Abstract
Whole breast irradiation represents an integral part of combined breast-conserving treatment of early breast cancer. A new concept includes replacing traditionally fractionated whole breast postoperative radiotherapy by accelerated partial breast irradiation. The latter involves a variety of techniques and may be applied intraoperatively or shortly after the surgery. The intraoperative techniques include photon or electron external beam irradiation and interstitial high dose rate (HDR) brachytherapy, whereas the postoperative techniques comprise interstitial brachytherapy, be it HDR, pulse dose rate (PDR) or low dose rate (LDR), intracavitary brachytherapy and external beam radiotherapy using electrons, photons or protons. This article presents accelerated partial breast irradiation techniques, ongoing phase III trials evaluating their value and recommendations for clinical practice.
Keywords: Breast cancer, Adjuvant radiotherapy, Whole breast irradiation, Accelerated partial breast irradiation
1. Background
Whole breast irradiation (WBI) represents an integral part of combined breast-conserving treatment of early breast cancer. The outcome of this procedure is similar to that of radical mastectomy.1–6 Postoperative radiotherapy reduces the risk of local recurrence, thus enhancing the chance for breast conservation, and increases the rate of 10-year survival by 5%.6 Conventionally fractionated radiotherapy usually lasts 5–7 weeks, which is associated with prolonged hospitalisation or the necessity of reporting at hospital for regular procedures. Radiotherapy following breast conserving surgery is often delayed, which may deteriorate treatment outcome.7–10 Accelerated partial breast irradiation (APBI) may be an alternative for a standard postoperative radiotherapy. The method involves a delivery of a radiation dose within a shortened period of time and to a limited area of the breast including the tumour bed with a margin. The fraction size is higher than with standard treatment and the entire therapy comprises usually 1–10 fractions administered over 1–5 days. Potential benefits of APBI include reduction of treatment costs, saving of time, shorter radiotherapy waiting lists, convenience for patients, less adverse effects and better quality of life.
The concept of APBI is based on the observation that most local recurrences tend to occur in a close proximity of the tumour bed and the probability of developing new cancer foci far from the tumour bed is the same for WBI and non-WBI patients. Furthermore, in patients treated with WBI, the likelihood of developing cancer at a remote site of the irradiated breast is similar to that in the contralateral breast.2,6,11–13
Early attempts at the application of ABPI using external beam radiotherapy were discouraging. The rates of local recurrence following the use of this technique were twice as high as those seen after WBI.14,15 Such a poor outcome might have been caused by inadequate patient selection, suboptimal surgery and imprecise radiotherapy treatment planning. Results of further studies on APBI free of these shortcomings were more promising.16,17 Note was taken, for example, that surgery should obligatorily be preceded by mammography. Additionally, histological type and size of tumour were taken into account in patient selection, as was an appropriate tumour-free margin. More emphasis was also put on precise radiotherapy treatment planning.
2. APBI techniques
2.1. External beam intraoperative radiotherapy (IORT)
Intraoperative radiotherapy involves a delivery of a single high dose of photons or electrons to a target volume identified during a surgical procedure. To this end, dedicated applicators are inserted into the excision cavity.
Electron beam intra-operative radiotherapy (ELIOT) uses electron beam with energy of 3–12 MeV to deliver a dose of 21 Gy prescribed to the 90% isodose at the depth of 1.5–3 cm (Figs. 1 and 2). Prior to radiotherapy, a shield is placed on the chest wall to protect underlying tissues. The European Institute of Oncology in Milan has the longest experience in this technique, as it has used it since 1999.18 Recently, results have been reported for 1822 patients treated with APBI between 2000 and 2008. The most common adverse effects were fat necrosis and fibrosis, which occurred in 4.2% and 1.8% of patients, respectively. After the median follow-up of 36.1 months, the rates of local recurrence, new primary cancer foci in the irradiated breast and distant metastases were 2.3%, 1.3% and 1.4%, respectively. The rates of 5- and 10-year overall survival were 97.4% and 89.7%, respectively.19
Fig. 1.
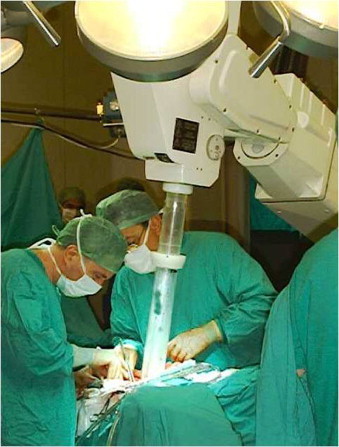
Insertion of the ELIOT applicator into the excision cavity during breast conserving surgery.
Courtesy: Prof. Roberto Orecchia.
Fig. 2.
Electron beam intra-operative radiotherapy.
Courtesy: Prof. Roberto Orecchia.
The other currently used IORT technique is Targeted Intra-Operative Radiotherapy (TARGIT) (Figs. 3 and 4). This modality includes insertion of a spherical applicator into a lumpectomy bed and delivery of 50 kV photon radiation. The size of the applicator depends on the bed volume. With the most commonly used technique, a dose of 20 Gy is achieved at 2 mm from the surface of the applicator, falling rapidly to 5 Gy at 1 cm from the surface. Irradiation is given over 20–25 min.20
Fig. 3.
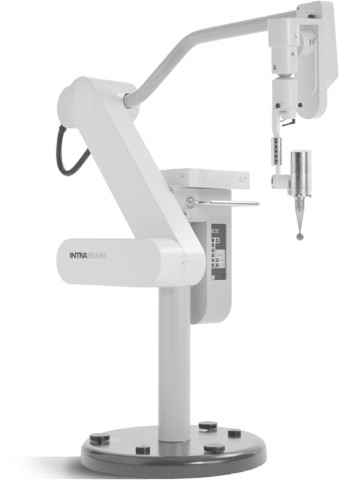
Mobile intraoperative radiation device (Intrabeam, ZEISS Corporation, Germany).
Courtesy: Prof. Michael Baum.
Fig. 4.
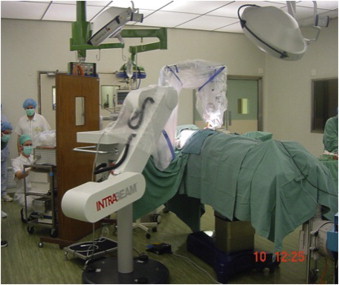
Targeted Intra-Operative Radiotherapy (TARGIT).
Courtesy: Prof. Michael Baum.
External beam IORT offers the benefit of delivering radiotherapy exactly to the target volume with little risk of geographical miss, effective protection of healthy tissues, convenience of use and shortening of total treatment time by virtue of the application of just one radiotherapy fraction. Limitations of this modality include the absence of final histopathological report at the time of radiotherapy, patient's longer stay at the operation theatre under general anaesthesia and the potential risk of late complications related to the administration of single high-dose radiation.
2.2. Interstitial brachytherapy
Interstitial brachytherapy resembles the method routinely used to boost a dose for the tumour bed after WBI. Several catheters, the number of which depends on the size and shape of the site left after an excised tumour, are inserted into the tumour bed under general anaesthesia (Fig. 5). Then, a target volume is set to include the bed and a margin of 1–2 cm. An optimal dose distribution is obtained by establishing the dwell positions for radioactive seed in particular catheters placed in the breast. Treatment is carried out using the afterloading technique (HDRs—high dose rates, PDRs—pulse dose rates, LDRs—low dose rates). The most commonly used fractionation schedule in APBI employing 192Ir HDR unit involves the administration of 34 Gy in 10 fractions over 5 days (2 fractions a day given at intervals of at least 6 h). The technique allows a good control of doses delivered to the skin and enables the shape of a reference isodose to be adjusted to the shape of the tumour bed.21 This modality, however, requires high skills and experience from the radiation oncologist. If postponed until after the surgery, it may necessitate another procedure in general anaesthesia. Another limitation is related to inhomogeneous dose distribution in the implant due to the presence of “hot spots”. The results of phase I and II trials using APBI interstitial brachytherapy are presented in Table 1.
Fig. 5.
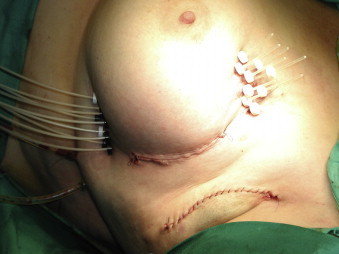
Flexible plastic catheters inserted into the tumour bed.
Table 1.
Recent studies of APBI with interstitial brachytherapy.
| Author (Ref.) | No. of patients | Median follow-up (months) | RT technique and dose | In-breast recurrence | Cosmesis good/excellent | Grade 3/4 toxicity |
|---|---|---|---|---|---|---|
| Arthur22 | 99 | 84 | LDR: 45 Gy/3.5–5 days HDR: 34 Gy/10 bid/5 days |
4% at 5 years | NR | 18% for LDR; 4% for HDR |
| Polgar23 | 45 | 133 | HDR: 30.3–36.4 Gy/7fr/4 days | 9.3% at 12 years | 78% | 1 G3 fibrosis 1 fat necrosis |
| King24 | 51 | 75 | LDR: 45 Gy/4 days HDR: 32 Gy/8 bid/4 days |
1.9% | 75% | 8% |
| Vicini25 | 199 | 65 | LDR: 50 Gy/4 days HDR: 32–34 Gy/8–10 bid |
1% at 5 years | 99% | 0 |
Abbreviations: bid, twice a day; HDR, high dose radiotherapy; LDR, low dose radiotherapy; NR, not reported; RT, radiotherapy.
2.3. Intracavitary brachytherapy
MammoSite is the most commonly used device for intracavitary brachytherapy. It is a silicone balloon with a centrally placed catheter holding a source of HDR radiation (Fig. 6). The balloon is inserted into the tumour bed intraoperatively or under USG guidance a few days after the surgery (Fig. 7). Having been placed in the bed, the balloon is inflated with a saline solution and a contrast agent to fill up the whole tumour cavity. The minimum acceptable distance between the balloon and the skin is 5 mm, and the recommended distance is 7 mm. Reducing the skin spacing substantially increases the risk of worse cosmetic effects.26 The volume to be irradiated covers the tumour bed with a margin of 1–1.5 cm and the duration of the therapy is approximately 1 week. The dose is fractionated as with interstitial HDR brachytherapy, i.e. 34 Gy in 10 fractions over 5 days. The method is simple, reproducible, fast to learn and easy to plan. It is less invasive than interstitial brachytherapy. However, the dose cannot be redistributed to fit possible irregularities of the tumour bed or reduced for the adjacent skin and chest wall. Additionally, the cost of the device is relatively high. Women with small breasts may find this method more painful and toxic than those with bigger breasts.
Fig. 6.

MammoSite brachytherapy device. (Hologic, Inc.)
Fig. 7.
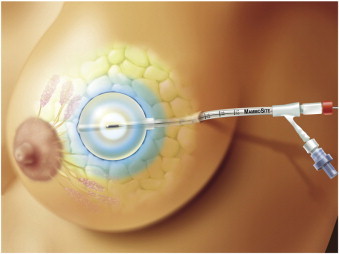
Illustration of MammoSite balloon inside tumour resection cavity.
So far, results of using MammoSite have been promising, with low rates of local recurrence (0–5.7%); however, the short follow-up periods of 7–65 months call for some caution. The cosmetic outcome is similar to that with WBI or PBI using interstitial brachytherapy.27 Most common adverse effects include erythema, dry and moist desquamation, telangiectasias, fat necrosis and development of seroma.
2.4. Postoperative external beam radiotherapy
APBI may be performed by postoperative irradiation of the tumour bed with external beam. Treatment may include multi-field 3D conformal techniques, intensity modulated radiation therapy (IMRT) or proton beam. The benefits of this modality include availability of final histopathological report at therapy onset, use of standard radiotherapy techniques, possibility to use widely available therapy planning and delivery equipment, and low invasiveness. As compared to the other APBI techniques, external beam radiotherapy offers a very homogeneous dose distribution. On the other hand, an irradiated volume has to be larger, as it encompasses the tumour bed along with a margin to allow for respiratory mobility and possible differences in patient setup at particular radiotherapy fractions.28
Current experience with this method is based on small-sized studies performed in single institutions. Although the results seem to be promising, typical follow-up periods of 10–18 months are too short for a reliable evaluation of this procedure.29–31
3. Phase III trials comparing WBI and APBI
So far, results of only two prospective randomised studies comparing WBI and APBI have been published.32,33 The study carried out by the National Institute of Oncology in Hungary included 258 patients who underwent breast-conserving surgery and had negative margins of T1 N0-1mi breast cancer.32 The patients assigned to PBI arm received interstitial HDR brachytherapy at a dose of 36.4 Gy in 7 fractions or limited-field electron beam irradiation at a dose of 50 Gy in 25 fractions. Standard arm included conventionally fractionated WBI (50 Gy in 25 fractions). The 5-year local recurrence rates with PBI and WBI were 4.7% and 3.4%, respectively (insignificant difference). There were also no differences for overall survival and disease-free survival. A very good cosmetic result was achieved in 77.6% and 62.9% patients treated with PBI and WBI, respectively (p = 0.009).
Recently, results of TARGIT-A, a multicentre, international trial, were presented.33 This study included 2232 patients aged 45 and older with early breast cancer. After having undergone breast-conserving surgery, the patients were randomly assigned to receive either intraoperative PBI of 20 Gy on a spherical applicator surface or WBI of 40–56 Gy with or without a boost to the tumour bed of 10–16 Gy (depending on the centre's decision). The PBI group patients with lobular carcinoma, extensive intraductal component or other factors considered adverse by a treating centre (e.g., G3, pN, LVI), were subsequently administered additional WBI. After a median follow-up of 4 years, the rates of local recurrence in the group treated with PBI and WBI were respectively 1.2% and 0.95% (p = 0.41). Radiotherapy toxicity (RTOG grade 3 and 4) in the PBI and WBI groups occurred in 0.5% and 2.1% of patients, respectively (p = 0.002), while seroma requiring more than three aspirations occurred in 2.1% and 0.8% of patients, respectively (p = 0.012). There was no difference between the groups in the incidence of haematoma, delayed wound healing or infections.
4. Ongoing studies
At present, several randomised studies are being conducted to compare treatment results using WBI and APBI in early-stage breast cancer patients after breast conserving surgery.33–41 Main inclusion criteria and treatment methods are shown in Table 2. Particular studies differ considerably in terms of radiotherapy techniques, dose fractionation and planning target volume.42 The trials also vary in patient selection with regard to age, histological type of cancer and presence of metastases to axillary lymph nodes. These differences may hinder interstudy comparison and metaanalyses.
Table 2.
Randomised trials comparing APBI and WBI.
| Trial (Ref.) | Main inclusion criteria | Experimental arm | Control arm |
|---|---|---|---|
| TARGIT33,34 | Stage I, II T ≤ 3 Age ≥ 45 years cN0 Negative margins |
IORT—single dose of 20 Gy, if high riska WBI is added (45–50 Gy) |
WBI 45–50 Gy in 15–25 fr, optional boost 10–16 Gy in 5–8 fr |
| ELIOT35,36 | Post quadrantectomy Stage I, II T ≤ 2.5 cm Age > 48 years N0, N1 Negative margins |
IORT—single dose of 21 Gy with electrons | WBI 50 Gy in 25 fr with boost 10 Gy in 5 fr |
| GEC-ESTRO37 | Post lumpectomy Stage 0, I, II T ≤ 3 cm Age ≥ 40 years ≤1 micrometastasis in axilla Margins ≥ 2 mm (≥5 mm for DCIS) |
Interstitial brachytherapy: HDR 32 Gy/8 fr, bid or HDR 30.3 Gy/7 fr, bid or PDR 50 Gy in hourly fractions of 0.6–0.8 Gy |
WBI 50–54 Gy, optional boost 10 Gy |
| NSABP B-39/RTOG38 |
Post lumpectomy Stage 0, I, II T ≤ 3 cm pN0, N1 Negative margins Age ≥ 18 years |
Interstitial brachytherapy 34 Gy/10 fr or MammoSite balloon catheter 34 Gy/10 fr or 3D-CRT 38.5 Gy/10 fr |
WBI 50–50.4 Gy, optional boost to 60–66.6 Gy |
| RAPID/Ontario Clinical Oncology Group39 | Post lumpectomy Stage 0, I, II T ≤ 3 cm Age ≥ 40 years pN0 Negative margins |
3D CRT 38.5 Gy/10 fr/5–8 days | WBI 42.5 Gy/16 fr/22 days or 50 Gy/25 fr/35 days (large breast), optional boost 10 Gy/4–5 fr |
| IMPORT-LOW40 | Post lumpectomy Stage I T ≤ 2 cm Age ≥ 50 years pN0 Margins ≥ 2 mm |
EBRT based on IMRT: Arm 1: 40 Gy/15 fr to region of primary tumour and 36 Gy/15 fr to low risk areas Arm 2: PBI 40 Gy/15 fr |
WBI 40 Gy/15 fr/3 weeks |
| IRMA41 | Age ≥ 49 years Post conservative breast surgery T < 3 cm pN0-N1 Margins ≥ 2 mm |
3D CRT 38.5 Gy/10 fr/5 days | WBI 45 Gy/18 fr, or 50 Gy/25 fr, or 50.4 Gy/28 fr, optional boost 10–16 Gy/5–8 fr |
Abbreviations: 3D CRT, three-dimensional conformal radiotherapy; bid, twice a day; DCIS, ductal carcinoma in situ; EBRT, external beam radiotherapy; ELIOT, electron intraoperative radiotherapy technique; GEC-ESTRO, Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology; HDR, high dose radiotherapy; IMRT, intensity modulated radiotherapy; IMPORT-LOW, intensity modulated and partial organ radiotherapy trial; IORT, intraoperative radiotherapy; PBI, partial breast irradiation; PDR, pulsed dose rate; TARGIT, targeted intraoperative radiotherapy; WBI, whole breast irradiation.
Pre-specified criteria, e.g., unsuspected lobular carcinoma, lymphovascular invasion, and others.
5. Conclusion
There are reasonable biological rationales for using APBI and the available results of this approach in selected groups of patients are encouraging. However, as local recurrences in breast cancer and some radiotherapy-specific complications may emerge after many years following treatment, full assessment of APBI value will only be possible after a long follow-up. Essential in this respect will be the results of ongoing large randomised clinical studies. The current knowledge does not allow to identify an optimal APBI technique. In consequence, this method may not yet be recommended as a routine alternative for WBI. Some scientific societies have already allowed restricted use of APBI in clinical practice in carefully selected patient groups (Table 3). The National Comprehensive Cancer Network (NCCN) recommends encouraging patients to participate in clinical trials. If not trial eligible, NCCN allows the use of APBI in accordance with ASTRO recommendations.43 Most recently, around a half of the panelists of the Expert Consensus on the Primary Therapy of Early Breast Cancer in St. Gallen 2011, accepted the use of APBI as definitive treatment in selected patients, and a strong majority accepted this method in patients above the age of 70.48
Table 3.
Major eligibility criteria for APBI in clinical setting.
| Society (Ref.) | Age | Tumour size | Histology | DCIS | Margin status | Induction therapy | Nodal status | Tumour grade | EIC |
|---|---|---|---|---|---|---|---|---|---|
| ASTRO44,45 | ≥60 years | ≤2 cm | Unifocal, unicentric invasive ductal carcinoma or other favourable types | Not allowed | Tumour-free within 2 mm | Not allowed | N0 | Any | Not allowed |
| GEC-ESTRO46 | ≥50 years | <3 cm | Unifocal, unicentric invasive ductal carcinoma or other favourable types | Not allowed | Tumour-free within 2 mm | Not allowed | N0 | Any | Not allowed |
| ABS47 | >50 years | ≤3 cm | Invasive ductal carcinoma | Not allowed | Negative | Not specified | N0 | Not specified | Not specified |
| ASBS47 | >45 years | ≤3 cm | Invasive ductal carcinoma and/or DCIS | Allowed | Negative | Not specified | SN negative | Not specified | Not specified |
Abbreviations: ABS, American Brachytherapy Society; ASBS, American Society of Breast Surgeons; ASTRO, The American Society for Radiation Oncology; DCIS, ductal carcinoma in situ; EIC, extensive intraductal component; GEC-ESTRO, Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology; SN, sentinel node.
References
- 1.Fisher B., Anderson S., Bryant J. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U., Cascinelli N., Mariani L. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Poggi M.M., Danforth D.N., Sciuto L.C. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 4.Van Dongen J.A., Voogd A.C., Fentiman I.S. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 5.Blichert-Toft M., Nielsen M., During M. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47:672–681. doi: 10.1080/02841860801971439. [DOI] [PubMed] [Google Scholar]
- 6.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.Hershman D.L., Wang X., McBride R. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Mikeljevic J.S., Haward R., Johnston C. Trends in post-operative radiotherapy delay and the effect on survival in breast cancer patients treated with conservation surgery. Br J Cancer. 2004;90:1343–1348. doi: 10.1038/sj.bjc.6601693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J., Barbera L., Brouwers M. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 10.Jack R.H., Davies E.A., Robinson D. Radiotherapy waiting times for women with breast cancer: a population-based cohort study. BMC Cancer. 2007;7:71. doi: 10.1186/1471-2407-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offersen B.V., Overgaard M., Kroman N. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90:1–13. doi: 10.1016/j.radonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Sanders M.E., Scroggins T., Ampil F.L. Accelerated partial breast irradiation in early stage breast cancer. J Clin Oncol. 2007;25:996–1002. doi: 10.1200/JCO.2006.09.7436. [DOI] [PubMed] [Google Scholar]
- 13.Huang E., Buchholz T.A., Meric F. Classifying local disease recurrences after breast conservation therapy based on location and histology. New primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 14.Fentiman I.S., Poole C., Tong D. Irridium implant treatment without external radiotherapy for operable breast cancer: a pilot study. Eur J Cancer. 1991;27:447–450. doi: 10.1016/0277-5379(91)90383-o. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro G.G., Magee B., Swindell R. The Christie Hospital breast conservation trial: an updated at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 16.Vicini F., Kini V.R., Chen P. Irradiation of the tumor bed alone after lumpectomy in selected patients with early-stage breast cancer treated with breast conserving therapy. J Surg Oncol. 1999;70:33–40. doi: 10.1002/(sici)1096-9098(199901)70:1<33::aid-jso6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.King T.A., Bolton J.S., Kuske R.R. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is, 1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 18.Orecchia R., Ivaldi G.B., Leonardi M.C. Integrated breast conservation and intraoperative radiation therapy. Breast. 2009;18:98–102. doi: 10.1016/S0960-9776(09)70283-8. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U., Orecchia R., Luini A. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124:141–151. doi: 10.1007/s10549-010-1115-5. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya J.S., Baum M., Tobias J.S. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol. 2001;12:1075–1080. doi: 10.1023/a:1011609401132. [DOI] [PubMed] [Google Scholar]
- 21.Wazer D.E. Point: brachytherapy for accelerated partial breast irradiation. Brachytherapy. 2009;8:181–183. doi: 10.1016/j.brachy.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Arthur D.W., Winter K., Kuske R.R. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72:467–473. doi: 10.1016/j.ijrobp.2007.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polgar C., Major T., Fodor J. Accelerated partial breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274–279. doi: 10.1016/j.radonc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 24.King T.A., Bolton J.S., Kuske R.R. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for Tis, 1,2 breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 25.Vicini F.A., Kestin L., Chen P. Limited-field radiation therapy in the management of early-stage breast cancer. J Natl Cancer Inst. 2003;95:1205–1211. doi: 10.1093/jnci/djg023. [DOI] [PubMed] [Google Scholar]
- 26.Vicini F., Beitsch P., Quiet C. First analysis of patient demographics, technical reproducibility, cosmesis, and early toxicity. Results of the American society of breast surgeons MammoSite breast brachytherapy registry trial. Cancer. 2005;104:133–134. doi: 10.1002/cncr.21289. [DOI] [PubMed] [Google Scholar]
- 27.Strauss J.B., Dickler A. Accelerated partial breast irradiation utilizing balloon brachytherapy techniques. Radiother Oncol. 2009;91:157–165. doi: 10.1016/j.radonc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Taghian A.G., Bourgier C. Counterpoint: conformal external beam for accelerated partial breast irradiation. Brachytherapy. 2009;8:184–188. doi: 10.1016/j.brachy.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Formenti S.C., Truong M.T., Goldberg J.D. Prone accelerated partial breast irradiation after breast conserving surgery: preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Kozak K.R., Smith B.L., Adams J. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Vicini F.A., Remouchamps V., Wallace M. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patient with early-stage breast cancer treated with breast conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247–1253. doi: 10.1016/s0360-3016(03)01573-6. [DOI] [PubMed] [Google Scholar]
- 32.Polgar C., Fodor J., Major T. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma-5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya J.S., Joseph D.J., Tobias J.S. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomized, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 34.Vaidya J.S., Tobias J.S., Baum M. Targeted intra-operative radiotherapy (TARGIT): an innovative approach to partial-breast irradiation. Semin Radiat Oncol. 2005;15:84–91. doi: 10.1016/j.semradonc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Orecchia R., Ciocca M., Lazzari R. Intraoperative radiation therapy with electrons (ELIOT) in early-stage breast cancer. Breast. 2003;12:483–490. doi: 10.1016/s0960-9776(03)00156-5. [DOI] [PubMed] [Google Scholar]
- 36.Orecchia R., Ciocca M., Tosi G. Intraoperative electron beam radiotherapy (ELIOT) to the breast: a need for a quality assurance programme. Breast. 2005;14:541–546. doi: 10.1016/j.breast.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Polgar C., Strnad O., Ciocca R., Major T. Brachytherapy for partial breast irradiation: the European experience. Semin Radiat Oncol. 2005;15:116–122. doi: 10.1016/j.semradonc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 38.McCormick B. Partial-breast radiation for early staged breast cancers: hypothesis, existing data, and a planned phase III trial. JNCCN. 2005;3:301–307. doi: 10.6004/jnccn.2005.0017. [DOI] [PubMed] [Google Scholar]
- 39.Ontario Clinical Oncology Group (OCOG), Canadian Institutes of Health Research (CIHR), Canadian Breast Cancer Research Alliance. RAPID: Randomized Trial of Accelerated Partial Breast Irradiation; 2010. Available from: http://clinicaltrials.gov/ct2/show/NCT00282035.
- 40.Coles C., Yarnold J. The IMPORT trials are launched (September 2006) Clin Oncol (R Coll Radiol) 2006;18:587–590. doi: 10.1016/j.clon.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Available from: http://groups.eortc.be/radio/res/irma/synopsis_trial_irma1.pdf.
- 42.Mannino M., Yarnold J. Accelerated partial breast irradiation trials: diversity in rationale and design. Radiother Oncol. 2009;91:16–22. doi: 10.1016/j.radonc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Available from: www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 44.Smith B.D., Arthur D.W., Buchholz T.A. Accelerated partial breast irradiation consensus statement from The American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Prosnitz L.P., Horton J., Wallner P.E. Accelerated partial breast irradiation: caution and concern from an ASTRO task force. Int J Radiat Oncol Biol Phys. 2009;74:981–984. doi: 10.1016/j.ijrobp.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Polgar C., Limbergen E., Potter R. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC/ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Dirbas F.M. Accelerated partial breast irradiation: where do we stand? JNCCN. 2009;7:215–225. doi: 10.6004/jnccn.2009.0015. [DOI] [PubMed] [Google Scholar]
- 48.Goldhirsch A., Wood W.C., Coates A.S. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]


