Abstract
Aim
To compare and evaluate the performance of two different volumetric modulated arc therapy delivery techniques.
Background
Volumetric modulated arc therapy is a novel technique that has recently been made available for clinical use. Planning and dosimetric comparison study was done for Elekta VMAT and Varian RapidArc for different treatment sites.
Materials and methods
Ten patients were selected for the planning comparison study. This includes 2 head and neck, 2 oesophagus, 1 bladder, 3 cervix and 2 rectum cases. Total dose of 50 Gy was given for all the plans. All plans were done for RapidArc using Eclipse and for Elekta VMAT with Monaco treatment planning system. All plans were generated with 6 MV X-rays for both RapidArc and Elekta VMAT. Plans were evaluated based on the ability to meet the dose volume histogram, dose homogeneity index, radiation conformity index, estimated radiation delivery time, integral dose and monitor units needed to deliver the prescribed dose.
Results
RapidArc plans achieved the best conformity (CI95% = 1.08 ± 0.07) while Elekta VMAT plans were slightly inferior (CI95% = 1.10 ± 0.05). The in-homogeneity in the PTV was highest with Elekta VMAT with HI equal to 0.12 ± 0.02 Gy when compared to RapidArc with 0.08 ± 0.03. Significant changes were observed between the RapidArc and Elekta VMAT plans in terms of the healthy tissue mean dose and integral dose. Elekta VMAT plans show a reduction in the healthy tissue mean dose (6.92 ± 2.90) Gy when compared to RapidArc (7.83 ± 3.31) Gy. The integral dose is found to be inferior with Elekta VMAT (11.50 ± 6.49) × 104 Gy cm3 when compared to RapidArc (13.11 ± 7.52) × 104 Gy cm3. Both Varian RapidArc and Elekta VMAT respected the planning objective for all organs at risk. Gamma analysis result for the pre-treatment quality assurance shows good agreement between the planned and delivered fluence for 3 mm DTA, 3% DD for all the evaluated points inside the PTV, for both VMAT and RapidArc techniques.
Conclusion
The study concludes that a variable gantry speed with variable dose rate is important for efficient arc therapy delivery. RapidArc presents a slight improvement in the OAR sparing with better target coverage when compared to Elekta VMAT. Trivial differences were noted in all the plans for organ at risk but the two techniques provided satisfactory conformal avoidance and conformation.
Keywords: Varian RapidArc, Elekta VMAT, Planning study
1. Background
Approximately 50% of patients diagnosed for cancer receive radiotherapy as a part of their oncologic management. So, balancing the potential for early and late toxicity against tumour control is particularly important. Intensity Modulated Radiation Therapy (IMRT) has been revealed in several instances to improve conformal avoidance when compared to 3D conformal techniques for various sites. The availability of more sophisticated techniques like intensity- modulated protons, helical tomotherapy and the newly introduced volumetric modulated arc therapy (Elekta's VMAT™ and Varian's RapidArc™), triggered interest in performing a new investigation to compare relevant dosimetric investigation when applied to tough planning sites.1–5 These techniques are believed to improve delivery efficiency compared to that of IMRT while maintaining similar treatment plan quality.4–6
Volumetric modulated arc therapy (VMAT) represents a new paradigm in the treatment of patients with external beam radiotherapy. VMAT uses one or more gantry arcs to deliver dose from a range of coplanar or non-coplanar directions. This new method of treatment delivery offers a challenge to classical dosimetry protocols that involve static fields of moderately large size. With VMAT, the beams are dynamic in orientation and aperture shape and may include small apertures. The scatter conditions are expected to differ in such beams, compared to classical open fields. So the calibration carried out under broad-beam conditions may not be relevant for VMAT. The concept of rotational intensity-modulated radiation therapy was first described by Rock Mackie in 1993. In 1995, Cedric Yu introduced an alternative approach to the delivery of rotational IMRT. Arc therapy can be used as an alternative to tomotherapy as proposed by Yu.1 According to Yu, with the increase in the number of gantry angles, the number of intensity levels at each gantry angle can be reduced without degrading plan quality. Also, arc therapy is efficient in treating a target wrapped around a critical structure, as demonstrated by Cotrutz et al.18
Varian adopted Otto's VMAT algorithm (Otto, 2008) and marketed it with the trade name, RapidArc™ (RA), in 2007. Elekta started to market their product with the trade name VMAT™. In this work, volumetric modulated arc therapy delivery by Varian machine will be referred as “RapidArc” and that of Elekta machine as “Elekta VMAT”. A recent meta-analysis has suggested that intensity modulated treatment delivery can reduce toxicities as compared to other conventional and conformal type of deliveries. However, the data regarding local control and overall survival are inconclusive.7 With emerging data demonstrating the advantages of IMRT, future development in advanced radiation delivery will include improvements in quality, efficiency, accuracy with the help of image guidance.6 A major advancement in intensity modulated arc therapy delivery was realized when Otto implemented the volumetric modulated arc therapy (VMAT) algorithm.4 Later, Yu et al.1,8 and Duthoy et al.9,10 analysed the benefits of using multiple coplanar or noncoplanar intensity-modulated arcs for complex treatments, demonstrating that this new approach offers equivalent or superior advantage. Recent studies by Bortfeld and Webb suggested that a single-arc VMAT technique shows superior dosimetric benefits over intensity modulated radiotherapy, although we have to accept a low dose spread over a large volume of tissue. Both Varian and Elekta implemented arc therapy which allows dose rate variations.
2. Aim
The aim of the present study is to compare and evaluate the plan quality, delivery efficiencies and performance of two different volumetric modulated arc therapy delivery techniques for different treatment sites.
3. Materials and methods
Ten cases were selected for the planning study. This included 2 head and neck, 2 oesophagus, 1 bladder, 3 cervix and 2 rectum. Total dose of 50 Gy was given for all the plans. All selected head and neck patients were post surgery cases. VMAT and RapidArc plans were generated for each of the cases, with clinically relevant planning constraints. Planning parameters were kept the same for both Elekta VMAT and RapidArc. All plans were evaluated based on the ability to meet the dose volume histogram, dose homogeneity index, radiation conformity index, estimated radiation delivery time, mean dose to healthy tissues and integral dose, for both Elekta VMAT and Varian RapidArc. Also, all the plans were generated by a single planner.
3.1. RapidArc planning (Eclipse)
RapidArc plans were created in the Eclipse planning system, which supports more than one arc. All the RapidArc treatment plans were created with 2 full arcs for 6 MV photons. The first arc was planned in the clockwise direction and second in the counter clockwise direction. The maximum dose rate was set at 600 MU/min. For all the plans, the collimator was rotated between 30° and 45° to cover the entire tumour and to reduce the tongue and groove effect.11 Couch parameters were also added while planning to account for the attenuation for mega voltage beams.12 The final calculations were performed using the Analytical anisotropic Algorithm (AAA). The total numbers of control points are 177 for each arc. In version 8.6, the optimization begins with 10 control points, gradually increasing to a maximum of 177. The arc optimization algorithm, PRO (Progressive Resolution Optimizer) used in RapidArc, optimizes leaf position, dose rate and gantry speed. The optimization is performed using progressive sampling in five resolution levels. In this version of the RapidArc planning, the optimization appears to switch to next MR level before the objective function has converged for a given level. The optimization can be paused in any resolution level by the planner. A Millennium MLC with 120 leaves with a spatial resolution of 5 mm at isocentre was used. Varian's MLC is mounted on a carriage and acts as a tertiary collimator below the conventional collimator jaws.
3.2. Elekta VMAT planning (Monaco)
Volumetric modulated arc therapy planning for Elekta synergy linac was done on the Monaco planning system, version 2.4. All VMAT plans consist of only a single arc with 360° gantry rotation. All the plans were generated for 6 MV photons with a maximum dose rate kept at 600 MU/min. The collimator was set at zero for all the plans. Elekta VMAT does not implement Otto's algorithm but uses a proprietary algorithm.13 The Monaco planning system optimizes treatment plans with the aid of sophisticated mathematical formulas, the cost functions that model several different kinds of effects of desired goal doses and their relationships for both targets and organs at risk. Monaco offers constrained optimization. The cost functions predict the clinical effect of the treatment plan and then compare the acceptability of the plan to the optimization objectives and constraints. The optimization algorithm works within parameters that impose several limits on the decision variables so that the dose delivered to the tumour is the maximum effective dose subject to given parameters. The VMAT sequencer uses a sweep sequencer because leaf speed is the most limiting factor of any modulated delivery. Even though it requires more MUs, the sweep sequencer minimizes leaf travel. This allows to avoid frequent beam stops, multiple arcs, and gantry moves without irradiation. Monte Carlo dose calculation algorithm is used for photon beam calculations. The Elekta MLC assembly consists of 40 opposed pairs of leaves. Each individual leaf projects to a width of 1 cm at the isocentre. Unlike with the Varian Millennium 120 leaf MLC, no leaf interdigitation is allowed for this type of design. Also the MLC on the Elekta linac is a secondary collimator with x- and y-back-up jaws placed under the MLCs. Each leaf can travel 12.5 cm across the centre and 20 cm from the centre. The back-up collimators always dynamically follow the MLC-shaped fields and lower the leakage radiation especially through the parked gaps. The minimum gap between the opposed leaves and the opposed adjacent leaves was set to be 0.5 cm as suggested by the vendor.
3.3. Radiation delivery (Varian)
For Varian machines before the latest TrueBeam, the linac and the MLC are controlled by separate computers that interact with each other; the control points designed for treatment delivery are decomposed into two groups of control parameters. (1) The MLC positions, as a function of delivered MUs, are sent to the MLC controller. (2) The gantry angle, as a function of cumulative MU, is sent as a segmented treatment table to the linac control system, which translates the segmented treatment table into commands that control the dose rate and gantry speed during dynamic arc delivery. For Varian linacs using a gridded gun, the instantaneous dose rate change in their C-series of machines is performed by the dose rate servo using pulse dropping. These older systems do not vary pulse width.
3.4. Radiation delivery (Elekta)
For Elekta machines, the coordination between the MLC and the linac delivery is performed by the ‘RT Desktop’. The methods of dose rate control are also different. For Elekta machines employing a non-gridded gun, the dose rate is achieved by varying the rate of the pulses and dose from each pulse is fixed. As a result, dose rates can be varied among discrete levels. The dose rate is automatically calculated to be the maximum that can be used without exceeding any of the movement speeds of leaves, jaws and the gantry. If the dose to be delivered in a given interval is too high, the gantry and the leaves slow down accordingly.
3.5. Evaluation tools
Evaluation of the plans was performed by means of standard DVH. For PTV D98% and D2% (dose received by the 98% and 2% of the volume) were defined for minimum and maximum doses. The homogeneity of the treatment plans was expressed in terms of (D5% − D95%)/D50% (Homogeneity Index) according to ICRU 83. The conformity of the plans was measured with a conformity index, CI95% defined as the ratio between the patient volume receiving at least 95% of the prescribed dose and the volume of the PTV. For organs at risk, the analysis includes the mean dose and the maximum dose. For healthy tissue, the integral dose, “doseInt”, is defined as the integral of the absorbed dose extended over all voxels but excluding those within the target volume. Doses to relevant OARs were evaluated according to the specific treatment sites. Also, patient specific quality assurance for all the treatment plans for both RapidArc and Elekta VMAT techniques were done using 2D array and Octavius Phantom. The TPS calculated fluence was compared to the delivered fluence in phantom using PTW verisoft. The acceptance criteria of 3 mm for the distance to agreement (DTA) and dose difference tolerance level of 3% were chosen for analysis. Also the percentage of the evaluated dose points passing the gamma index was kept at a limit of greater than or equal to 95%.
3.6. Gamma analysis
Gamma analysis is used to compare two 2D dose maps. Generally, the dose distribution comparison is sub divided into regions of high and low dose gradients. In low gradient regions, the doses are compared directly with an acceptance tolerance placed on the difference between the two dose maps. In high dose gradient regions, a small spatial error, in either of the dose maps or small miss alignment results in large dose difference between the dose maps. Dose difference in the high dose gradient regions may therefore be relatively unimportant and the concept of DTA distribution is used to determine the acceptability of agreement. The DTA is the distance between a dose point in a dose map and the nearest point in the other dose map that exhibits the same dose. The dose difference and DTA evaluations complement each other when used as determinants of agreement accuracy between the dose maps. The simultaneous use of DTA and a percent dose difference (DD) was proposed by Low et al.14. These parameters can help evaluate the agreement of the two distributions in terms of misalignment and difference, respectively. So in this study the various gamma index constraints which are a combination of particular DTA value with specific dose difference tolerance value were used. In this study gamma analysis was done for the whole arc.
4. Results and discussion
All plans sufficiently respected the planning objectives and can be clinically accepted. Tables 1 and 2 provide an overview of the numerical findings from an average DVH analysis on PTV and healthy tissues which are reported as mean values ± standard deviation (SD) to assess for the relative inter-patient variability. Dosimetric results for organs at risk (OAR) for all the ten patients were reported independently as the analysis was done for different sites (Tables 3–12). The results are very homogeneous within the different approaches, as suggested by the small standard deviations and frequent statistical significance despite rather small absolute differences. Both delivery techniques, if considered from a clinical perspective appear to be equivalent. Figs. 1 and 2 show the treatment planning window for Varian RapidArc and Elekta VMAT, respectively.
Table 1.
Dosimetric results for the PTV.
| Parameter | RapidArc | VMAT | P |
|---|---|---|---|
| D98% (Gy) | 48.95 ± 1.05 | 47.66 ± 1.19 | 0.007 |
| D2% (Gy) | 54.54 ± 1.15 | 53.81 ± 1.22 | 0.170 |
| HI | 0.08 ± 0.03 | 0.12 ± 0.02 | 0.031 |
| CI95% | 1.08 ± 0.07 | 1.10 ± 0.05 | 0.377 |
| MU | 515.86 ± 125.61 | 640.43 ± 173.98 | 0.035 |
| Beam on time (min) | 2.59 ± 0.05 | 3.59 ± 0.56 | 0.007 |
P – paired t-test analysis: RapidArc vs. VMAT.
Table 2.
Dosimetric results for healthy tissue.
| Organ | Parameter | RapidArc | VMAT | P |
|---|---|---|---|---|
| Healthy tissue | Mean (Gy) | 7.83 ± 3.31 | 6.92 ± 2.90 | 0.003 |
| V2 Gy (%) | 58.77 ± 12.41 | 46.87 ± 14.19 | 0.006 | |
| V5 Gy (%) | 44.99 ± 13.63 | 34.09 ± 12.96 | 0.047 | |
| V10 Gy (%) | 35.55 ± 12.98 | 23.00 ± 9.87 | 0.049 | |
| V20 Gy (%) | 17.29 ± 8.09 | 12.28 ± 7.38 | 0.134 | |
| V30 Gy (%) | 6.19 ± 3.18 | 5.18 ± 3.29 | 0.475 | |
| V40 Gy (%) | 1.95 ± 1.24 | 1.64 ± 1.35 | 0.502 | |
| Int. dose (×104 Gy cm3) | 13.11 ± 7.52 | 11.50 ± 6.49 | 0.010 |
P – paired t-test analysis: RapidArc vs. VMAT.
Table 3.
Head and neck—case study 1.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Brain max: dose (Gy) | 44.72 | 47.72 |
| D2% (Gy) | 22.76 | 24.17 |
| Cord max: dose (Gy) | 35.69 | 37.17 |
| D2% (Gy) | 34.28 | 35.49 |
| Left parotid mean dose (Gy) | 21.94 | 23.54 |
| D50% (Gy) | 21.60 | 20.26 |
| Right parotid mean dose (Gy) | 22.20 | 21.93 |
| D50% (Gy) | 21.28 | 18.95 |
| Larynx mean dose (Gy) | 34.65 | 41.69 |
| D50% (Gy) | 33.97 | 41.53 |
| Mandible max: dose (Gy) | 54.91 | 52.28 |
Table 4.
Head and neck—case study 2.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Cord max: dose (Gy) | 37.50 | 39.12 |
| D2% (Gy) | 35.45 | 36.82 |
| Left parotid mean dose (Gy) | 22.26 | 24.85 |
| D50% (Gy) | 21.72 | 20.38 |
| Right parotid mean dose (Gy) | 24.32 | 22.25 |
| D50% (Gy) | 22.65 | 19.84 |
| Mandible max: dose (Gy) | 52.25 | 50.73 |
Table 5.
Oesophagus—case study 1.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Cord max: dose (Gy) | 36.76 | 33.22 |
| D2% (Gy) | 39.10 | 37.17 |
| Lung mean dose (Gy) | 18.07 | 15.41 |
| V5 Gy (%) | 89.22 | 89.04 |
| V10 Gy (%) | 88.03 | 84.90 |
| V15 Gy (%) | 71.18 | 44.36 |
| V20 Gy (%) | 35.95 | 22.45 |
| V30 Gy (%) | 9.09 | 7.22 |
| V40 Gy (%) | 1.11 | 1.51 |
Table 6.
Oesophagus—case study 2.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Cord max: dose (Gy) | 37.36 | 39.57 |
| D2% (Gy) | 32.62 | 36.00 |
| Heart mean dose (Gy) | 21.39 | 26.33 |
| D35% (Gy) | 22.94 | 28.84 |
| Lung mean dose (Gy) | 22.03 | 15.25 |
| V5 Gy (%) | 83.22 | 82.74 |
| V10 Gy (%) | 81.85 | 75.98 |
| V15 Gy (%) | 79.36 | 46.95 |
| V20 Gy (%) | 65.74 | 23.94 |
| V30 Gy (%) | 23.66 | 9.03 |
Table 7.
Bladder—case study 1.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Rectum mean dose (Gy) | 20.84 | 25.36 |
| D30% (Gy) | 22.77 | 27.85 |
| Femoral head left mean dose (Gy) | 22.94 | 13.31 |
| D15% (Gy) | 27.18 | 20.84 |
| Femoral head right mean dose (Gy) | 22.50 | 13.15 |
| D15% (Gy) | 26.98 | 20.04 |
Table 8.
Cervix—case study 1.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Rectum mean dose (Gy) | 36.76 | 33.22 |
| D30% (Gy) | 39.10 | 37.17 |
| Femoral head mean dose (Gy) | 22.17 | 11.05 |
| D15% (Gy) | 29.22 | 14.31 |
| Bladder mean dose (Gy) | 36.42 | 31.31 |
| D35% (Gy) | 38.14 | 35.40 |
Table 9.
Cervix—case study 2.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Rectum mean dose (Gy) | 29.65 | 35.18 |
| D30% (Gy) | 33.25 | 39.91 |
| Femoral head mean dose (Gy) | 24.73 | 14.96 |
| D15% (Gy) | 29.87 | 22.75 |
| Bladder mean dose (Gy) | 30.40 | 31.93 |
| D35% (Gy) | 32.24 | 35.69 |
Table 10.
Cervix—case study 3.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Rectum mean dose (Gy) | 32.73 | 37.58 |
| D30% (Gy) | 32.25 | 38.25 |
| Femoral head mean dose (Gy) | 26.20 | 16.55 |
| D15% (Gy) | 31.41 | 24.23 |
| Bladder mean dose (Gy) | 32.15 | 33.42 |
| D35% (Gy) | 35.26 | 36.68 |
Table 11.
Rectum—case study 1.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Femoral head left mean dose (Gy) | 23.07 | 21.11 |
| D15% (Gy) | 28.99 | 30.20 |
| Femoral head right mean dose (Gy) | 23.73 | 18.54 |
| D15% (Gy) | 30.33 | 27.94 |
| Bladder mean dose (Gy) | 33.30 | 29.37 |
| D35% (Gy) | 35.11 | 31.73 |
Table 12.
Rectum—case study 2.
| Parameter | RapidArc | VMAT |
|---|---|---|
| Femoral head left mean dose (Gy) | 24.55 | 22.75 |
| D15% (Gy) | 26.80 | 27.50 |
| Femoral head right mean dose (Gy) | 24.53 | 20.64 |
| D15% (Gy) | 30.55 | 28.13 |
| Bladder mean dose (Gy) | 32.72 | 30.55 |
| D35% (Gy) | 33.84 | 30.04 |
Fig. 1.

RapidArc treatment planning window in Eclipse TPS.
Fig. 2.
VMAT treatment planning window in Monaco TPS.
4.1. Planning target volume (PTV)
Target coverage was almost similar for both RapidArc and Elekta VMAT techniques. RapidArc plans resulted in a better conformity (CI95% = 1.08 ± 0.07) than VMAT plans (CI95% = 1.10 ± 0.05). The dose inhomogeneity in PTV was higher for Elekta VMAT plans with HI equal to 0.12 ± 0.02 when compared to RapidArc with 0.08 ± 0.03. The average MU (±SD) needed to deliver the dose of 200 cGy per fraction was 516 ± 126 MU for RapidArc and 640 ± 174 MU for Elekta VMAT. The total number of segments for each RapidArc plan is 354. For Elekta VMAT, the segments varied from 81 to 213, with a standard deviation of ± 43.92 between the plans. The radiation beam on time (after patient setup) was 2.52–2.64 min for RapidArc and 2.82–4.22 min for Elekta VMAT. The average beam on time for all RapidArc plans (2.59 ± 0.05) was found to be significantly lower when compared with Elekta VMAT (3.59 ± 0.56). Also the gamma analysis result for the pre-treatment quality assurance shows good agreement with the planned and delivered fluence for 3 mm DTA, 3% DD for all the evaluated points inside the PTV, for both Elekta VMAT and RapidArc techniques. The percentage dose points failed the gamma criteria by less than 5% in the analysed area. The results were displayed in Fig. 3.
Fig. 3.
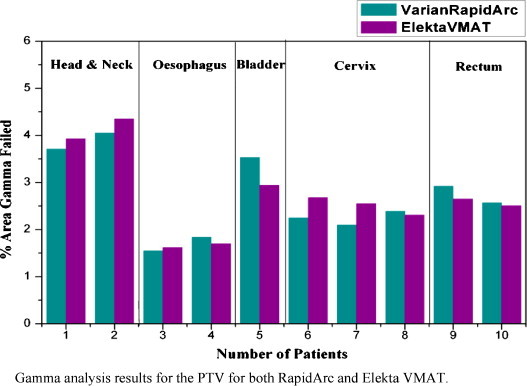
Gamma analysis results for the PTV for both RapidArc and Elekta VMAT.
4.2. Healthy tissue
Significant changes were observed between the RapidArc and Elekta VMAT plans in terms of the mean dose and integral dose. The Integral dose is computed as the mean dose times the volume of healthy tissue irradiated. VMAT plans show a reduction in the healthy tissue mean dose (6.92 ± 2.90) Gy when compared to RapidArc (7.83 ± 3.31) Gy. The integral dose is found to be lower with VMAT (11.50 ± 6.49) × 104 Gy cm3 when compared to RapidArc (13.11 ± 7.52) × 104 Gy cm3. Also Elekta VMAT plans show a reduction in the volume of healthy tissue irradiation at lower doses mainly with a reduction observed at V10 Gy (35.55 ± 12.98 with RapidArc and 23.00 ± 9.87 with Elekta VMAT), V5 Gy (44.99 ± 13.63 with RapidArc and 34.09 ± 12.96 with Elekta VMAT), V2 Gy (58.77 ± 12.41 with RapidArc and 46.87 ± 14.19 with Elekta VMAT) when compared to RapidArc plans.
4.3. Organs at risk
Both the technique satisfied the planning objective for all organs at risk. The different characteristics of patients prevent the possibility to present an average conclusion and therefore the analysis was done separately for all the cases. The results are displayed in Tables 3–12.
This study demonstrates that treatment techniques differ in terms of the trade-off between treatment planning time, treatment delivery time, and overall plan quality. It is true that IMRT treatment plans can be created in a much shorter period of time as compared to either Elekta VMAT or RapidArc plans, but has the lowest estimated treatment delivery time compared to IMRT. Also with respect to plan quality, RapidArc appears to meet the most dose–volume criteria and can, on average, produce plans with the most homogeneity within the target volume. The conformity indices show that RapidArc is better than Elekta VMAT, but the difference does not appear to be clinically significant. Both RapidArc and Elekta VMAT spare critical structures by redistributing the normal tissue dose to less critical regions and reducing the high dose volume to cover just the target. Elekta VMAT shows superiority in delivering less dose to healthy tissues particularly in the lower volumes. Significant reductions of dose to healthy tissues were observed with Elekta VMAT at V10 Gy, V5 Gy, V2 Gy. The reason may be the jaw tracking capability during delivery. This can also reduce the interleaf leakage in Elekta VMAT delivery. Also for beam collimation systems in which the jaws cannot track the MLC-shaped apertures, the gap between the closed opposing leaves outside the aperture gives additional leakage radiation. If the collimator angle is fixed at zero, leaving the gap parallel with the rotational axis, the leakage radiation from the gap will be focused on the rotational axis or form a cylindrical high dose shell around the rotational axis outside the target. One way to spread this leakage dose is to set the appropriate collimator angle during planning. So, all the RapidArc plans were generated by setting a collimator angle between 30° and 45°. The ability of rotating the collimator angle during delivery may increase the optimization freedom and produce a better plan quality as proposed by Zhang et al.19 Webb20 demonstrated that if the collimator angle is also included as an optimization parameter, additional freedom can be gained for improved deliverability, leading to a reduction in the number of parked gaps and unwanted leakage dose. The latest machine from Varian (True Beam) with the jaw tracking ability will be able to deliver lesser dose to the normal structures.
The intercomparison of data from different treatment planning systems may be stringent due to a number of complicating issues.15 A single arc delivered in less than 2 min may unduly compromise the plan quality for very complex cases and feel that the plan quality for Elekta VMAT and RapidArc are similar. For RapidArc, the estimated treatment delivery time is the sum of the time spent at each of the 177 segments within an arc. The time is the sum of all angular increments per segment divided by the gantry rotation rate. All the RapidArc cases in this study were planned using double arc. The use of two arcs was found to be necessary to achieve the high degree of conformal avoidance required by the planning objective. Adding a second arc provided the planning system with additional freedom for achieving better treatment plans.16 This is especially true for MLCs which cannot (geometrically) cover a large target with one dynamic sequence. For tumours that are not centrally located and surrounded by parallel organs, the use of partial arcs is often desirable. For tumours in the brain and in the head-and-neck region, it is often advantageous to use multiple non-coplanar arcs for better tumour targeting and critical structure avoidance. In principle, the more freedom we give to the planning system, the better quality of plans can be generated. However, with photon beams, there is a limit to the treatment plan quality. This limit is often not set by the degree of intensity modulation but by the physics of photon dose deposition. Also, it should be noted that there are many other issues in addition to plan quality that are associated with different delivery techniques. These include the efficiency of planning, delivery, quality assurance (QA), the complexity and reliability of delivery, and the total MUs required in delivering the prescribed doses and the total leakage radiation received by the patient outside the target region.
Both Elekta VMAT and RapidArc plans show clinically acceptable plan quality, but each technique has its advantages and shortcomings in different types of cases. The influence of more efficient modulated approaches such as VMAT on secondary cancer risk is not clear to date. But the improved treatment efficiency may reduce secondary malignancies due to less scatter dose from reducing the MUs. A different characteristic of the dose distribution with higher non-target tissue receiving 5–40 Gy with the VMAT technique may well increase the number of secondary malignancies.17 Duthoy et al. could perform VMAT for large volumes with four rotations in patients to undergo a whole abdomen and pelvic radiation therapy with a mean treatment time of 13.8 min with only 444 MU for a fraction dose of 1.5 Gy.10 The same author reported treatment time of 6.3 min (3–6 arcs and 456 MU) for 7 patients with rectal cancer at a prescription dose of 1.8 Gy.9 While no formal comparison with IMRT was made, these data implied the potential of the rotational approach. Both the Elekta VMAT and RapidArc algorithms used in this study are robust and can produce plans that can cover any complex and large targets with a high degree of conformity.
5. Conclusion
It is well known that planning comparisons such as our study are susceptible to bias. It should be stressed that this study will not give a general conclusion about superiority of one technique or the other, but results have to be interpreted in the context of the specific planning algorithm and study protocol. The study demonstrated that the variable gantry speed with variable dose rate is important for efficient arc therapy delivery. RapidArc plans had less estimated beam on time, higher conformity index and better homogeneity index. The mean dose to healthy tissue and integral dose reduced significantly with Elekta VMAT. The conclusions of this study are that the two techniques are practically equivalent. Trivial differences were noted in all the plans for organ at risk but the two techniques provided satisfactory conformal avoidance and conformation.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Yu C.X. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol. 1995;40:1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 2.Cameron C. Sweeping-window arc therapy: an implementation of rotational IMRT with automatic beam-weight calculation. Phys Med Biol. 2005;50:4317–4336. doi: 10.1088/0031-9155/50/18/006. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich S., Nill S., Oelfke U. Development of an optimization concept for arc-modulated cone beam therapy. Phys Med Biol. 2007;52:4099–4119. doi: 10.1088/0031-9155/52/14/006. [DOI] [PubMed] [Google Scholar]
- 4.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 5.Wang C., Luan S., Tang G. Arc-modulated radiation therapy (AMRT): a single-arc form of intensity-modulated arc therapy. Phys Med Biol. 2008;53:6291–6303. doi: 10.1088/0031-9155/53/22/002. [DOI] [PubMed] [Google Scholar]
- 6.Yu C.X., Amies C.J., Svatos M. Planning and delivery of intensity-modulated radiation therapy. Med Phys. 2008;35(12):5233–5241. doi: 10.1118/1.3002305. [DOI] [PubMed] [Google Scholar]
- 7.Veldeman L., Madani I., Hulstaert F., De Meerleer G., Mareel M., De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9(4):367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 8.Yu C.X., Li X.A., Ma L. Clinical implementation of intensity-modulated arc therapy. Int J Radiat Oncol Biol Phys. 2002;53(2):453–463. doi: 10.1016/s0360-3016(02)02777-3. [DOI] [PubMed] [Google Scholar]
- 9.Duthoy W., De Gersem W., Vergote K. Clinical implementation of intensity modulated arc therapy (IMAT) for rectal cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):794–806. doi: 10.1016/j.ijrobp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Duthoy W., De Gersem W., Vergote K. Whole abdominopelvic radiotherapy (WAPRT) using intensity modulated arc therapy (IMAT): first clinical experience. Int J Radiat Oncol Biol Phys. 2003;57(4):1019–1032. doi: 10.1016/s0360-3016(03)00663-1. [DOI] [PubMed] [Google Scholar]
- 11.Ling C.C., Zhang P., Archambault Y., Bocanek J., Tang G., Losasso T. Commissioning and quality assurance of RapidArc radiotherapy delivery system. Int J Radiat Oncol Biol Phys. 2008;72(2):575–581. doi: 10.1016/j.ijrobp.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 12.Vanetti E., Nicolini G., Clivio A., Fogliata A., Cozzi L. The impact of treatment couch modelling on RapidArc. Phys Med Biol. 2009;54(9):N157–N166. doi: 10.1088/0031-9155/54/9/N03. [DOI] [PubMed] [Google Scholar]
- 13.Elekta Inc. 2009. VMAT technology overview. Available from http://www.elekta.com/healthcare_us_vmat_technology__zoverview.php [cited 05.05.09] [Google Scholar]
- 14.Low D.A., Harms W.B., Mutic S., Purdy J.A. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25(5):656–661. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 15.Rosenow U.F. Quality assurance in radiation treatment planning. Principle considerations and a practical proposal. Rep Pract Oncol Radiother. 1996;1(2):72–78. [Google Scholar]
- 16.Ryczkowski A., Piotrowski T. Tomotherapy archive structure and new software tool for loading and advanced analysis of data contained in it. Rep Pract Oncol Radiother. 2011;16(2):58–64. doi: 10.1016/j.rpor.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall E., Wuu C.S. Radiation-induced secondary cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 18.Cotrutz C., Kappas C., Webb S. Intensity modulated arc therapy (IMAT) with centrally blocked rotational fields. Phys Med Biol. 2000;45:2185–2206. doi: 10.1088/0031-9155/45/8/309. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P., Happersett L., Yang Y., Yamada Y., Mageras G., hunt M. Optimization of collimator trajectory in volumetric modulated arc therapy: development and evaluation for paraspinal SBRT. Int J Radiat Oncol Biol Phys. 2010;77:591–599. doi: 10.1016/j.ijrobp.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Webb S. Does the option to rotate the Elekta Beam Modulator MLC during VMAT delivery confer advantage? – a study of ‘parked gaps’. Phys Med Biol. 2010;55:303–319. doi: 10.1088/0031-9155/55/11/N01. [DOI] [PubMed] [Google Scholar]


