Abstract
Acute radiation syndrome or acute radiation sickness is classically subdivided into three subsyndromes: the hematopoietic, gastrointestinal and neurovascular syndrome but many other tissues can be damaged. The time course and severity of clinical signs and symptoms are a function of the overall body volume irradiated, the inhomogeneity of dose exposure, the particle type, the absorbed dose and the dose rate. Classical pathophysiology explain the failure of each of these organs and the timing of appearance of their signs and symptoms due to radiation-induced cytocidal effects of a great number of parenchymal cells of hierarchically organized tissues. Contemporaneously, many other radiation-induced effects has been described and all of them may lead to tissue injury with their corresponding signs and symptoms that can be expressed after short or long period of time. Radiation-induced multi-organ involvement is thought to be due to radiation-induced systemic inflammatory response mediated by released pro-inflammatory cytokines.
Keywords: Radiobiology, Acute radiation syndrome, Hematopoietic syndrome, Gastrointestinal syndrome, Neurovascular syndrome, Radiation-induced multi-organ involvement, Radiation-induced multi-organ failure
1. Introduction
Acute radiation syndrome or acute radiation sickness is a term used to describe a constellation of signs and symptoms that occurs after whole-body or significant partial-body irradiation of certain amount of radiation (>0.5 Gy) delivered at high-dose rate. These signs and symptoms are the consequences of severe radiation damage to specific tissues of specific organs (mono-organ involvement) although many tissues can be damaged (multi-organ involvement). The first description of acute radiation syndrome was made by De-Coursey after atomic bomb explosions in Japan during World War II in 1945.1 Other sources of human data come from radiation accidents as Chernobyl nuclear power plant disaster as well as clinical data of patients undergoing total body irradiation as part of their cancer therapy. This syndrome differs from others radiation exposures as acute partial irradiation, chronic radiation syndrome or external and internal particle contamination. The purpose of this paper is to describe the underlying pathologic mechanisms that explain the clinical course of humans exposed acutely to a large single radiation exposure until recovery or death.
Classically, acute radiation syndrome is subdivided into three subsyndromes: the hematopoietic syndrome, the gastrointestinal syndrome and the neurovascular syndrome. Classical radiobiology explain the failure of each of these organs by radiation-induced death (cytocidal effects) of a great number of parenchymal cells (target cell theory) but today we know that radiation not only cause lethal effects but also functional and indirect effects in many cells (multi-cellular target theory).2 Contemporaneously, a new syndrome called radiation-induced multi-organ dysfunction is believed to be part of acute radiation syndrome. The pathophysiological mechanism of radiation induced multiple organ failure is not clear but evidence has accumulated suggesting a major role of damaged endothelial cells leading to a radiation-induced systemic inflammatory response syndrome mediated by the release of inflammatory cytokines.3,20
The time course and severity of clinical signs and symptoms are a function of the overall body volume irradiated, the inhomogeneity of dose exposure, the absorbed dose, the dose rate and the particle type. There are four clinical phases in the development of radiation sickness:
-
-
Prodromal phase: is the initial phase of acute illness. Signs and symptoms appear within 1–3 days after the exposure and are characterized by nausea, vomiting, anorexia, fever, headache and early skin erythema. Depending on the dose received these symptoms can be mild viral like or severe. The onset of vomiting is also related with absorbed dose and can be seen within few minutes after a high dose exposure.
-
-
Latent phase: is a delusive phase characterized by improvement of symptoms and an apparent cure. Individuals look and fell good but laboratory tests become abnormal with lymphopenia and granulocytopenia. This phase is also dose dependent and may last hours to weeks.
-
-
Manifest illness phase: in this phase specific signs and symptoms of each syndrome appear depending on the dose. The hematopoietic syndrome develops at doses of between 1 and 8 Gy although slight decrease in blood cell counts can be seen with doses below 1 Gy. The gastrointestinal syndrome occurs at doses of between 5 and 20 Gy and the cerebrovascular syndrome at doses higher than 20 Gy.
-
-
Final phase: recovery or death depending on the absorbed dose, dose rate and the heterogeneity of exposure.
A good summary of signs and symptoms and their correlation with absorbed doses has been published by the International Atomic Energy Agency (IAEA) and is reproduced in Tables 1–3.4 The time course and severity of clinical signs and symptoms are summarized in Table 4.5
Table 1.
Signs and symptoms of prodromal phase.4
| Signs and symptoms | Mild (1–2 Gy) | Moderate (2–4 Gy) | Severe (4–6 Gy) | Very severe (6–8 Gy) | Lethal (>8 Gy) |
|---|---|---|---|---|---|
| Vomiting Onset % of incidence |
≥2 h after exposure 10–50 |
1–2 h after exposure 70–90 |
<1 h after exposure 100 |
<30 min after exposure 100 |
<10 min after exposure 100 |
| Diarrhea Onset % of incidence |
None | None | Mild 3–8 h <10 |
Heavy 1–3 h >10 |
Heavy Within min 100 |
| Headache Onset % of incidence |
Slight | Mild | Moderate 4–24 h 50 |
Severe 3–4 h 80 |
Severe 1–2 h 80–90 |
| Consciousness Onset % of incidence |
Unaffected | Unaffected | Unaffected | May be altered | Unconsciousnes s/min 100 at >50 Gy |
| Body temperature Onset % of incidence |
Normal | Increased 1–3 h 10–80 |
Fever 1–2 h 80–100 |
High fever <1 h 100 |
High fever <1 h 100 |
Table 2.
Signs and symptoms of latent phase.4
| Signs and symptoms | Mild (1–2 Gy) | Moderate (2–4 Gy) | Severe (4–6 Gy) | Very severe (6–8 Gy) | Lethal (>8 Gy) |
|---|---|---|---|---|---|
| Latency period | 21–35 days | 18–28 days | 8–18 days | ≤7 days | None |
| Lymphocytes G/L (days 3–6) | 0.8–1.5 | 0.5–0.8 | 0.3–0.5 | 0.1–0.3 | 0.0–0.1 |
| Granulocytes G/L | >2.0 | 1.5–2.0 | 1.0–1.5 | ≤0.5 | ≤0.1 |
| Diarrhea | None | None | Rare | Appears on days 6–9 | Appears on days 4–5 |
| Depilation | None | Moderate, beginning on day 15 or later | Moderate, beginning on day 11–21 | Complete earlier than day 11 | Complete earlier than day 10 |
Table 3.
Signs and symptoms of critical phase.4
| Signs and symptoms | Mild (1–2 Gy) | Moderate (2–4 Gy) | Severe (4–6 Gy) | Very severe (6–8 Gy) | Lethal (>8 Gy) |
|---|---|---|---|---|---|
| Onset of symptoms | >30 days | 18–28 days | 8–18 days | <7 days | <3 days |
| Clinical manifestations | Fatigue, weakness | Fever, infections, weakness, depilation | High fever, infections, bleeding, depilation | High fever, diarrhea, vomiting, dizziness, desorientation, hypotension | High fever, diarrhea, unconsciousness |
| Lymphocytes G/L (days 3–6) | 0.8–1.5 | 0.5–0.8 | 0.3–0.5 | 0.1–0.3 | 0.0–0.1 |
| Platelets G/L | 60–100 | 30–60 | 25–35 | 15–25 | <20 |
| % of incidence | 10–25 | 25–40 | 40–80 | 60–80 | 80–100 |
| Lethality | 0% | 0–50% | 20–70% | 50–100% | 100% |
| Onset time | 6–8 week | 4–8 week | 1–2 week | 1–2 week |
Table 4.
The time course and severity of clinical signs and symptoms.
| Absorbed dose level | Prodromal phase | Latent phase | Manifest illness | Final phase |
|---|---|---|---|---|
| 0.5–1.5 Gy | Absence of symptoms or nausea and vomiting for 1 day | 1 day–several weeks | No symptoms or weakness, nausea and vomiting, temporary hair loss | Recovery |
| 1.5–4 Gy | Nausea, vomiting, fatigue, weakness, diarrhea for up to two days | 1–3 weeks | Hematopoietic syndrome (HS): leucopenia and trombocitopenia, hair loss | Recovery possible with supportive care |
| 4–6 Gy | Nausea, vomiting, weakness, diarrhea for up to two days | <1–3 weeks | HS: bleeding, immunosuppression and sepsis, permanent hair loss | Death without supportive care |
| 6–15 Gy | Severe nausea and vomiting, diarrhea in shorter period of time | Several days | HS + gastrointestinal syndrome: diarrhea, bleeding, fluid loss and electrolyte imbalance | Variable with supportive care |
| >15 Gy | Immediate severe nausea and vomiting | Non-existent | Neurovascular syndrome | Death within 48 h |
Modified from Ref. 5.
To understand the signs and symptoms due to parenchymal cell depletion caused by radiation (target cell theory) and their onset is useful to know how mammalian tissues are organized.
2. Type of organization of mammalian adult normal tissues
A tissue is a group of cells from the same origin that carry out a specific function. Mammalian adult normal tissues are populations of particular cell lineage (same type of cells) that maintain their number and have a particular type of organization. Under normal conditions, the number of cells is homeostatically controlled and well balanced between the production of news cells and the loss of adult mature cells dying after a finite lifespan. In contrast to tumors, physiologic growth or post-injury status, adult normal tissues are population of cells in equilibrium.
We can distinguish two models of tissue organization6:
-
-Hierarchical model organization (H-type model), also called tissues with rapid cell turnover or proliferative tissues. The main characteristic of this organization is the presence of several cell compartments (compartmental tissues)(Fig. 1a) where the tissue-specific function is restricted to mature non-proliferative cells. Three compartments can be distinguished:
-
•Stem cell compartment: is an small compartment composed by stem cells capable of unlimited divisions and a self-renewing capacity to maintain their number and provide cells to enter in the amplification compartment. These cells are incapable of tissue-specific function. The crypt cells in the intestinal epithelium, bone marrow stem cells or stem cells in the basal layer of the epidermis are examples of this compartment.
-
•Differentiating compartment or amplification compartment: in this compartment, the descendants of the stem cells are in the process of maturation. They are actively proliferating and differentiating. They are acquiring functional capacity while their proliferation becomes increasingly limited (subclonogenicity) and they are the precursors of mature cells. Cells of the basal layer of epidermis and erythroblasts are examples of this compartment.Both compartments are constituted by proliferating cells, stem cells more slowly than precursors cells.
-
•Post-mitotic compartment or mature functioning compartment: is a compartment composed by mature, fully differentiated and functional competent cells. They are not able to divide and give the function of the tissue. After their lifespan, variable between different tissues, they die. Examples of this compartment are the cells in the surface layers of epidermis, cells at the top of villi of intestinal mucosa or mature circulating blood cells.
-
•
Cell depletion caused by any kind of injury will be compensated by triggering the surviving stem cells to proliferate quickly. This phenomenon is known as compensatory repopulation or accelerated proliferation (Fig. 1b).
Fig. 1.
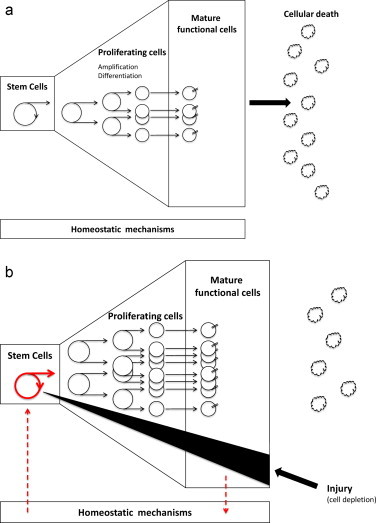
(a) Hierarchical model of tissue organization. (b) Response of hierarchical model of tissue organization in front of a cell depletion caused by injury.
Examples of hierarchically organized tissues are the haematopoietic tissue, buco-pharyngeal and intestinal mucosa, testicular epithelium and epidermis. These tissues have a rapid cell turnover, for example the turnover of epidermis is nearly three weeks and three days for intestinal mucosa. The average life of granulocytes is less than one day, 8–10 days for platelets and 120 days for erythrocytes.
Flexible model organization (F-type model), also called tissues with slow cell turnover. They have no compartments (mono-compartmental tissues) and no hierarchy (Fig. 2a). The tissue is composed of identical cells with tissue-specific function and clonogenicity (capacity for cell renewal). The archetype of non-hierarchically organized tissue are the hepatocytes. All of them are functionally competent, the majority residing in phase G0 although they retain their proliferative potential. These tissues have a slow cell turnover, the average life of hepatocytes is one year.
Fig. 2.
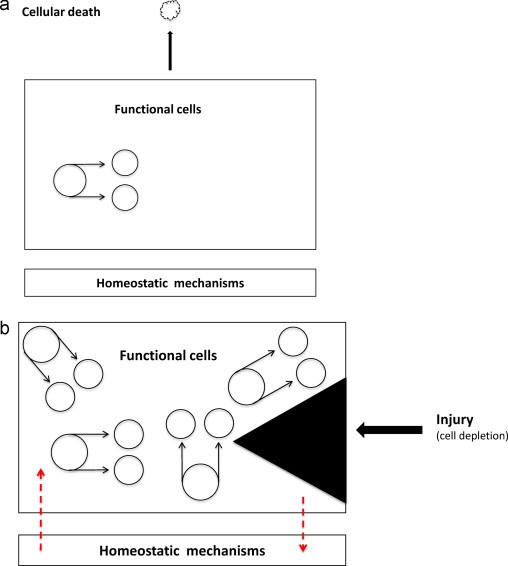
(a) Flexible model of tissue organization. (b) Response of flexible model of tissue organization in front of a cell depletion caused by injury.
Cell depletion caused by any kind of injury as after partial hepatectomy, will be compensated by triggering the surviving functional cells to proliferate parasynchronously faster (accelerated rate of proliferation) (Fig. 2b).
The differential characteristics of the two models are shown in Table 5. It has been proposed that these two models are extreme ends of a spectrum of proliferative tissues and that some real tissues may be more appropriately represented by hybrid models incorporating some properties of each of the two models.7 In any case, the distinction between these two models is useful to understand the differences in the radio-response.
Table 5.
Differences between H-type and F-type models of normal tissues and their response to irradiation.6
| Properties | H-type model | F-type model |
|---|---|---|
| Proliferative capacity of functional cells | None | Infinite |
| Physiological rate of turnover of functional cells | Rapid | Slow |
| Time-scale of expression of radiation injury | Early | Late |
| Time onset of functional damage | Dose-independent | Dose-dependent |
| Dose-response relationship | Short shoulder | Large shoulder |
| α/β ratio (Gy) | Large (∼10–15 Gy) | Short (∼2–3 Gy) |
3. Target cell theory
The historical mechanistic model of injury is that radiation-induced normal tissue damage consists of two distinct and separate phases, an acute and a late phase each due to the depletion of different target cell populations. Early or acute effects result from the death of a large number of proliferating cells hierarchically organized and delayed or late effects result from the death of a large number of parenchymal cells organized in a flexible manner. As the most important mode of cell death following irradiation is mitotic cell death that results from DNA damage, the time between irradiation and manifestation of injury (latency interval) will depend on target cell characteristics and tissue organization.
Hierarchically organized tissues composed by proliferating cells with rapid cell turnover will express their radiation damage early dying in mitosis within a few days. Mature functional cell of this kind of tissues are non-proliferating, not cycling, then they will remain relatively unaffected by radiation because they cannot die in mitosis. After their lifespan, they will die at their physiologic rate. For this reason, the rate of radiation-induced depopulation of functional cells is largely dose-independent. In summary, irradiation of hierarchical tissues depletes stem and progenitor proliferating cells but has not direct effect on mature functional competent cells. Tissue injury and the development of signs and symptoms will result from the failure of proliferating cells to replenish the post-mitotic compartment of mature cell which continue to be lost at the normal physiologic rate. The intensity of signs and symptoms is related with the absorbed dose and the number of stem cells killed but the time of onset of these reactions correlates with the lifespan of mature cells and the latent period is predictable and short (Fig. 3).
Fig. 3.
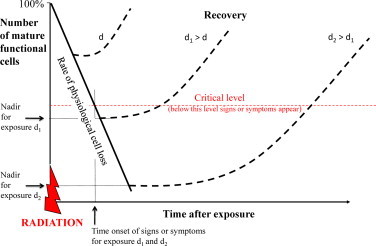
Graph showing the dose-independence of onset of signs and symptoms and the dose-dependence of their severity.
On the other hand, non-hierarchically organized or flexible tissues are composed of functional slowly proliferating cells with slow cell turnover. The expression of injury caused by mitotic cell death and the development of signs and symptoms is dose-dependent and the latency period is long of months or years.
4. Hematopoietic syndrome
Hematopoietic stem cell and progenitors cells of the bone marrow that are rapidly dividing cells (stem cell compartment and differentiating compartment) are highly sensitive to the effects of ionizing radiation. Animal studies indicate that hematopoietic stem cells have a D0 of about 0.95 Gy.8 It means that a dose of 0.95 Gy reduces the population of stem cells to 37%. For this reason, hematopoietic syndrome is seen with radiation exposures exceeding 1 Gy. At doses below 1 Gy, surviving proliferating cells (through accelerated proliferation) will be able to replenish mature functioning compartment and only an insignificant clinical decrease in blood cell counts can be seen. As the absorbed dose increases, more and more hematopoietic stem cell and precursors cells will be killed and only a few cells or no cells will enter in post-mitotic compartment. Mature circulating cells unaffected by radiation will die after their lifespan at their physiological rate. The onset of signs and symptoms will appear depending on the physiological cellular loss rate of circulating cells and the dose-dependent reduced supply of mature cells from the depleted proliferating compartments. The balance between these two phenomena results in different degree of pancytopenia with predisposition to infection due to leucopenia and bleeding due to thrombocytopenia. The severity of signs and symptoms (hypoplasia or aplasia of the bone marrow) and the probability to recover will depend on the absorbed dose, the dose rate and the overall bone marrow volume irradiated. If no regeneration, death usually occurs due to infection and/or hemorrhage at doses of 4.5–6 Gy without supportive care.
Lymphopenia resulting from radiation-induced apoptosis occurs before the onset of other cytopenias. It can be seen within the first 6–24 h after a moderate or high dose exposure. The rate of lymphocytic depletion and its nadir is dose-dependent and can be predicted. This predictability has led to the development of models using lymphocyte depletion kinetics as an element of biodosimetry if absorbed dose is unknown.9 But, as radiation also perturbs recirculation properties of lymphocytes, their decline cannot be taken as an indicator of the extent of stem cell damage.10
The onset time and the duration of the nadir of other cytopenias is also dose-dependent. Granulocytes count may transiently increase due to the release of mature cells (not affected by radiation) stored in the bone marrow into the blood. This transient increase called abortive rise or stress reaction may be clinical useful because its prognostic significance.11 After their lifespan of about 7–24 h, granulocytes disappear from the blood and are replaced in different degrees depending on the absorbed dose and the number of few injured or intact stem cells. The higher the dose, the earlier disappearance of granulocytes.
Circulating platelets that have a lifespan of about 10 days disappear progressively from the blood within this period of time and are replaced in different degree or not depending on the level of damaged stem cells.
Analyzing the early blood cell change patterns (mainly granulocytes and platelets) after an unknown dose exposition, biomathematical models has been developed to predict the effect of radiation on the hematopoietic stem cell pool (possibility of a reversible damage to hematopoiesis or a complete and irreversible damage of the stem cell pool).12
Anaemia not due to acute hemorrhage is an unusual finding because mature red blood cells have a long lifespan of about four months.
Flt-3 ligand (FL) is a cytokine that acts alone or in combination with other cytokines such as Stem Cell Factor mainly on stems cell and progenitor cells regulating cell differentiation and proliferation (homeostatic mechanism).13 High levels of FL has been found in patients with aplastic anaemia as well as in patients undergoing chemo and radiotherapy for bone marrow transplantation. It was hypothesized that plasma levels of FL might be used as an indicator of aplasia after radiation exposure. Bertho et al. showed an increase in plasma FL concentration as early as day 2 after whole or partial body irradiation in non-human primates. Increase in plasma FL concentration on day 5 post-exposure was correlated with radiation dose and with the severity of radiation-induced aplasia. Subsequent decrease in FL concentration was also correlated with the recovery of blood cells population suggesting that monitoring plasma levels of FL can be used as a bioindicator of bone marrow function.14 These findings were confirmed in patients undergoing chemo and radiotherapy as a conditioning regimen for stem cell transplantation15 and used to assess the severity of radiation-induced damage in a recent accidental exposure.16
5. Gastrointestinal syndrome
Gastrointestinal syndrome occurs at doses of between 6 and 15 Gy. Clinical signs and symptoms are due to the lack of replacement of cells in the surface of the villi because stem and proliferating cells located in the crypts are damaged by radiation and die in mitosis. Between 7 and 10 days after exposure, the denudation of intestinal mucosa produces watery diarrhea, dehydratation and electrolyte loss, gastrointestinal bleeding and perforation. The breakdown of the mucosal barrier facilitates the entry of bacterias into the bloodstream. Of course, the immunosuppression associated with the hematopoietic syndrome favours opportunistic infections and thrombocytopenia favours hemorrhage. Death from the gastrointestinal syndrome is due to sepsis, bleeding, dehydratation and multisystem organ failure.
Recently it has been shown that plasma citrulline, a nonprotein amino acid product of glutamine metabolism in small bowell enterocytes, is a reliable marker of anatomic enterocyte mass.17 Radiation-induced enterocyte damage and the consequently reduction in small-bowell epithelial cell mass can decrease citrulline concentration. Citrulline concentration significantly decrease as a function of the radiation dose and the volume of small bowel treated and it has been proposed as a biomarker for radiation-induced small-intestinal mucosal damage.18
6. Neurovascular syndrome
The exact pathobiology of cerebrovascular syndrome is by no means clear. A hypothesis is the damaging effects of radiation on endothelial cells and vascular leak with edema and consequently an increase in intracranial pressure. Cerebrovascular syndrome occurs at doses higher than 20 Gy and is characterized by a very short prodromal and latent phases followed by neurological symptoms as headache, abnormal cognition, neurological deficits and finally somnolent state, loss of consciousness and death.
7. Other signs and symptoms
The exact mechanism of radiation-induced nausea and vomiting is poorly understood. It is believed that enterochromaffin-like cells of the gastrointestinal tract release 5-hydroxytryptamine (5-HT) also called serotonine in response to radiation exposure and serotonine acts on the 5-HT receptor cells stimulating the medullary vomiting center. Serotonine can also be released after radiation exposure from the chemoreceptor trigger zone located in the area postrema at the base of the fourth ventricle.
An indirect proof is the significant benefit obtained with 5-hydroxytryptamine receptor antagonists in the prevention of nausea and emesis of patients treated with external beam radiation therapy on the upper abdomen or total-body irradiation.19,20 The estimate dose threshold for vomiting at the prodromal phase is 1.5 Gy and the time of onset of vomiting is highly dependent on absorbed dose of acute external exposure with a linear correlation (Table 1).21 The higher the dose, the earlier onset of vomiting.
A cutaneous syndrome can be seen in the form of erythema at doses around 3 Gy. At higher doses basal layer stem cells are sterilized dying by mitotic death and no cells will enter in the differentiating compartment. Mature cells dying after their lifespan of around three weeks will be not replaced by new cells and clinical manifestations will appear at this time. Moist desquamation of the skin with pruritus, blisters, bullae and ulceration can be seen.
Moderate depilation due to radiation damage to hair follicle will appear within two weeks after an exposure of around 4 Gy but can be complete and earlier than 10 days for doses higher than 6–8 Gy.
Parotiditis with gland tumefaction and slight pain can be seen after low exposures of 2.00 Gy. Radiation causes apoptotic cell death of serous acinar cells in salivary glands causing acute xerostomia. These damaged cells release their intracellular amylase into the bloodstream. Changes in the serum amylase following irradiation of the salivary glands was first described by Kasima in 1965 showing a maximal increase in amylasemia 24 h after irradiation depending on dose and volume of gland irradiated.22 It has been shown a sigmoid relationship between dose to parotids and hyperamylasemia with no significant increase in serum enzyme levels for doses lower than 1 Gy and a maximum level between doses of 4 and 10 Gy.23 However, a wide interindividual variability precludes its use as a biological dosimeter.
8. Multi-cellular target theory
Old thinking about the physiopathology of normal tissue radiation toxicity has changed in the last years because our better understanding of radiation injury mechanisms at a molecular level. At present it is believed that normal tissue response to radiation is an integrated response involving cell death (target cell theory) but also the production of cytokines, reactive oxygen species and alterations in gene expression of many cells. A part from cytocidal effects, radiation can produce indirect and functional effects. Examples of indirect effect are the bystander effect and the secretion of pro-inflammatory cytokines that can induce an inflammatory response. For instance, endothelial radiation-induced dysfunction can produce leucocyte adhesion and chemotaxis with cytokine production and reactive oxygen species generation. These changes produce an oxidative imbalance with hypercoagulability, leucocyte chemotaxis and again cytokine production leading to an inflammatory response. Functional effects are non-lethal effects on different intracelular and extracelular molecules and changes in gene expression in irradiated cells. Examples of functional effects are the activation of proteases or activation of latent growth factors.2 All these effects may lead to tissue injury with their corresponding signs and symptoms that can be expressed after short or long period of time. This biological complexity means that initial radiation-induced damage, namely cytocidal, indirect or functional effects is a continuum complex of events that can be manifested in different ways and at different times.
9. Radiation-induced multi-organ dysfunction (failure) syndrome
The dogma that failure of a single critical organ explains the pathophysiology of acute radiation syndrome is today outdated. Recent advances in the study of clinical case histories of patients involved in different radiation accidents occurred between 1945 and 2000 showed that a part from classical syndromes, other organs systems can be involved. In particular signs or symptoms due to cardiovascular, respiratory, liver and urogenital damage can also be seen. These observations leads to the concept of radiation-induced multi-organ involvement (RI-MOV) and multi-organ failure (RI-MOF).3,24,25 The hypothesis is that the symptomatology of organ system involvement is not only due to the radiation-induced depletion of proliferating cells of rapid turnover tissues but also due to radiation-induced changes in the vascular system and specifically in the endothelial cells leading the development of an uncontrolled systemic inflammatory response. Moreover, the last two reported examples of large-field high-dose accidental irradiation in Tokai-mura and Nesvizh showed that after appropriate and extensive treatment, it is possible to “bridge” the acute phase of radiation-induced hematopoietic and gastrointestinal syndromes but sequentially new clinical findings involving other organs and cell system appear.26,27 For instance, it appears that cytokines play a central role in mediating central nervous system response following irradiation. It has been shown that radiation response of central nervous system is characterized by local production of pro-inflammatory cytokines in different brain structures causing a stimulation of inflammatory cascade, interaction with other inflammatory mediators and upregulation of the inflammatory process that leads to neurotoxicity.28 In the same way, radiation-induced endothelial dysfunction can cause increased permeability, endothelial cell apoptosis, coagulation disorders, the expression of adhesion molecules, inflammatory cytokines and chemokines with transmigration of leukocytes and the release of proteases and reactive oxygen species that can contribute to tissue injury.29 These alterations in endothelial cell integrity and function could play a critical role in mediating organ dysfunction after acute radiation exposure. An extensive review of the hypothetical pathways involved in the initiation and development of radiation-induced multi-organ dysfunction syndrome has been recently published and is highly recommended.30
References
- 1.De-Coursey E. Human pathological anatomy of ionizing radiation effects of the atomic bomb explosions. Mil Surg. 1948;102:427–432. [PubMed] [Google Scholar]
- 2.Denham J.W., Hauer-Jensen M., Peters L.J. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001;50:1105–1106. doi: 10.1016/s0360-3016(01)01556-5. [DOI] [PubMed] [Google Scholar]
- 3.Gourmelon P., Benderitter M., Bertho J.M. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98:825–832. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- 4.International Atomic Energy Agency . IAEA; Vienna: 1998. Diagnosis and treatment of radiation injuries. Safety reports series 2. [Google Scholar]
- 5.Pedigo T. Mosby/Jems; 2005. Radiological weapons in Currance PL. Medical response to weapons of mass destruction. [Google Scholar]
- 6.Michalowski A. Effects of radiation on normal tissues: hypothetical mechanisms and limitations of in situ assays of clonogenicity. Radiat Environ Biophys. 1981;19(3):157–172. doi: 10.1007/BF01324183. [DOI] [PubMed] [Google Scholar]
- 7.Wheldon T.E., Michalowski A.S. Alternative models for the proliferative structure of normal tissues and their response to irradiation. Br J Cancer. 1986;53:382–385. [PMC free article] [PubMed] [Google Scholar]
- 8.Till J.E., McCulloch E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 9.Goans R.E., Holloway E.C., Berger M.E., Ricks R.C. Early dose assessment following severe radiation accidents. Health Phys. 1997;72:513–518. doi: 10.1097/00004032-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fliedner T.M. Nuclear terrorism: the role of hematology in coping with its health consequences. Curr Opin Hematol. 2006;13:436–444. doi: 10.1097/01.moh.0000245696.77758.e6. [DOI] [PubMed] [Google Scholar]
- 11.Dainiak N., Waselenko J.K., Armitage J.O. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 12.Fliedner T.M., Graessle D., Meineke V., Dörr H. Pathophysiological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: an essential basis for an evidence-based clinical triage. Exp Hematol. 2007;35:8–16. doi: 10.1016/j.exphem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Lyman S.D., James L., Johnson L. Cloning of the human homologue of the murine Flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 14.Bertho J.M., Demarquay C., Frick J. Level of Flt3-ligand in plasma: a possible new bio-indicator for radiation-induced aplasia. Int J Radiat Biol. 2001;77:703–712. doi: 10.1080/09553000110043711. [DOI] [PubMed] [Google Scholar]
- 15.Prat M., Frick J., Laporte J.P. Kinetics of plasma FLT3 ligand concentration in hematopoietic stem cell transplanted patients. Leuk Lymph. 2006;47:77–80. doi: 10.1080/10428190500175122. [DOI] [PubMed] [Google Scholar]
- 16.Bertho J.M., Roy L. A rapid multiparametric method for victim triage in cases of accidental protracted irradiation or delayed analysis. BJR. 2009;82:764–770. doi: 10.1259/bjr/49063618. [DOI] [PubMed] [Google Scholar]
- 17.Crenn P., Vahedi A., Lavergne-Slove L. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowell disease. Gastroenterology. 2003;124:1210–1219. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 18.Lutgens L.C., Deutz N., Granzier-Peeters R. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer T.R., Friedman C.J., Bushnell W. Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Transplant. 2000;26:203–210. doi: 10.1038/sj.bmt.1702479. [DOI] [PubMed] [Google Scholar]
- 20.Salvo N, Doble B, Khan L, et al. Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: a systematic review of randomized trials. Int J Radiat Oncol Biol Phys, in press. [DOI] [PubMed]
- 21.Osovets S.V., Azizova T.V., Day R.D. Assessment of risks and dose thresholds for some effects of acute exposure. Health Phys. 2011;100:176–184. doi: 10.1097/HP.0b013e3181ebd123. [DOI] [PubMed] [Google Scholar]
- 22.Kashima H.K., Kirkham W.R., Andrews J.R. Post-irradiation sialadenitis: a study of the clinical features, histopathologic changes and serum enzyme variations following irradiation of human salivary glands. Am J Roentgenol. 1965;94:271–291. [Google Scholar]
- 23.Dubray B., Girinsky T., Thames H.D. Post-irradiation hyperamylasemia as a biological dosimeter. Radiot Oncol. 1992;24:21–26. doi: 10.1016/0167-8140(92)90349-y. [DOI] [PubMed] [Google Scholar]
- 24.Fliedner T.M., Dörr H.D., Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR Suppl. 2005;27:1–8. [Google Scholar]
- 25.Fliedner T.M., Chao N.J., Bader J.L. Stem cells, multiorgan failure in radiation emergency medical preparedness: a U.S./European consultation workshop. Stem Cells. 2009;27:1205–1211. doi: 10.1002/stem.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HiramaT, Akashi M. Multi-organ involvement in the patients who survived the Tokai-mura criticality accident. BJR Suppl. 2005;27:17–20. [Google Scholar]
- 27.Baranov A.E., Selidovkin G.D., Butturini A., Gale R.P. Hematopoietic recovery after 10-Gy acute total body irradiation. Blood. 1994;83:596–599. [PubMed] [Google Scholar]
- 28.Gourmelon P., Marquette C., Agay D. Involvement of the central nervous system in radiation-induced multi-organ dysfunction and/or failure. BJR Suppl. 2005;27:62–68. [Google Scholar]
- 29.Gaugler M.H. A unifying system: does the vascular endothelium have a role to play in multi-organ failure following radiation exposure? BJR Suppl. 2005;27:100–105. [Google Scholar]
- 30.Williams JP and Mcbride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol, in press; available online. [DOI] [PMC free article] [PubMed]


