Abstract
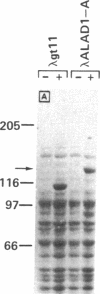
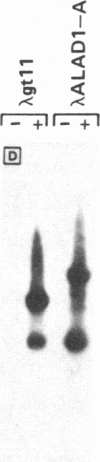
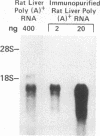
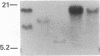
We have isolated several cDNA clones encoding delta-aminolevulinate dehydrase [ALAD; porphobilinogen synthase; 5-aminolevulinate hydro-lyase (adding 5-aminolevulinate and cyclizing), EC 4.2.1.24], the second enzyme in the heme biosynthetic pathway. We used a rabbit polyclonal antibody developed against the purified 35-kDa subunit of rat liver ALAD to screen a lambda gt11 cDNA expression library constructed from rat liver mRNA. A prototype clone (ALAD-1) contained a 680-base-pair insert and expressed a 140-kDa beta-galactosidase gene cDNA insert fusion protein immunoreactive with both polyclonal and monoclonal anti-ALAD. Identity of ALAD-1 was verified by hydridization to ALAD mRNA that had been enriched via immunopurification of rat liver polysomes with anti-ALAD. Intensity of such hybridization to a 1500-nucleotide-long mRNA was approximately equal to 200-fold greater than that realized with whole liver mRNA, a result consistent with the degree of immunoenrichment of ALAD mRNA found independently by analysis of cell-free translation products. A second ALAD cDNA clone (ALAD-3) was isolated when the rat liver cDNA expression library was rescreened with ALAD-1. The identity of both ALAD cDNA clones was established by correspondence between their nucleotide sequence and the reported amino-terminal protein sequence of bovine ALAD. Hybridization of ALAD cDNA to mouse genomic DNA indicates that the previously unexplained incremental differences in ALAD enzymatic activity among inbred mouse strains has arisen through alterations in ALAD gene dose.
Full text
PDF

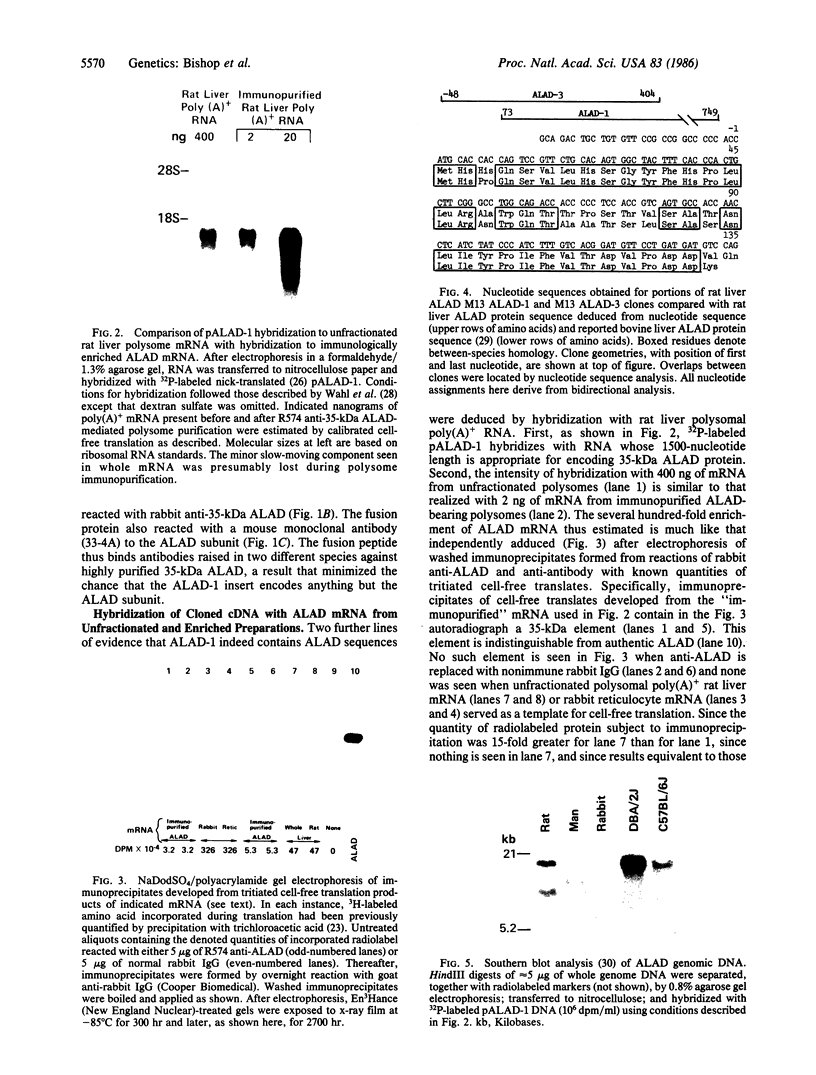
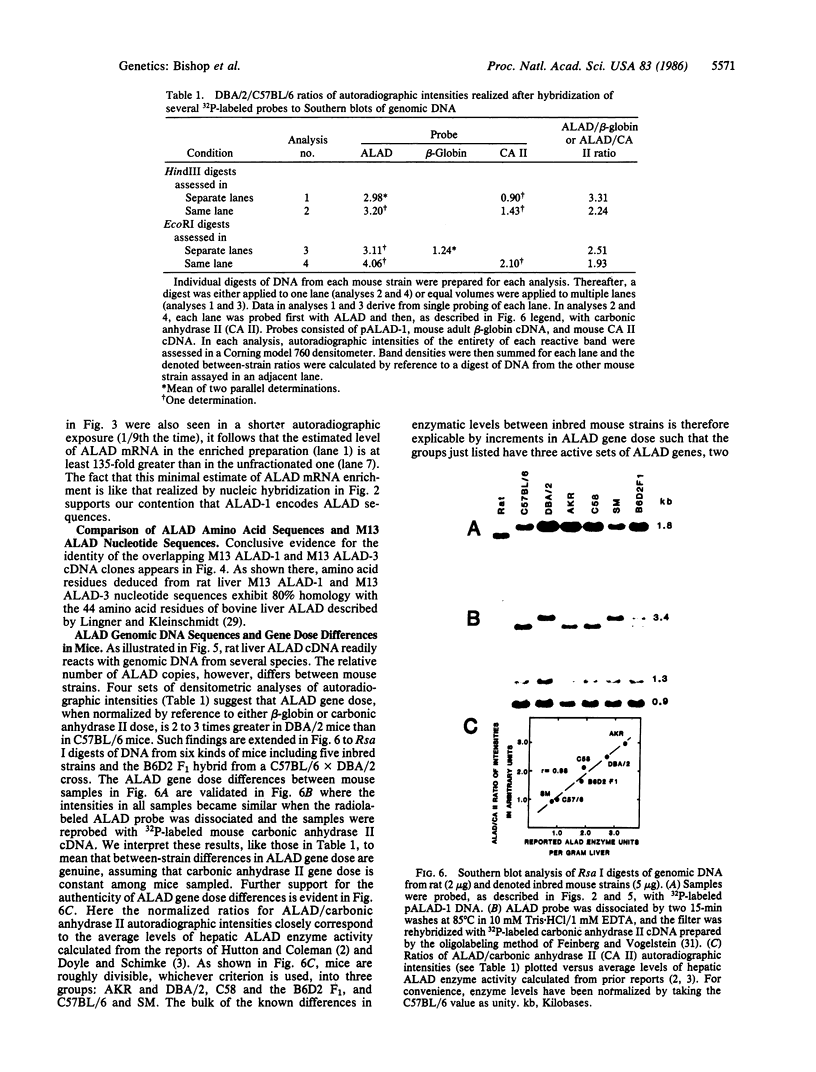

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Bhown A. S., Bennett J. C. High-sensitivity sequence analysis of proteins recovered from sodium dodecyl sulfate gels. Methods Enzymol. 1983;91:450–455. doi: 10.1016/s0076-6879(83)91042-x. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Smith K. D., Noyes A. N., Mullen M. A. Immunological characterization of rabbit hemoglobin alpha and beta chain-synthesizing polysomes. J Biol Chem. 1974 Nov 25;249(22):7210–7219. [PubMed] [Google Scholar]
- Boyer S. H., Smith K. D., Noyes A. N., Young K. E. Adjuvants to immunological methods for mRNA purification. Application to the isolation of mRNA for carbonic anhydrase I from rabbit reticulocytes. J Biol Chem. 1983 Feb 25;258(4):2068–2071. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Curtis P. J. Cloning of mouse carbonic anhydrase mRNA and its induction in mouse erythroleukemic cells. J Biol Chem. 1983 Apr 10;258(7):4459–4463. [PubMed] [Google Scholar]
- Despaux N., Comoy E., Bohuon C., Boudène C. Purification and properties of human erythrocyte delta-amino-levulinic acid dehydratase (EC 4-2-1-24). Biochimie. 1979;61(9):1021–1028. doi: 10.1016/s0300-9084(80)80256-2. [DOI] [PubMed] [Google Scholar]
- Doyle D., Schimke R. T. The genetic and developmental regulation of hepatic delta-aminolevulinate dehydratase in mice. J Biol Chem. 1969 Oct 25;244(20):5449–5459. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gibbs P. N., Chaudhry A. G., Jordan P. M. Purification and properties of 5-aminolaevulinate dehydratase from human erythrocytes. Biochem J. 1985 Aug 15;230(1):25–34. doi: 10.1042/bj2300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Coleman D. L. Linkage analyses using biochemical variants in mice. II. Levulinate dehydratase and autosomal glucose 6-phosphate dehydrogenase. Biochem Genet. 1969 Oct;3(5):517–523. doi: 10.1007/BF00485612. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lingner B., Kleinschmidt T. N-Terminale Sequenz einer Porphobilinogen-Synthase. Z Naturforsch C. 1983 Nov-Dec;38(11-12):1059–1061. doi: 10.1515/znc-1983-11-1229. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Nakamura H., Hamaguchi Y. Colcemid treatment of myeloma prior to cell fusion increases the yield of hybridomas between myeloma and splenocyte. Biochem Biophys Res Commun. 1984 Nov 14;124(3):903–908. doi: 10.1016/0006-291x(84)91043-x. [DOI] [PubMed] [Google Scholar]
- Nakakuki M., Yamauchi K., Hayashi N., Kikuchi G. Purification and some properties of delta-aminolevulinate synthase from the rat liver cytosol fraction and immunochemical identity of the cytosolic enzyme and the mitochondrial enzyme. J Biol Chem. 1980 Feb 25;255(4):1738–1745. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28(2-3):133–145. doi: 10.1159/000459097. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shemin D. 5-Aminolaevulinic acid dehydratase: structure, function, and mechanism. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):109–115. doi: 10.1098/rstb.1976.0004. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]