Abstract
Computed tomography and magnetic resonance imaging-based techniques of functional imaging are proven to be sensitive and reliable tools for detection and staging of head and neck cancer.
These new techniques enable to visualize and differentiate subtle pathologic changes before they become evident on standard cross-sectional images.
However, it is their role in evaluating possible recurrence and estimation of treatment response that holds the biggest promise.
This article describes the role and usefulness of diffusion and perfusion in detecting recurrence and follow-up in patients with head and neck squamous cell carcinoma.
Keywords: Perfusion imaging, Diffusion-weighted imaging, Functional imaging, Squamous cell carcinoma, Recurrence
1. Introduction
Head and neck imaging has been revolutionized during last 20 years. In squamous cell carcinoma (SCC), multislice computed tomography (CT) and new sequences in magnetic resonance (MR) imaging help in tumor staging, which influences patient management and prognosis. New imaging modalities have the ability to differentiate neoplastic from inflammatory lesions; provide essential information about the deep extension of clinically detected masses and delineate additional clinically unsuspected lesions.1–6 They have proven accuracy in detection of soft tissue extent, lymph node involvement, bone marrow infiltration and perineural spread.5–10
The latest developments in imaging patients with head and neck SCC are based on the observation that most pathological processes become evident before they proceed in gross anatomical distortion. Methods of functional imaging depict and differentiate diseases by assessing functional (vascularization, blood flow, lymphatic flow), metabolic (e.g. glycolysis) or molecular phenomena (proliferation, epidermal growth factor receptor expression and apoptosis). This information is readily used for the detection of recurrent disease and assessment of early response.
Antonio Mancuso, one of America's leading experts in head and neck radiology, used to say that there are three basic strategies in the war against head and neck squamous cell cancer.11 The first one is prevention. The second one is to kill the cancer completely the first time it is treated. The third strategy is the salvage of initial treatment failures. All physicians who treat head and neck cancer realize that salvage comes at a considerable cost in terms of increased morbidity and markedly diminished chances of disease-free survival at most primary sites. When tumor recurs, it will usually be difficult to recognize within deformed tissues or post-radiotherapy changes. It may also be biologically more aggressive and will have a tendency to spread along nerves and vessels. A failure at the primary site then must be discovered early, before its margins become untreatably diffused into surrounding tissues, if there is to be a reasonable hope for successful salvage.
The risk of developing local or regional recurrence or distant metastases remains high, despite aggressive surgical and radiotherapy treatment3,6; since the early 1990s, many radiological studies have been performed and their potential in the detection of recurrent cancer is being evaluated.
Nowadays, we have a broad availability of tools for detecting asymptomatic recurrence. Having more experience and more data concerning the value of functional imaging, we must begin to apply these techniques as part of strategy in the war against head and neck cancer.11
2. Perfusion imaging
Perfusion is defined as blood flow through a unit volume of tissue per unit of time.2,8,12 Perfusion imaging may be performed with either CT or MR. This technique adds little time to conventional MR/CT examinations and can also be obtained noninvasively. Following 30–60 s. of dynamic acquisition, basic parameters of perfusion – blood flow (BF), blood volume (BV), mean transit time (MTT), time to peak (TTP) and permeability surface (PS) can be generated mathematically by a computer, on a separate workstation with commercially available software. Perfusion characteristics of tissue usually demonstrate changes in blood flow or blood volume in a pre-defined region of interest, depending on the underlying pathologic processes. This technique has been previously studied in characterizing brain ischemia, particularly in identifying infarcted tissue versus penumbra.13 Neoplastic tissue also demonstrates noticeable changes in perfusion parameters. Some studies have shown that squamous cell carcinoma of the oral cavity/oropharynx demonstrated increased blood volume and blood flow in comparison to normal tissue12,14 (Fig. 1). These findings have been attributed to tumor's neovascularity. Also, other studies have proven, that with analysis of perfusion parameters of a given structure, it is possible to depict malignant infiltration before it becomes visible in standard cross-sectional imaging.15 Generally, these findings will add substantial additional information regarding tumor extent and staging. Such imaging may also be useful in qualitative analysis of tumor tissue. In a very recent study by Faggioni et al.,14 the role of computed tomography perfusion (CTP) was evaluated with 64-row multislice CT. Data from this study showed that CTP could provide useful information for differentiation between SCC and normal tissue. The authors found significantly higher BF and BV values in SCC compared to normal tissue. This finding was attributed to the enlarged neovascular bed as a consequence of tumoral neoangiogenesis. This holds promise for a better delineation of the extent of tumor, more accurate staging and earlier depiction of squamous cell carcinoma recurrence.
Fig. 1.
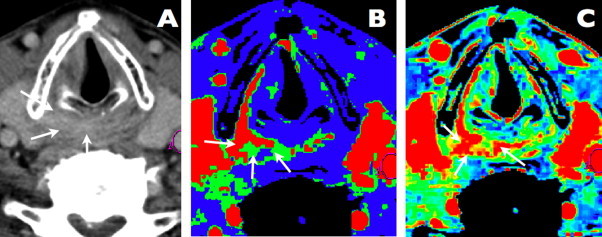
A case of hypopharyngeal squamous cell carcinoma. Axial contrast-enhanced CT scan. Slight contrast enhancement and pathological mass is visible in the right pyriform sinus apex and postcricoid area (arrows). Perfusion map of blood flow. The area of a high blood flow (mean value = 132 ml/100 g/min), representing malignant infiltration, is visible in right pyriform sinus apex, postcricoid area and along the right thyroid cartilage (arrows). Perfusion map of blood volume. The area of elevated blood volume (mean value = 5.72 ml/100 g) is marked with arrows and represents malignant infiltration.
The evaluation of perfusion images clearly improves the depiction of malignant infiltration of muscles, with much better overall results when compared to standard CT/MR images.12,14,15 These standard modalities may fail to depict small areas of malignant tissue and may be unable to distinguish tumor from inflammation, but there is evidence, that the combined use of CT/MR and perfusion results in correct staging of the majority of head and neck tumors and accurate detection of recurrence.
Recent studies have demonstrated that squamous cell carcinomas of head and neck, with increased blood volume/flow, are more chemosensitive than other lesions with relatively decreased perfusion parameters.16 This is probably due to increased oxygenation and metabolism in such lesions. In view of these findings, perfusion techniques may be particularly useful in determining which patients would benefit from such medical treatment, as opposed to surgical therapies which may not always preserve organ function. It has been described by Surlan-Popovic18 that during chemotherapy responders show a significant constant reduction of BF and significant initial reduction of BV, while non-responders demonstrate an acute increase of BF, BV and PS. In patients receiving induction chemotherapy, tumors with high values of BV (>5.0 ml/100 ml) respond much better than tumors with low BV.
Another special area of interest is the question of tumor recurrence. Evaluation of post-treatment neck remains challenging despite availability of modern imaging studies. Postradiotherapy changes include fibrosis, edema, and soft tissue thickening, sometimes also necrosis. In this setting, the identifying recurrent tumor and distinguishing between carcinoma and a benign process is usually very challenging. Also, many recurrences present with multicentric tumor foci, localized below unchanged mucosa, and masked by post-treatment edema. Conventional CT and MRI do not allow precise differentiation between recurrent tumor and the post (chemo)radiotherapy changes. They would demonstrate increased contrast enhancement within the treated neck, but this may be due to persistent inflammation, edema or fibrosis. Recent studies have demonstrated that in cases of recurrent squamous cell carcinomas CT perfusion may play a significant role in the detection of malignant tissue. This technique may permit better differentiation between local tumor recurrence and nonspecific post-therapy tissue changes. As shown by Bisdas and Hermans,16–19 values of perfusion parameters are significantly higher in tumor tissue compared with normal structures, including tissue changes after chemoradiotherapy. Specifically, blood volume and blood flow values within recurrent tumor are markedly elevated (Fig. 2).19
Fig. 2.
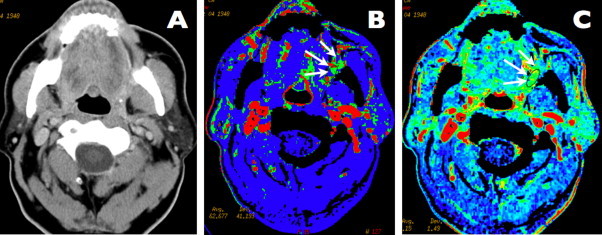
A case of left-sided retromolar trigone carcinoma. Axial contrast-enhanced CT scan showing no visible abnormality in the oral cavity and oropharynx. Perfusion map of blood flow. The area of a high blood flow (mean value = 103 ml/100 g/min), representing malignant submucosal infiltration, is visible in the left retromolar trigone area, pharyngeal mucosal space and buccal space (arrows). Perfusion map of blood volume. The area of a high blood volume (mean value = 4.6 ml/100 g), is marked with arrows and represents malignant infiltration.
3. Diffusion imaging
Diffusion-weighted imaging (DWI) is one of the best investigated tools for detection and characterization of tumors in the last few years. Technical improvements and higher magnetic fields have accelerated research in diffusion techniques.2
DWI is a noninvasive technique that characterizes tissue on the basis of differences in tissue water mobility. The motion of tissue water molecules caused by their thermal kinetic energy (Brownian motion) is limited by the presence of cellular membranes and macromolecules. Diffusion-weighted images are obtained by applying pairs of opposing magnetic field gradients around the refocusing pulse of a T2-weighted sequence. Water molecules will be dephased by the first gradient and rephased by the second gradient. If the water molecules are stationary, no net dephasing is expected. Movement of the tissue water molecules between the two opposing gradients will result in dephasing, depicted as a signal loss on the diffusion-weighted images.9,19 The amount of signal loss is proportional to the degree of water molecule movement, as well as the strength and duration of the applied gradients. This last factor is expressed by the b-value. By repeating the sequence with different b-values, the signal loss can be quantified by calculating the apparent diffusion coefficient (ADC).
Hypercellular tissue, which is present within malignant tumors, has low ADC values (Fig. 3). Changes in tissue, like edema, inflammation and necrosis, are expected to have low cellularity and demonstrate high ADC values, as clinically validated.5,10,20–22
Fig. 3.
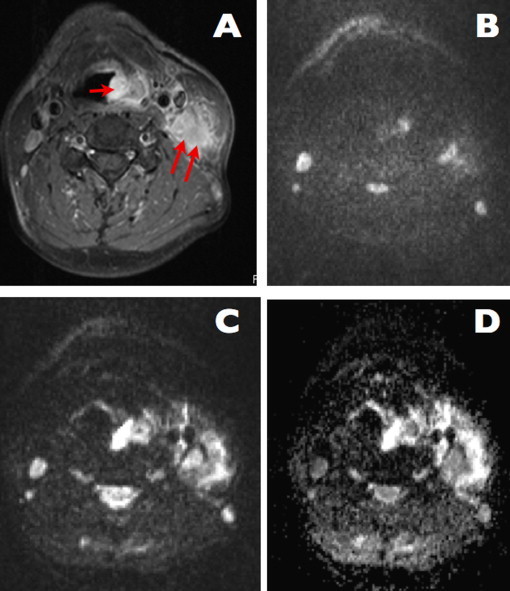
A case of left-sided laryngeal cancer with lymph nodes metastases (images courtesy of B. Bobek-Billewicz). (A) Axial T1-weighted MR image, with contrast enhancement. Pathological subglottic mass is visible (arrow); large metastatic lymph node is also visible on the left side (two arrows). (B and C) Diffusion-weighted images, b0 and b1200, respectively. Both the tumor and the lymph node show a persisting high signal on the b1200 image, indicating diffusion restriction. These findings are strongly indicative of the presence of malignant tissue and metastatic adenopathy. (D) Corresponding ADC-map reveals low ADC-values in the tissue and in the node, indicating presence of diffusion restriction.
A promising application of DWI for head and neck cancer is to make the distinction between post-treatment changes after radiotherapy and tumor recurrence (Figs. 4 and 5). Because the recurrence rates after (chemo)radiotherapy for advanced-stage tumors (T3 and T4) range from 25% to 50%, early diagnosis of cancer recurrence is essential. Several studies have demonstrated that diffusion-weighted MRI delivers promising results in differentiation between recurrent head and neck tumors and postoperative or postradiation changes.18,19 Signal intensity on native b0 images is significantly lower for cancer than for non-tumoral post-radiotherapeutic tissue. Signal intensity on native b1000 images is significantly higher for cancer than for non-tumoral tissue. ADC values are significantly lower for cancer than for non-tumoral tissue. When compared with computed tomography and FDG-PET, DW-MRI has higher sensitivity and better accuracy in detecting recurrence; it also has fewer false-positive results in persistent primary site abnormalities and in persistent adenopathies.22 It may help in the detection of subcentimetric nodal metastases. Overall, the method seems superior to CT, classical MR or other functional studies (positron emission tomography) for the differential diagnosis in post-treatment setting.
Fig. 4.
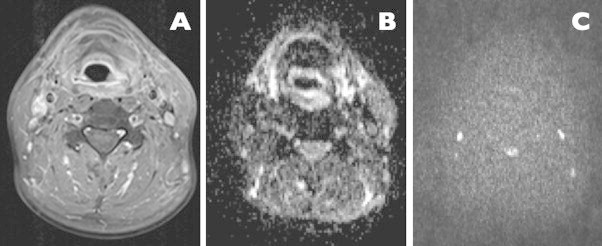
A case of left hypopharyngeal carcinoma treated with radiotherapy, follow-up study, 6 months after completion of treatment (images courtesy of B. Bobek-Billewicz). Axial contrast-enhanced MR image, no visible area of pathological contrast enhancement is visible diffusion-weighted b0 image, which is symmetric with no areas of restricted diffusion. Diffusion-weighted b1200 image, showing no areas of diffusion restrictions, which indicates a complete remission and successful treatment.
Fig. 5.
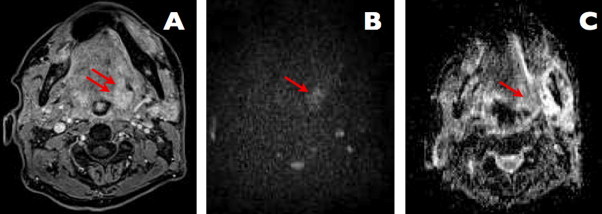
A case of left-sided floor of the mouth carcinoma, treated with chemo-radiotherapy, follow-up study 8 months after completion of treatment (images courtesy of B. Bobek-Billewicz). Axial contrast-enhanced MR image, distinct area of pathological contrast enhancement is visible on the left side (arrows). Diffusion-weighted b1200 image; area of persisting high signal is visible, indicating diffusion restriction (arrow); this indicates the presence of malignant tissue. Corresponding ADC map, which reveals low ADC values in the tissue (arrow), indicating the presence of restricted diffusion and suggesting recurrence.
However, as stated by Hermans and Vandecaveye,5,9,19 the difficult interpretation of DWI in the head and neck and the lack of method standardization to date are a true challenge, and one needs a lot of additional work to make this technique efficient for routine use. Also, this technique is sensitive to artifacts which may substantially reduce the quality of images at the level of skull base and thoracic inlet.
Another issue is the characterization of neck lymph nodes, which remains a difficult issue with anatomy-based imaging methods.23 DWI holds promise in this regard.5,22,24 On the ADC map, malignant nodes usually show a low signal intensity and benign nodes reveal high signal intensity. The mean ADC value of metastatic lymph nodes tends to be significantly lower than that of benign cervical lymph nodes. When the ADC value of 1.38 × 10−3 mm2/s is used as a threshold value for differentiating malignant from benign lymph nodes, the accuracy for detecting metastatic nodes reaches 96%, sensitivity 98%, specificity 88%, and the positive predictive value is as high as 98.5%.
In conclusion, diffusion-weighted MRI with ADC mapping is a new promising technique that can differentiate malignant from benign lymph nodes and delineate the solid viable part of the lymph node for biopsy.
As DWI allows differentiation between inflammatory and neoplastic tissue, another possible application could be the monitoring of tumor response during radiotherapy. For instance, high pretreatment ADC values are suggestive of necrosis, which may predict poor outcomes because of hypoxia-mediated radioresistance. Some recent studies4,15 have demonstrated, that cellular death and vascular changes caused by therapy could be detected by DWI before a reduction of lesion diameter becomes apparent at morphological imaging. However, DWI based on many b-values and ADC measurements should be used as an adjunctive, but not substitutive, tool to the standard MR imaging examination to assess the response of cancer to non-surgical treatment. The possibility of distinguishing responders from non-responders, as well as early identification of non-responder tumors, would help in addressing cervical cancer patients to stop ineffective treatments and to avoid delays in starting alternative, potentially more effective treatments.
4. Conclusion
Imaging postsurgical and post-radiotherapy patients can be challenging due to anatomical alterations and post-treatment changes. Also, clinical evaluation of such patients remains difficult. The knowledge about new powerful techniques of functional imaging, their advantages and their proper application will help to improve the salvage rate and will reduce the morbidity of treatment for recurrent head and neck squamous cell cancer, by earlier and more reliable detection of residual/recurrent disease.
Acknowledgements
Author is very grateful to Professor Barbara Bobek-Billewicz, Head of Department of Radiology, from Maria Curie-Sklodowska Oncology Institute, Gliwice Branch, for her expert advice concerning MR diffusion examination and permission to use examples of diffusion studies.
References
- 1.Lemort M., Canizares Ch., Kampouridis S. Advances in imaging head and neck tumours. Curr Opin Oncol. 2006;18:234–239. doi: 10.1097/01.cco.0000219251.15041.b1. [DOI] [PubMed] [Google Scholar]
- 2.Shah G.V., Wesolovski J., Ansari S.A. New directions in head and neck imaging. J Surg Oncol. 2008;97:644–648. doi: 10.1002/jso.21022. [DOI] [PubMed] [Google Scholar]
- 3.Weber A.L., Romo L., Hashmi S. Malignant tumors of the oral cavity and oropharynx: clinical, pathologic and radiologic evaluation. Neuroimag Clin N Am. 2003;13:443–464. doi: 10.1016/s1052-5149(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 4.Wippold F.J. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging. 2007;25:453–465. doi: 10.1002/jmri.20838. [DOI] [PubMed] [Google Scholar]
- 5.Vandecaveye V., De Keyzer F., Nuyts S. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67:960–971. doi: 10.1016/j.ijrobp.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Yousem D.M. Dashed hopes for MR imaging of the head and neck: the power of the needle. Radiology. 1992;184:25–26. doi: 10.1148/radiology.184.1.1609088. [DOI] [PubMed] [Google Scholar]
- 7.Laubenbacher C., Saumweber D., Wagner-Manslau C. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med. 1992;36:1747–1757. [PubMed] [Google Scholar]
- 8.Trojanowska A., Trojanowski P., Bisdas S. Squamous cell cancer of hypopharynx and larynx—evaluation of metastatic nodal disease based on coputed tomography perfusion studies. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.084. Feb 14 [Epub ahead of print] PMID: 21324623. [DOI] [PubMed] [Google Scholar]
- 9.Hermans R. Vandecaveye V: diffusion-weighted MRI in head and neck cancer. Cancer Imaging. 2007;7:126–127. doi: 10.1102/1470-7330.2007.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Takashima S., Takayama F. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–630. doi: 10.1148/radiol.2202010063. [DOI] [PubMed] [Google Scholar]
- 11.Mancuso A. The “third-best” strategy for treating head and neck cancer. AJNR Am J Neuroradiol. 1999;20:1191–1192. [PMC free article] [PubMed] [Google Scholar]
- 12.Trojanowska A., Grzycka-Kowalczyk L., Trojanowski P. Computed tomography perfusion examination is helpful in evaluating the extent of oropharyngeal and oral cavity cancer. Pol Przegl Radiol. 2011;76(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- 13.Lui W., Tang E.R., Allmendinger A.M. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010;31(9):1552–1563. doi: 10.3174/ajnr.A2026. October 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faggioni L., Neri E., Cerri F. 64-row MDCT perfusion of head and neck squamous cell carcinoma: technical feasibility and quantitative analysis of perfusion parameters. Eur Radiol. 2010 doi: 10.1007/s00330-010-1898-0. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi D., Hoeffner E.G., Carlos R.C. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract: initial results. J Comput Assist Tomogr. 2003;27:687–693. doi: 10.1097/00004728-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi D., Chepeha D.B., Miller T. Correlation between initial and early follow-up CT perfusion parameters with endoscopic tumor response in patients with advanced squamous cell carcinomas of the oropharynx treated with organ-preservation therapy. AJNR Am J Neuroradiol. 2006;27(1):101–106. [PMC free article] [PubMed] [Google Scholar]
- 17.Bisdas S., Baghi M., Smolarz A. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using firstpass contrast-enhanced computed tomography studies. Invest Radiol. 2007;42:172–179. doi: 10.1097/01.rli.0000252496.74242.0b. [DOI] [PubMed] [Google Scholar]
- 18.Surlan-Popovic K., Bisdas S., Rumboldt Z. Changes in perfusion CT of advanced squamous cell carcinoma of the head and neck treated during the course of concomitant chemoradiotherapy. Am J Neuroradiol. 2010;31:570–575. doi: 10.3174/ajnr.A1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermans R. Diffusion-weighted MRI in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:72–78. doi: 10.1097/MOO.0b013e32833555f2. [DOI] [PubMed] [Google Scholar]
- 20.Abdel Razek A.A., Kandeel A.Y., Soliman N. Role of diffusion-weighted echo-planar MR imaging in differentiation of residual or recurrent head and neck tumors and posttreatment changes. Am J Neuroradiol. 2007;28:1146–1152. doi: 10.3174/ajnr.A0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandecaveye V., de Keyzer F., Nuyts S. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67:960–971. doi: 10.1016/j.ijrobp.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Abdel Razek A.A., Soliman N.Y., Elkhamary S. Role of diffusion weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468–1477. doi: 10.1007/s00330-005-0133-x. [DOI] [PubMed] [Google Scholar]
- 23.Lebioda A., Makarewicz R., Żyromska A. Measurement of tumour volume by MRI to evaluate risk of pelvic nodal metastases in early cervical carcinoma patients. Rep Pract Oncol Radiother. 2009;14:146–150. [Google Scholar]
- 24.Sumi M., Sakihama N., Sumi T. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. Am J Neuroradiol. 2003;79:681–687. [PMC free article] [PubMed] [Google Scholar]


