Abstract
Background
The biological effects of ionizing radiation have long been thought to results from direct targeting of the nucleus leading to DNA damage. Over the years, a number of non-targeted or epigenetic effects of radiation exposure have been reported where genetic damage occurs in cells that are not directly irradiated but respond to signals transmitted from irradiated cells, a phenomenon termed the “bystander effects”.
Aim
We compared the direct and bystander responses of human A 549, BEAS-2-B and NHDF cell lines exposed to both photon (6 MV) and electron (22 MeV) radiation inside a water phantom. The cultures were directly irradiated or exposed to scattered radiation 4 cm outside the field. In parallel, non-irradiated cells (termed bystander cells) were incubated in ICM (irradiation conditioned medium) collected from another pool of irradiated cells (termed donor cells).
Materials and methods
In directly irradiated cells as well as ICM-treated cells, the frequency of micronuclei and condensation of chromatin characteristic for the apoptotic process were estimated using the cytokinesis-block micronucleus test.
Results
In all tested cell lines, radiation induced apoptosis and formation of micronuclei. A549 and BEAS-2B cells cultured in ICM showed increased levels of micronuclei and apoptosis, whereas normal human fibroblasts (NHDF line) were resistant to bystander response. In A549 and BEAS-2B cells placed outside the radiation field and exposed to scattered radiation the formation of micronuclei and induction of apoptosis were similar to that after ICM-treatment.
Conclusion
Results suggest that the genetic damage in cells exposed to scattered radiation is caused by factors released by irradiated cells into the medium rather than by DNA damage induced directly by X rays. It seems that bystander effects may have important clinical implications for health risk after low level radiation exposure of cells lying outside the radiation field during clinical treatment.
Keywords: X-irradiation, Apoptosis, Micronuclei, Bystander effect, Scattered radiation
1. Background and the aim of the work
Recently, attention in radiobiological studies turned to non-targeted effects, specially to radiation-induced bystander effects. These are induced by signals emitted by directly irradiated cells and manifest in non-irradiated cells as a reduced clonogenic survival,1,2 cytogenetic damage,3 apoptosis and modulation of expression of specific proteins.4,5 The bystander effect has been observed in experiments performed in vitro using very low doses of α-particles6,7 as well as after conventional irradiation with γ-rays and X-rays.8 Bystander effects are caused by molecular signals released from irradiated cells and these signals can be transmitted through gap junction intercellular communication and through a medium transfer mechanism. It has been shown that medium collected from irradiated cells (ICM – irradiation conditioned medium) and transferred to non-irradiated cells induce in them genetic changes characteristic for bystander responses.1 It has been well documented that bystander effects predominate after low dose exposure and saturate at higher doses and cause a deviation from the linear non-threshold (LNT) model in the low dose region of radiation.3,9,10 In our previous work we compared the dose depth distribution of ionizing radiation in a water phantom with the frequency of occurrence of apoptotic and micronucleated human cells that were exposed to radiation in a beam axis or outside the radiation field. Our measurements showed discrepancy between physical dose distribution observed on different depths in a water environment and biological effects.11 This discrepancy was especially significant for cells exposed in a beam axis at higher depths and cells placed outside the radiation field during exposure to radiation. The numbers of apoptotic and micronucleated cells were greater than those that should result from the corresponding received dose. Observed effects were explained to result from low dose scattered radiation, since with increasing medium depth as well as distance of beam of axis, its proportion to the incident radiation became greater. In the present work we tested whether bystander effects can play a role in the response of cells to direct irradiation (irradiation in beam axis) and scattered radiation (outside the radiation field). We explored this issue by using the medium transfer technique in which medium of exposed cells (termed donor cells) was transferred to non-irradiated cells (termed bystander cells). The genetic changes (formation of micronuclei and induction of condensation of chromatin characteristic for apoptotic process) in control, directly exposed as well as ICM-received cells were estimated using the cytokinesis-block micronucleus test. This study was presented at ESTRO 29 in Barcelona 12-16.09.2010.
2. Materials and methods
2.1. Cell culture
Human lung carcinoma cells (A549 line), normal bronchial epithelial cells (BEAS-2B line) and normal fibroblasts (NHDF line) were used in the experiments. Cells were grown in a monolayer in tissue culture flasks containing DMEM/F12 medium supplemented with 10% fetal bovine serum (Immuniq) in a humidified atmosphere of 5% CO2 at 37 °C. Before irradiation, the cells were detached by means of 10 mM EDTA and 0.25% trypsin. Next, 20 μl aliquots of cell suspension, containing approximately 1 × 103 cells, were transferred to Eppendorf‘s tubes and then the tubes were filled with medium up 0.5 ml so that cells could be irradiated without the presence of air.
2.2. Irradiation of cells
Dose was calculated in a planning treatment system (Eclipse – Varian Medical Systems), algorithm Monte Carlo, on the basis of measurements conducted in a water phantom of 35 cm × 35 cm × 35 cm. The cells in tubes were placed horizontally in a stand and irradiated with 5 Gy defined at the “build-up” depth in a water phantom in two variants: directly on the axis in the beam field or 4 cm outside the field, i.e. with scattered radiation (dose of 0.2 Gy), as shown in Fig. 1. The field size was 15 cm × 15 cm in SSD. Experiments were performed for electron (22 MeV) and photon (6 MV) radiation generated in a linear accelerator Clinac series Varian Medical System, for 300 MU/min accelerator mode and dose of 5 Gy in build-up (3 cm) depth in a water environment.
Fig. 1.

The conventional scheme of the irradiation set-up.
2.3. Conditioned medium transfer
To obtain the conditioned medium (ICM), we employed the described technique1 and the experimental protocol is presented in Fig. 2. Briefly, donor cells that had been placed in a water phantom and irradiated, were transferred to plastic dishes and incubated for 1 h in 37 °C in a normal culture medium (5 ml). Then, the ICM was collected, centrifuged and filtered through a 0.22 μm pore filter to ensure that no cells were present in the transferred medium. The filtrate was immediately added to non-irradiated (bystander) cells. Control cell cultures were incubated in a normal medium.
Fig. 2.

Preparation of conditioned medium (ICM).
2.4. Cytokinesis-block micronucleus test
After irradiation, the cells were transferred from Eppendorf's tubes into plastic dishes (50 mm diameter), supplemented with up to 5 ml of the culture medium and incubated at 37 °C. The micronucleus test was performed according to the standard procedure.12 The cytochalasin B was added to medium of irradiated and ICM-treated cultures to a final concentration of 2 μg/ml and cells were incubated for 48 h prior to fixation. The cells were fixed in situ with a cold solution of 1% glutaraldehyde (Sigma) in phosphate buffer (pH = 7.5) and stained by Feulgen reaction. At least 500 binucleate cells (Fig. 3A) were examined for the presence of micronuclei under microscope. The fraction of cells showing condensation of chromatin characteristic for the apoptosis process (Fig. 3B) was also recorded in 1000 cells in each plate.
Fig. 3.

Images of damaged cells scored 2 days after irradiation or icubation in ICM. (A) Micronucleated cell and (B) apoptotic cell.
Published data concerning the bystander effect have commonly classified apoptotic cells on the basis of morphological criteria.13 In all our experiments the frequency of apoptosis was assessed by microscopic observation. In selected cell cultures, we additionally performed annexin V-FITC flow-cytometry analysis. Both of these methods showed a similar tendency as the one we presented earlier.14
2.5. Statistical analysis
Student's t-test was used to determine the significance of differences (in terms of the number of micronuclei formed and induction of apoptosis) between cells irradiated and non-irradiated, as well as between cells cultured in a normal medium and in ICM.
3. Results and discussion
We compared the biological responses of cells incubated in ICM with those of cells exposed directly to radiation. It is well documented that following irradiation cells release molecular signals which can induce genetic changes in non-irradiated cells distant from the target cells. This phenomenon, defined as “bystander effect” may reflect the occurrence of at least two separate mechanisms for the transmission of signals from irradiated cells to non-irradiated ones. One line of evidence indicates that the bystander effect is dependent on intracellular communication through gap junctions; the other explanation is that irradiated cells secrete cytokines or other signaling factors into the medium (referred to as ICM – irradiated conditioned medium).1,15 Genotoxic action of ICM is inactivated by heating,1 which suggests that molecular factors released from irradiated cells are proteins. In our control experiments no changes in the number of micronucleated or apoptotic cells were observed when the non-irradiated cells were cultured in medium irradiated in the absence of cells (data not shown), suggesting that genetic changes in these cells are mediated by factors released by irradiated cells into the medium.
Fig. 4 shows the frequency of micronucleated A549 cells that were exposed to electron and photon radiation or incubated in ICM collected from donor cells irradiated in the same conditions. The number of damaged cells increased over the value of control not only in cells directly irradiated in a beam axis but also in cells exposed to scattered radiation. In cultures incubated in ICM the frequency of micronucleated cells increased nearly two-fold in comparison with the control cells incubated in a normal medium. The ICM collected from cells irradiated with photon and electron radiation caused a significant increase in the frequency of damaged cells and induced the same number of micronuclei, independent of the radiation dose and the positioning with respect to beam axis during exposure.
Fig. 4.
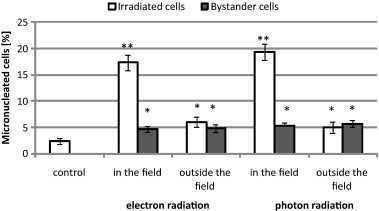
The percentage level of micronucleated A549 cells in directly irradiated or incubated in ICM cultures. Significant at *p < 0.01 and **p < 0.001 compared with control.
Fig. 5 shows the increase in the number of cells showing condensation of chromatin in both directly irradiated and ICM-received A549 cells. In directly irradiated cells, the number of apoptotic like cells increased nearly 3-fold in comparison with the number of spontaneous apoptosis. In cells exposed to scattered radiation, we observed nearly a 2-fold increase of the number of apoptotic cells compared to the control. A significant increase of the number of apoptosis was also observed in ICM-received cells. The level of induced apoptosis was similar in bystander cells incubated in ICM irrespective of the kind of irradiation of donor cells. The results for the micronuclei induction in irradiated and ICM-treated BEAS-2B cells are presented in Fig. 6. In directly irradiated cells the number of micronuclei increased about 10-fold over the control, whereas in cells exposed to scattered radiation we observed a 2-fold increase. The ICM obtained in different kind of irradiation induced also a two-fold increase of the number of chromosomal damage compared to non-treated control. As shown in Fig. 7, irradiation in a beam axis caused about a 4-fold increase in the number of apoptotic BEAS-2B cells. Over a 2-fold increase in the number of apoptosis was observed in cells exposed outside the radiation field as compared with the control. ICM collected from donor cells exposed to photon and electron radiation, both direct and scattered, induced a comparable increase in the number of apoptotic cells.
Fig. 5.

The percentage level of apoptotic A549 cells indirectly irradiated or incubated in ICM cultures. Significant at *p < 0.01 and **p < 0.001 compared with control.
Fig. 6.

The percentage level of micronucleated BEAS-2B cells in directly irradiated or incubated in ICM cultures. Significant at *p < 0.05 and **p < 0.001 compared with control.
Fig. 7.

The percentage level of apoptotic BEAS-2B cellsin directly irradiated or incubated in ICM cultures. Significant at *p < 0.0.01 and **p < 0.001 compared with control.
Figs. 8 and 9 show the results for irradiated and ICM-treated normal human fibroblasts (NHDF). We observed a significant increase in comparison with the control in the frequency of micronuclei in directly irradiated cells but no significant change in the level of micronuclei in cells exposed to scattered radiation (Fig. 8). There were also no differences in the number of micronuclei between control and ICM-received cells. Fig. 9 indicates that the number of apoptotic cells increased nearly 2-fold over the spontaneous level in directly irradiated cells. In cells exposed to scattered radiation, the level of apoptotic like cells increased over the control value but these differences were not significant. ICM did not affect the frequency of apoptotic cells in any treatment kind.
Fig. 8.

The percentage level of micronucleated NHDF cells in directly irradiated or incubated in ICM cultures. Significant at **p < 0.001 compared with control.
Fig. 9.

The percentage level of apoptotic NHDF cells in directly irradiated or incubated in ICM cultures. Significant at *p < 0.001 and **p < 0.01 compared with control.
In human cancer, A549 cells and normal bronchial epithelial BEAS-2B cells we observed for medium-mediated bystander effects that were independent of radiation type and radiation dose. When these cells were exposed to scattered radiation, the formation of micronuclei and induction of apoptosis were similar to that caused by ICM, suggesting that genetic changes are caused by factors released by irradiated cells into the medium rather than by radiation-induced ionization of DNA. Otherwise, normal human fibroblasts (NHDF) were resistant to the radiation-induced bystander effect. It has been shown that the bystander effect is cell specific since it was observed in epithelial cells,1,8,16 leukemia cells,17 glioma cells18 and primary human fibroblasts,13 but not in fibroblasts line MSU1.1.1 The above described results and observations from the present work indicate that normal human fibroblasts are resistant to radiation-induced bystander signals, while normal epithelial cells are sensitive to these signals. These data suggest that epithelial cells can be more sensitive to induction of genetic alterations leading to cancer development than fibroblasts. This suggestion can be confirmed by published data indicating that over 90% of human cancers arise from epithelial tissues.19 Although bystander effects may have potential disadvantages, such as risk of late organ damage or induction of secondary tumors,9,20 they may also deliver benefits to clinical radiotherapy, including more effective killing of cancer cells. It is especially important in modern radiotherapy techniques, such as intensity modulated radiation therapy (IMRT) that aims at reducing the radiation dose to normal tissues.21,22 It has been demonstrated that bystander effects predominate after low dose exposure and saturate at higher one and can be induced when a few cells in a population are irradiated with dose of about 0.1 Gy.1,16,18
4. Conclusion
Genetic changes observed in cells exposed to scattered radiation outside the field were at least in part induced by low dose irradiation. Additionally, low-dose scattered irradiation induces, among other things, the bystander effect and this effect can predominate the response of cells exposed outside the radiation field. The genetic damage of cells exposed outside the radiation field can be caused by factors released by irradiated cells into the medium rather than induced directly in DNA by X rays. It seems that bystander effects may have important clinical implications for health risk after low level radiation exposure of cells lying outside the radiation field during clinical treatment.
Acknowledgment
This study was supported by a grant from the Polish Ministry of Science and Higher Education, No. NN 402 4447 33.
References
- 1.Mothersill C., Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of irradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 2.Sawant S.G., Zheng W., Hopkins K.M. The radiation-induced bystander effect for clonogenic survival. Radiat Res. 2002;157:361–364. doi: 10.1667/0033-7587(2002)157[0361:tribef]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Morgan W.F., Sowa M.B. Non-targeted bystander effects induced by ionizing radiation. Mutat Res. 2006;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Azzam E.I., de Toledo S.M., Spitz R. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from α-particle-irradiated normal human fibroblasts cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 5.Koturbash I., Loree J., Kutanzi K. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int J Radiat Oncol Biol Phys. 2008;70:554–562. doi: 10.1016/j.ijrobp.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Little J.B., Nagasawa H., Pfenning T. Radiation-induced genomic instability: delayed mutagenic and cytogenetic effects of X rays and alpha particles. Radiat Res. 1997;148:299–307. [PubMed] [Google Scholar]
- 7.Ghandhi S.A., Yaghoubian B., Amundson S.A. Global gene expression analyses of bystander and alpha particle irradiated human lung fibroblasts: synchronous and differential responses. BMC Med Genomics. 2008;1:63. doi: 10.1186/1755-8794-1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaugler M.H., Neunlist M., Bonnaud S. Intenstinal epithelial cell dysfunction is mediated by an endothelial-specific radiation-induced bystander effect. Radiat Res. 2007;167:185–193. doi: 10.1667/rr0702.1. [DOI] [PubMed] [Google Scholar]
- 9.Mothersill C., Seymour C.B. Radiation-induced bystander effects – implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 10.Averbeck D. Does scientific evidence support a change from the LNT model for low-dose radiation risk extrapolation. Health Phys. 2009;97:493–504. doi: 10.1097/HP.0b013e3181b08a20. [DOI] [PubMed] [Google Scholar]
- 11.Konopacka M., Rogoliński J., Ślosarek K. Comparison of dose distribution of ionizing radiation in a water phantom with frequency of cytogenetic damage in a human bronchial cells. In: Dőssel, Schlegel, editors. World congress on medical physics and biomedical engineering, Munich, Germany, IFMBE proceedings, vol. 25/3. 2009. pp. 379–382. [Google Scholar]
- 12.Fenech M. The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect. 1993;101(Suppl. 3):101–107. doi: 10.1289/ehp.93101s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belyakov O.V., Malcolmson A.M., Folkard M., Prise K.M., Michael B.D. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer. 2009;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogoliński J., Konopacka M., Sochanik A., Ślosarek K. Cell monolayer positioning with respect to beam field of photon radiation affects cell viability and causes chromosomal damage. ESTRO 29, September 12–16, 2010, Barcelona, Spain. Radiother Oncol: J Eur Soc Ther Radiol Oncol. 2010;96:S605. [Google Scholar]
- 15.Mothersill C., Seymour C.B. Cell–cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- 16.Konopacka M., Rogoliński J. X-irradiation of human bronchial cancer cells causes the bystander effects in normal bronchial cells in vitro. Neoplasma. 2010;57:151–154. doi: 10.4149/neo_2010_02_151. [DOI] [PubMed] [Google Scholar]
- 17.Konopacka M., Rzeszowska-Wolny J. The bystander effect-induced formation of micronucleated cells is inhibited by antioxidants, but the parallel induction of apoptosis and loss of viability are not affected. Mutat Res. 2006;593:32–38. doi: 10.1016/j.mrfmmm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Shao C., Folkard M., Michael B.D., Prise K.M. Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int J Cancer. 2005;116:45–51. doi: 10.1002/ijc.21003. [DOI] [PubMed] [Google Scholar]
- 19.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 20.Munro A.J. Bystander effects and their implications for clinical radiotherapy. J Radiat Prot. 2009;29:A133–A142. doi: 10.1088/0952-4746/29/2A/S09. [DOI] [PubMed] [Google Scholar]
- 21.Chełmiński K., Bulski W., Georg D. Energy dependence of radiochromic dosimetry films for use in radiotherapy verification. Rep Pract Oncol Radiother. 2010;15(2):40–46. doi: 10.1016/j.rpor.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sas-Korczyńska B., Śladowska A., Rozwadowska-Bogusz B., Dyczek S. Comparison between intensity modulated radiotherapy (IMRT) and 3D tangential beams technique used in patients with early-stage breast cancer who received breast-conserving therapy. Rep Pract Oncol Radiother. 2010;15(4):79–86. doi: 10.1016/j.rpor.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


