians, pharmacists, social workers, and others may be the ideal model to improve the overall quality of cancer care in the elderly.29
19. Conclusion
The conclusions on breast cancer in older women are similar to those on other forms of cancer in the elderly. The main determinants of outcome and survival are tumor characteristics and comorbidities, not age itself. Furthermore, otherwise-healthy older women can and should be treated with the same standard adjuvant chemotherapy as younger women.30 There is a strong evidence that older women tend not to have their breast cancer managed in accordance with evidence-based national clinical guidelines, often with the omission of surgery, even when there is no clinical reason for this conservatism. There is a growing body of evidence around the usefulness of integrating the principles and practices of elderly care into oncology. Breast Cancer Care is calling for older women to be specifically and effectively targeted by health promotion campaigns as the people most at risk of developing breast cancer and for older patients to receive personalised care plans for their breast cancer treatment, based on that patient's individual circumstances rather than their chronological age.7
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Gennari R., Curigliano G., Rotmensz N. Breast carcinoma in elderly women – features of disease presentation, choice of local and systemic treatments compared with younger postmenopausal patients. Cancer. 2004;101:1302–1310. doi: 10.1002/cncr.20535. [DOI] [PubMed] [Google Scholar]
- 2.Spazzapan S., Crivellari D., Bedard P. Therapeutic management of breast cancer in the elderly. Expert Opin Pharmacother. 2011;12(April (6)):945–960. doi: 10.1517/14656566.2011.540570. [DOI] [PubMed] [Google Scholar]
- 3.Singletary S.E., Shallenberger R., Guinee V.F. Breast cancer in the elderly. Ann Surg. 1993;218:667–671. doi: 10.1097/00000658-199321850-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildiers H., Kunkler I., Biganzoli L. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 5.Cardia J., Calçada C., Pereira H. Treatment of lung cancer in the elderly: Influence of comorbidity on toxicity and survival. Rep Pract Oncol Radiother. 2011;16(2):45–48. doi: 10.1016/j.rpor.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louwman W.J., Janssen-Heijnen M.L.G., Houterman S. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Cancer. 2005;41:779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Extermann M., Aapro M., Bernabei R.B. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Audisio R.A. The surgical risk of elderly patients with cancer. Surg Oncol. 2004;13:169–173. doi: 10.1016/j.suronc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Fennessy M., Bates T., MacRae K. Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg. 2004;91:699–704. doi: 10.1002/bjs.4603. [DOI] [PubMed] [Google Scholar]
- 10.Pierga J.Y., Girre V., Laurence V. Characteristics and outcome of 1755 operable breast cancers in women over 70 years of age. Breast. 2004;13:369–375. doi: 10.1016/j.breast.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Balducci L., Extermann M. Cancer and aging: an evolving panorama. Hematol Oncol Clin North Am. 2000;14:1–16. doi: 10.1016/s0889-8588(05)70274-4. [DOI] [PubMed] [Google Scholar]
- 12.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1(Suppl. 2):18–24. [PubMed] [Google Scholar]
- 13.Barthélémy P., Heitz D., Mathelin C. Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol. 2011;79(2):196–204. doi: 10.1016/j.critrevonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Muss H.B., Woolf S., Berry D. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo M.I., Cullen J., Hwang Y.T. Breast cancer treatment in older women: does getting what you want improve your long-term body image and mental health? J Clin Oncol. 2004;22:4002–4009. doi: 10.1200/JCO.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Barni S., Cabiddu M., Petrelli F. Benefit of adjuvant chemotherapy in elderly ER-negative breast cancer patients: benefits and pitfalls. Expert Rev Anticancer Ther. 2010;10(2):185–198. doi: 10.1586/era.09.188. [DOI] [PubMed] [Google Scholar]
- 17.Talarico L., Chen G., Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. JCO. 2004;22(22):4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 18.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19.Cutuli B. Breast cancer irradiation in elderly. Cancer Radiother. 2009;(6–7):615–622. doi: 10.1016/j.canrad.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Smith B.D., Gross C.P., Smith G.L. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 21.Hughes K.S., Schnaper L.A., Berry D. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 22.EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 23.Silliman R.A., Guadagnoli E., Rakowski W. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20:2680–2688. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 24.Blackman S.B., Lash T.L., Fink A.K. Advanced age and adjuvant tamoxifen prescription in early-stage breast carcinoma patients. Cancer. 2002;95:2465–2472. doi: 10.1002/cncr.10985. [DOI] [PubMed] [Google Scholar]
- 25.Kudachadkar R., O’Regan R.M. Aromatase inhibitors as adjuvant therapy for postmenopausal patients with early stage breast cancer. CA Cancer J Clin. 2005;55:145–163. doi: 10.3322/canjclin.55.3.145. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R., Adams J.E., Coleman R.E. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 27.Partridge A.H., Wang P.S., Winer E.P., Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 28.Mauri D., Polyzos N.P., Salanti G., Pavlidis N., Ioannidis J.P. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100(24):1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 29.Schofield P., Carey M., Love A., Nehill C., Wein S. ‘Would you like to talk about your future treatment options’? Discussing the transition from curative cancer treatment to palliative care. Palliat Med. 2006;20:397–406. doi: 10.1191/0269216306pm1156oa. [DOI] [PubMed] [Google Scholar]
- 30.Holmes C.E., Muss H.B. Diagnosis and treatment of breast cancer in the elderly. CA Cancer J Clin. 2003;53:227–244. doi: 10.3322/canjclin.53.4.227. [DOI] [PubMed] [Google Scholar]


