Abstract
There have been significant improvements in the radiotherapeutic management of patients with high risk prostate cancer. Randomized trials have clearly demonstrated improved outcomes with the combination of radiotherapy in conjunction with androgen deprivation. While these trials have utilized low doses of radiotherapy in the range of 70 Gy, recent studies have suggested that significant benefits of combined androgen deprivation therapy with dose escalated radiotherapy are also observed. The use of high radiation dose levels in the setting of high risk prostate cancer is important, and strategies which combine external beam radiotherapy with a brachytherapy boost may provide an opportunity for even greater intensification of the radiation dose to the prostate target. Systemic therapies, second generation anti-androgen therapy and novel targeted agents integrated with radiotherapy will open up new vistas and challenges for further improved outcomes in patients with high-risk disease.
Keywords: High-risk prostate cancer, External beam radiation therapy, Androgen-deprivation therapy, Anti-androgen agents, Androgen receptor
1. Background
External beam radiation therapy (EBRT) is considered a standard treatment intervention for patients with high-risk prostate cancer. However, EBRT alone is generally inadequate to achieve durable disease control for high-risk patients, and long-term outcomes with radiation therapy used as monotherapy for this cohort are far from optimal. From the radiotherapy-only arms of various randomized trials where low doses of EBRT in the ranges of 65–70 Gy were administered, the 10-year prostate-specific antigen (PSA) failure rates were greater than 75%.1–4 Randomized trials1–4 for high-risk prostate cancer have evaluated the combination of EBRT plus androgen-deprivation therapy (ADT) and have consistently demonstrated improved outcomes with combined-modality therapy for this high-risk population (Table 1). The greatest benefit may be seen among those patients with high-grade cancers. This notion is further supported by a meta-analysis of five Radiation Therapy Oncology Group (RTOG) trials incorporating 2743 patients5 where it was demonstrated that patients with Gleason 8–10 or T3 disease experience superior survival outcomes when treated with ADT in conjunction with EBRT compared with EBRT alone.
Table 1.
Outcomes for combined androgen-deprivation therapy and radiotherapy in high-risk disease.
| Study | PSA failure | Distant metastasis | Prostate cancer death | Overall survival |
|---|---|---|---|---|
| Pilepich et al.4 | 65% at 10 years | 35% at 10 years | 23% at 10 years | 43% at 10 years |
| Horwitz et al.3 | 52% at 10 years | 23% at 10 years | 11% at 10 years | 52% at 10 years |
| Pilepich et al.2 | 31% at 10 years (PSA < 1.5 ng/mL) | 24% at 10 years | 16% at 10 years | 49% at 10 years |
| Bolla et al.1 | 24% at 5 years | 10% at 5 years | 6% at 5 years | 78% at 5 years |
PSA = prostate-specific antigen.
Clinical evidence suggests that not only is the use of ADT in conjunction with EBRT an important element in the management of high-risk prostate cancer, but in addition the use of longer-durations of ADT may be associated with improvement in survival outcomes. RTOG 92-02 included over 1500 patients and compared 28 months of ADT (consisting of 2 months neo-adjuvant ADT, 2 months concurrent ADT with EBRT, and 24 months adjuvant ADT) with 4 months of ADT (consisting of 2 months neo-adjuvant ADT and 2 months concurrent ADT with EBRT without adjuvant ADT). In a subset analysis, a 10% survival advantage was noted among the Gleason 8–10 cohort who received the long-term ADT regimen compared with the short-course treatment.3 European Organisation for Research and Treatment of Cancer (EORTC) 22961 randomized high-risk patients to 6 months ADT (neo-adjuvant and concurrent ERBT) or 3 years of ADT (additional 30 months adjuvant ADT). Nine hundred seventy patients were randomized to this trial, and with a median follow-up of 6.4 years a 4% survival advantage was observed for the group treated with long-term ADT, with significant improvement in other parameters including progression-free survival outcomes and biochemical relapse–free survival outcomes.1
Nevertheless, the optimal duration of ADT when administered with radiation therapy (RT) remains to be defined. In a subset analysis of a Phase III trial from Canada that randomized high-risk patients to 3 versus 8 months of neo-adjuvant ADT, patients were reported to have an improvement in 5-year disease-free survival outcomes from 42% to 71% (P < 0.01); however, no advantage was noted for overall survival.6 To date, while it is common practice for patients with high-risk disease to receive 2–3 years of adjuvant ADT, current trials have never established if ADT courses with durations of only 12 or 18 months may be sufficient, especially in the setting of escalated doses of radiotherapy at 80 Gy or higher. Previously published trials comparing ADT plus EBRT versus EBRT alone utilized low doses of radiotherapy, often in the absence of targeted conformal treatment delivery such as intensity-modulated radiotherapy. Therefore the optimal duration of ADT remains unclear with the use of high-dose conformal EBRT. Only randomized trials evaluating various ADT durations in the setting of high-dose radiotherapy administration will be able to resolve these clinical uncertainties.
2. Is there an established role for ADT in the era of dose-escalated IMRT for high-risk patients?
Prior randomized trials1–4 in locally advanced prostate cancer demonstrating the benefit of concomitant and adjuvant ADT in conjunction with EBRT have all been in the setting of low-dose radiotherapy. In retrospect, the dose levels of 65–70 Gy (in the absence of conformal radiotherapy techniques) routinely utilized in these studies would be considered inadequate by current standards and associated with an increased likelihood of local tumor failure. Zelefsky et al. have recently shown that even in the setting of dose levels of 81 Gy and higher, the use of ADT for higher-risk patients provides an incremental benefit for improved PSA relapse-free survival outcomes and distant metastases-free survival outcomes. Zelefsky et al. reviewed the outcome of 2551 patients treated with three-dimensional conformal radiotherapy (3D-CRT)/IMRT with dose levels ranging from 64.8 Gy to 86.4 Gy.7 In this experience the median duration of ADT was 6–8 months. The use of ADT for intermediate- and high-risk patients was associated with significantly improved biochemical tumor control and distant metastases-free survival outcomes. The benefit of ADT was observed even among patients who were treated to dose levels of 81 Gy or higher. These data suggest that ADT (at least 6–8 months and probably longer) is still required in conjunction with high-dose radiotherapy for high-risk patients. We concur and believe that for high-risk prostate cancer patients, longer courses of ADT such as 2–3 years would be more appropriate in the setting of high-dose radiotherapy and represent our current practice.
3. Dose intensification with EBRT and brachytherapy
Another important direction to improve the prostate cancer-specific outcomes of high-risk patients includes the intensification of the radiation dose with the addition of brachytherapy to external RT and ADT. It would appear that the use of traditional escalated radiation dose levels in the range of 78–80 Gy may still be inadequate to eradicate locally advanced prostate cancer. A meta-analysis of the Phase III dose-escalation trials in patients for high-risk prostate cancer that comprised over 2800 patients demonstrated continued improvements in biochemical control outcomes with escalation of doses from 64 Gy to 81 Gy, and there was no apparent suggestion of a plateau being reached.8 Some reports have in fact noted that dose-escalation even beyond 80 Gy has been associated with similar improvements in tumor control outcomes.9,10 In one report from the Fox Chase Cancer Center investigators noted that patients who received doses of 80 Gy or more had improved local and distant control compared with those treated with doses less than 80 Gy.9 Pahlajani et al. noted that for high-risk patients treated with EBRT there appears to be an overall survival advantage with dose escalation up to 84 Gy compared with doses <80 Gy.10
Several reports have noted improved outcomes with combined-modality regimens of EBRT and brachytherapy. In one study, 1342 patients with PSA >20 ng/mL, cT3 or higher, or biopsy Gleason 8–10 prostate cancer were retrospectively studied. Patients were treated with brachytherapy alone or with supplemental ADT, RT or both; the analysis was adjusted for age, year of treatment, and other known prostate cancer prognostic factors. After a median follow-up of 5.1 years, there was a significant reduction in the risk of prostate cancer-specific mortality in men treated with brachytherapy and both ADT and combined with external beam RT, further highlighting the need for greater dose intensification in high-risk patients.11
Stock et al.12 reported the outcome of combined brachytherapy with EBRT and ADT for high-risk prostate cancer. These investigators reported on 181 prostate cancer patients with Gleason scores 8–10 who were treated with a Pd-103 implant (prescription dose 100 Gy), 45 Gy of EBRT, and 9 months of ADT. The median follow-up was 5.5 years. The 8-year PSA relapse-free survival and freedom from distant metastases were 73% and 80%, respectively. The pretreatment PSA level significantly affected freedom from biochemical failure, with 8-year rates of 72%, 82%, and 58% for patients with PSA level of ≤10, >10–20 and >20 ng/mL, respectively (P = 0.006). The PSA level had no significant effect on rates of distant metastases. The Gleason score had the most significant effect on freedom from biochemical failure in a multivariate analysis, and was the only factor to significantly affect rates of distant metastases; the 8-year FBF rates were 84%, 55%, and 30% for scores of 8, 9, and 10, respectively (P = 0.003). The corresponding freedom from distant metastases and prostate cancer-specific survival rates were 86%, 76%, 30% and 92%, 80%, 62.5%, respectively (P < 0.001).
4. Radiation therapy: adjuvant treatment with chemotherapy
In line with the adjuvant approach following radical prostatectomy for high-risk prostate cancer, a feasibility trial to combine RT (70 Gy) and ADT (3 years) with adjuvant docetaxel (weekly 20 mg/m2 during RT followed by 3 cycles of 60 mg/m2 every 3 weeks) was performed.13 Of 50 patients enrolled, 46 patients completed a full-dose chemoradiation regimen. With a median follow-up of 54 months, the 5-year clinical disease-free survival was 66.72% and the 5-year survival was 92.15%. The authors concluded that 3D-CRT with androgen deprivation and concurrent weekly docetaxel, followed by 3 cycles of adjuvant docetaxel may be considered as feasible in high-risk prostate cancer and deserved to be evaluated in a Phase III randomized trial.
5. New horizons
The overwhelming focus of present translational research has been to develop effective treatments for castrate-resistant prostate cancer, yet castrate-resistant prostate cancer for many patients can be avoided altogether by improving upfront radiotherapy for localized, high-risk disease (see Fig. 1). To improve outcomes of definitive therapy for high-risk patients, it is critical first to determine the mechanism by which ADT potentiates prostate cancer radioresponse. This has never been more clinically relevant given the recently demonstrated success of second-generation anti-androgens in the treatment of castration-resistant patients.14,15
Fig. 1.
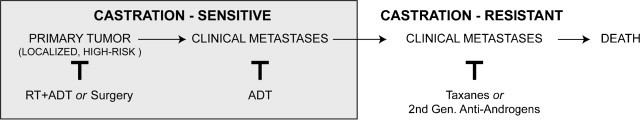
Natural history and therapeutic sequence of prostate cancer. Prostate cancer is classified by sensitivity to castration (induced chemically or by orchiectomy). Initially disease is castration sensitive, and for localized, high-risk disease the most common upfront treatment is radiotherapy (RT) plus androgen deprivation therapy (ADT).
Mechanisms proposed to explain how ADT potentiates prostate cancer radioresponse include an increase in cell death when ADT is combined with irradiation, decreased tumor cell hypoxia,16 decreased DNA repair,17 and decreased AR-mediated cell growth without evidence of direct synergy.18 Our group has recently demonstrated that the androgen receptor (AR) in fact regulates a transcriptional program of DNA repair genes in a direct manner, providing perhaps the most compelling mechanism by which ADT improves the outcome of radiotherapy (RT). Using a xenograft model of CRPC, we demonstrated that second-generation anti-androgen therapy downregulates DNA repair genes, and then by deploying RNA-seq and ChIP-seq technologies defined which of these genes represent bona fide AR target genes. We next established that these AR-mediated transcriptional changes are associated with decreased DNA repair when prostate cancer cells are treated with irradiation plus ADT, and conversely with increased DNA repair when cells are irradiated in the presence of androgen. However, perhaps most surprisingly, we also demonstrated that primary prostate cancer tumors display a significant spectrum of AR transcriptional output and that this variation directly correlates with the expression of a set of DNA repair genes (unpublished data).
The observation that a wide spectrum of AR signaling in primary tumors that correlates with DNA repair gene expression has potentially significant clinical implications. The heterogeneity of AR output observed in patient samples raises the tantalizing possibility that this may underlie heterogeneity in response from patients who receive ADT and radiotherapy. Specifically, inhibiting AR signaling in those patients with high AR transcriptional output may result in more clinical benefit than inhibiting AR signaling in those patients with low AR transcriptional output. In this manner, prostate cancer may share more in common with the other classic hormonally-driven tumor, breast cancer. Just as breast cancer patients are not indiscriminately treated with tamoxifen but rather selected for hormonal therapy based upon ER/PR positivity, it may be possible now to personalize adjuvant hormonal therapy for prostate cancer patients. To test this hypothesis, archived samples from landmark clinical trials must be transcriptionally profiled and AR output must be associated with clinical outcome. In collaboration with the RTOG we are actively engaged in this endeavor.
Once we can identify those patients whose tumors depend upon AR signaling, it is imperative that we take advantage of new, more potent anti-androgens by integrating these agents as part of definitive radiotherapy. These anti-androgens, such as abiraterone acetate and enzalutamide, have transformed the treatment of castration-resistant disease by more completely inhibiting both the production of endogenous androgens (abiraterone) and more effectively inhibiting the androgen receptor (enzalutamide). The rationale for integrating these drugs as part of definitive radiotherapy for high-risk prostate cancer is that, if moderate androgen deprivation in the form of bicalutamide and leuprolide have clinical benefit, then more potent androgen deprivation in the form of a second-generation anti-androgen like enzalutamide and leuprolide would be predicted to have even more clinical benefit. In contrast to bicalutamide, which is a partial AR agonist, enzalutamide is a far more potent AR antagonist, as demonstrated by comparing the expression of AR target genes, AR ChiP, and AR nuclear localization between the two drugs.19
The next question is how to design adjuvant therapy for those patients whose tumors exhibit low AR output and therefore appear to not depend as much upon AR signaling. The challenge of how to create adjuvant therapy for the low AR output patient can be approached using one of two broad strategies: first, manipulating the prostate cancer cell's biology to create (or perhaps recreate) AR dependence; and second, to inhibit targets that may radiosensitize prostate cancer cells independent of AR biology altogether. With respect to the first strategy, recent work has demonstrated that there exists an inverse relationship between AR signaling and PI3K signaling in which inhibition of one pathway activates the other and visa versa.20 Specifically, Carver et al. demonstrated that in those tumors in which PTEN is deleted, AR transcriptional output is decreased and that PI3K inhibition activates AR signaling. On the other hand, AR inhibition was found to activate AKT signaling. Deploying combined pharmacologic inhibition of AR and PI3K in a PTEN-deficient transgenic mouse model of prostate cancer resulted in near-complete regression. In light of these data, it may be possible to create AR dependence/sensitivity by treating patients with a PTEN-deleted tumor with a drug that inhibits PI3K signaling along with an antiandrogen. Therefore, for the patient with a PTEN-deleted tumor who is high risk and requires radiosensitization, a compelling treatment regimen to test would be a second-generation antiandrogen plus a PI3K signaling inhibitor (either PI3K, AKT, or mTOR inhibitor) delivered along with RT.
The second strategy to treat patients whose tumors exhibit low AR output would be to target aberrant pathways independent of AR signaling. One target that has garnered significant interest recently is PARP1, which recent work has found to play a role in mediating the effects of the predominant ETS gene fusion, TMPRSS2:ERG.21 Brenner et al. demonstrated that TMPRSS2:ERG interacts in a DNA-dependent manner with both PARP1 and DNA-PKcs, both of which are required for ETS-mediated transcription and cell invasion. Therefore, in the large number of patients with an ETS gene fusion, a reasonable therapeutic strategy to test would be to combine radiotherapy with PARP inhibition. Additional recent evidence has shown that there may be some connection between PARP1 and AR signaling. Schiewer et al. demonstrated that PARP-1 is an important cofactor for AR activity, whereby PARP-1 activity is required for AR function.22 Our own recent data demonstrate that PARP-1 is a direct AR target gene whose expression is in part regulated by AR activity (unpublished data). Given that PARP1 levels are only partially decreased after AR inhibition, in conjunction with the evidence that AR may promote DNA repair through the increased expression of a number of different DNA genes, we hypothesize that combined blockade of AR and PARP-1 may maximally radiosensitize the cell compared to either AR or PARP-1 inhibition alone.
6. Conclusions
High-risk prostate cancer treated with radiotherapy is associated with improved outcomes when high-dose radiotherapy is used in conjunction with ADT. It would appear that the dose intensification with ultra-high radiation doses can be achieved most effectively when combining EBRT with a brachytherapy boost. This approach can facilitate the delivery of greater radiobiologic dose levels than that of high-dose radiotherapy delivered with IMRT alone. Longer courses of ADT appear to be associated with improved outcomes in the high-risk prostate cancer population compared with shorter courses. Future directions using second-generation anti-androgen agents more targeted to the androgen receptor hold great promise for novel radiotherapeutic strategies for high-risk prostate cancer.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgment
The authors wish to thank Eve S. Ferdman for her editorial assistance.
References
- 1.Bolla M., Van Tienhoven G., Warde P. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 2.Pilepich M.V., Winter K., Lawton C.A. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz E.M., Bae K., Hanks G.E. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 4.Pilepich M.V., Winter K., John M.J. Phase III Radiation Therapy Oncology Group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 5.Roach M., 3rd, Lu J., Pilepich M.V. Predicting long-term survival, and the need for hormonal therapy: a meta-analysis of RTOG prostate cancer trials. Int J Radiat Oncol Biol Phys. 2000;47:617–627. doi: 10.1016/s0360-3016(00)00577-0. [DOI] [PubMed] [Google Scholar]
- 6.Crook J., Ludgate C., Malone S. Final report of multicenter Canadian Phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:327–333. doi: 10.1016/j.ijrobp.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky M.J., Pei X., Chou J.F. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 9.Eade T.N., Hanlon A.L., Horwitz E.M., Buyyounouski M.K., Hanks G.E., Pollack A. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahlajani N., Ruth K.J., Buyyounouski M.K. Radiotherapy doses of 80 Gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:1949–1956. doi: 10.1016/j.ijrobp.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Amico A.V., Moran B.J., Braccioforte M.H. Risk of death from prostate cancer after brachytherapy alone or with radiation, androgen suppression therapy, or both in men with high-risk disease. J Clin Oncol. 2009;27:3923–3928. doi: 10.1200/JCO.2008.20.3992. [DOI] [PubMed] [Google Scholar]
- 12.Stock R.G., Cesaretti J.A., Hall S.J., Stone N.N. Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy, external beam radiotherapy and hormonal therapy. BJU Int. 2009;104:1631–1636. doi: 10.1111/j.1464-410X.2009.08661.x. [DOI] [PubMed] [Google Scholar]
- 13.Bolla M., Hannoun-Levi J.M., Ferrero J.M. Concurrent and adjuvant docetaxel with three-dimensional conformal radiation therapy plus androgen deprivation for high-risk prostate cancer: preliminary results of a multicentre phase II trial. Radiother Oncol. 2010;97:312–317. doi: 10.1016/j.radonc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 15.de Bono J.S., Logothetis C.J., Molina A. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain R.K., Safabakhsh N., Sckell A. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Ubaidi F.L., Schultz N., Loseva O., Egevad L., Granfors T., Helleday T. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res. 2013;19:1547–1556. doi: 10.1158/1078-0432.CCR-12-2795. [DOI] [PubMed] [Google Scholar]
- 18.Pollack A., Salem N., Ashoori F. Lack of prostate cancer radiosensitization by androgen deprivation. Int J Radiat Oncol Biol Phys. 2001;51:1002–1007. doi: 10.1016/s0360-3016(01)01750-3. [DOI] [PubMed] [Google Scholar]
- 19.Tran C., Ouk S., Clegg N.J. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carver B.S., Chapinski C., Wongvipat J. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner J.C., Ateeq B., Li Y. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–668. doi: 10.1016/j.ccr.2011.04.010. Erratum in: 2013;23:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiewer M.J., Goodwin J.F., Han S. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]


