Abstract
Background
Sentinel node biopsy (SNB) is a gold standard in staging of early breast cancer. Nowadays, routine mapping of lymphatic tract is based on two tracers: human albumin with radioactive technetium, with or without blue dye. Recent years have seen a search for new tracers to examine sentinel node as well as lymphatic network. One of them is indocyanine green (ICG) visible in infrared light.
Aim
The aim of this study is to evaluate clinical usage of ICG in comparison with standard tracer, i.e. nanocoll, in SNB of breast cancer patients.
Materials and methods
In the 1st Department of Surgical Oncology and General Surgery, Greater Poland Cancer Centre, Poznań, 13 female breast cancer patients have benn operated since September 2010. All these patients had sentinel node biopsy with nanocoll (human albumin with radioactive technetium), and with indocyanine green. The feasibility of this new method was assessed in comparison with the standard nanocoll.
Results
A lymphatic network between the place of injection of ICG and sentinel node was seen in infrared light. An area where a sentinel node was possibly located was confirmed by gamma probe. Sensitivity of this method was 100%.
Conclusion
SNB using ICG is a new, promising diagnostics technique. This procedure is not without drawbacks; nevertheless it opens new horizons in lymphatic network diagnostics.
Keywords: Sentinel node biopsy, Lymphatic flow, Breast cancer
1. Background
Sentinel node biopsy (SNB) is a standard procedure in the staging of early breast cancer. On the one hand, it is an easy and safe way of providing information concerning the staging of a neoplastic disease and, on the other hand, it gives most patients a possibility to have the lymphatic system of the armpit spared.
As regards negative results of the SNB, randomised multi-centre clinical research proved the absence of a significant difference in local relapses and survival rate between patients after resection and those with saved lymphatic system of the armpit.1–3 The SNB provides benefits of much smaller incidence of complications and traumas as well as a better quality of life in comparison with axillary lymphadenectomy. At present, the standard procedure used in mapping lymph vessels and sentinel lymph node involves the application of tracers based on albumin marked with radioactive technetium in combination with or without blue dye. Either technique has its advantages and disadvantages. Originally, Guliano in breast cancer, just like Morton in skin melanoma, used blue dye to locate the sentinel node.4,5 Later, Krag et al. used technetium sulphur colloid, which enabled transcutaneous identification of the sentinel node.6 At present some centres use a radiocolloid by itself without blue dye. In recent years, there has been a search for an alternative method of visualisation of the lymph vessels and sentinel node. Indocyanine green is a promising tracer which, after being administered intradermally, flows to the sentinel node and is visible in infrared light also through the skin. The application of the method is at the stage of clinical research.
2. Aim
The aim of the study was to evaluate the usefulness of the new method of sentinel lymph node mapping in comparison with the radioactive technetium nanocolloid, which is a routinely used tracer.
3. Materials and methods
Since September 2010, 13 consecutive women with diagnosed breast cancer have undergone surgery in the 1st Department of Surgical Oncology and General Surgery, Greater Poland Cancer Centre, Poznań. The group of patients had an early breast cancer. Two of them had DCIS (ductal carcinoma in situ), 11 had invasive cancers (2 cases of T1a, 5 cases of T1b, 4 cases of T1c). In two cases, (both T1c) additional lymphadenectomy was done following intraoperative pathological examination of sentinel node because of metastases. The age of these patients ranged between 41 and 69.
A new system of sentinel node location was implemented in the patients. In order to visualise the sentinel lymph node, one day before the surgery the patients underwent a routine intradermal injection of radioactive technetium nanocolloid above the breast tumour. In the case of microcalcifications scattered over the whole breast, the tracer was administered periareolarly from four needle injections. The patients underwent lymphoscintigraphy. Immediately before the surgery, indocyanine green (ICG) (concentration 5 mg/ml, volume 1–2 ml) was also administered intradermally above the tumour, or periareolarly in the case of microcalcifications scattered over the whole breast. Then, after 5–10 min following the ICG injection, the lymph channels connecting the place of administration with the lymphatic system were visualised as along with the sentinel lymph node itself by means of a PDE (Photodynamic Eye) camera from Hamamatsu Photonics K.K., Hamamatsu, Japan (Fig. 1). The fluorescence phenomenon was used, which consists in the emission of light of appropriate wavelength from the substance which absorbs light of a different wavelength. The PDE camera emits infrared light with the wavelength of 760–805 nm and ICG exposed to the activity of that sends light radiation with the wavelength of about 820–830 nm. The sent light and emitted light are called excitation light (LED) and fluorescence light respectively. The image was seen in infrared light emitted by the camera. CCD (charged coupled device) is a device that usually consists of three main components: an NIR (near-infrared) sensitive image intensifier, 16-bit dynamic-range frame transfer CCD camera, and light emitting diodes (LED). In comparison with the IC-View camera; Pulsion Medical Systems, Munich, Germany, which we used previously, the new system has more LEDs, which enables much better quality of obtained image and improved sensitivity of the examination. In each case, the ICG intercepted by the sentinel node was confirmed by means of a gamma probe of the camera routinely used for detection of gamma radiation transcutaneously during the SNB (Fig. 2), after opening the wound and once more after sentinel node resection.
Fig. 1.

Place of injection of ICG intradermally above the breast cancer with two lymphatic channels passing to one sentinel node.
Fig. 2.
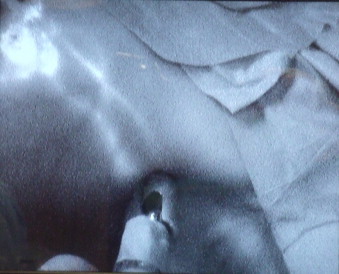
Lymphatic channels after ICG injection ending in the sentinel node-confirmed with the gamma probe.
4. Results
10 min after the administration of ICG in all patients, the PDE camera visualised the lymph ducts channels from the place of administration of the tracer with the lymphatic system of the armpit. In the place of atrophy of the lymph duct passage to deeper structures of the armpit, including the sentinel lymph node was suspected. The area was checked with a gamma camera and in all cases the place transcutaneously showed radiation captured from the sentinel node. After making an incision in the skin and dissection of tissues, the sentinel node with intense ICG capture was visualised in the PDE camera (Fig. 3). In each case the node showed captured radiation in the gamma camera. In 5 cases green colour of the node was seen without camera (Fig. 4). The sensitivity of the method in the abovementioned group was calculated at 100%. No side effects of the procedure were observed.
Fig. 3.
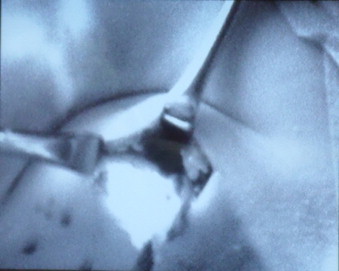
Sentinel node stained with ICG after skin incision and tissue dissection.
Fig. 4.
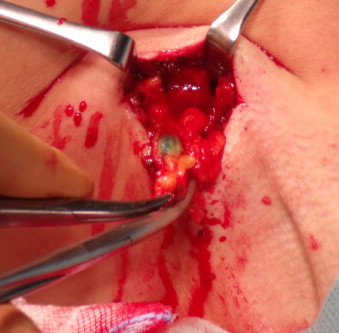
Sentinel node with ICG-view without camera-green colour of the node.
5. Discussion
ICG is a non-toxic substance, which has been used in ophthalmology, transplantology or diagnostics and surgery of the alimentary tract for many years.7–9 It is characterised by short half-life in the organism and a strong tendency to compound with serum proteins, especially with α1 lipoprotein. After being injected and compounding with serum proteins, ICG easily moves along the lymph ducts to nodal stations. Thus, it is possible to visualise not only the place of administration of the dye and the lymph duct, but also and above all the sentinel lymph node.10
Since 2005, when Kitai et al. first suggested the application of indocyanine green for mapping the lymph channels and sentinel lymph node in breast cancer, the interest in the new technique has increased considerably and studies on the application of the method in marking the sentinel node in other cancers have been done.11 Throughout the detection process, it is necessary to use equipment with an infrared emitting camera and indocyanine green showing emission of light. In published articles, the tracer was administered intradermally or subcutaneously and then after 5–10 min the lymph vessel, and sentinel lymph node were visualised. Until now, there have been no decisions concerning the dose and, according to Sevick-Muraca, it is sufficient to apply ICG in concentration of 10–100 μg/ml up to 25 mg/ml, as suggested by Kitai et al.11,12 Another important problem is the necessity to switch off the light in order to visualise the ICG-dyed lymphatic system. Owing to the new equipment from Hamamatsu, it is possible to keep the light on in the operating theatre, except for intense surgical lightening that needs to be switched off. When older generation cameras were used, a complete darkness was required during a surgery, which resulted in many technical problems and considerably extended the surgery time. Until now, we have had only a few publications describing the problem of SNB detection with ICG in breast cancer (Table 1).
Table 1.
ICG and SNB in patients with breast cancer.
| Number of patients | Type and dose of ICG | Injection site | Positive sentinel lymph node | Metastatic involvement of SLN detected (%) | False negative (%) | |
|---|---|---|---|---|---|---|
| Kitai et al.11 | 18 | ICG, 5 ml (25 mg) | Subcutaneous in areola | 17/18 (94%) | 6/6 (100%) | 0/11 (0) |
| Tagaya et al.13 | 25 | ICG, 1 ml (5mg) | Subdermally in areola | 25/25 (100%) | 8/8 (100%) | 0/17 (0) |
| Murawa et al.14 | 30 | ICG, 5 mg, 10 mg, 15 mg | Intradermal in periareolar region | 29/30 (97%) | 19/21 (90%) | 2/21 (10%) |
| Troyan et al.15 | 6 | ICG:HAS, 1.6 ml (12.5 μg) | Four deep peritumoural injection and four subcutaneous periotumoural injection | 5/6 (83%) | 3/9 (33%) | – |
| Hirche et al.16 | 43 | ICG, average 11 mg | Subareolar region | 42/43 (94.4%) | 17/18 (94.4%) | 1/43 (5.6%) |
| Hojo et al.17 | 141 | Combination of 1% blue dye 2 ml + ICG 2 ml | Skin overlying the tumour and subareolar region | 140/141 (99.3%) | 5/31 (16.1%) | – |
Currently used radioactive tracers are safe and emit insignificant amounts of radiation, but the application of ICG eliminates the problem completely. Another benefit arising from the use of ICG is the safety of application.18 At present, the risk of an anaphylactic reaction has been estimated at 0.05% and side effects have only been observed with doses considerably exceeding those recommended by the FDA (Food and Drug Administration).19 Such tracers as isosulfan dye in SNB have been reported to cause anaphylaxis at a rate between 0.7% and 1.1%, whereas the rate for patent blue was 0.4%.20–23 Another important argument supporting the application of ICG is real-time visualisation of not only the sentinel lymph node but also the whole lymph vessel during the surgery. The time necessary to prepare the substance and the delay between the injection and visualisation of the lymph vessel are also worth mentioning. For ICG, it is 15 min and the whole procedure is performed perioperationally, while it takes up to 29 h to prepare the radiocolloid, if the radiopharmaceutical is administered one day before the surgery. The inconvenience of applying ICG in the SNB is that it requires the light to be switched off. In recognising the problem, efforts by Troyan et al. have resulted in development of the FLARE™ imaging system which offers a possibility to view surgical anatomy with two independent channels of NIR fluorescence.15 However, it is necessary to mention the enormous cost of the appliance, which exceeds 120,000 dollars. Another challenge is the depth at which ICG detection is possible. At present, the limit is 1 cm of the tissue, which makes detection of deeper lymph nodes impossible. The application of ICG in combination with other substances may improve the parameter. An appropriate length of training to become familiar with the new technique is also necessary. At present, for a standard SNB it is necessary to perform 20 biopsies on one's own.24
SNB with the application of indocyanine green is a new promising diagnostic technique. It is not free from disadvantages. However, it opens a completely new scope in diagnostics of the lymphatic system.
Acknowledgement
This study was supported financially by Greater Poland Cancer Centre No. 1/2010.
References
- 1.Olson J.A., Jr., McCall L.M., Beitsch P. Impact of immediate versus delayed axillary dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 2.Krag D.N., Anderson S.J., Julian T.B. Technical outcomes of sentinel lymph node resection and conventional axillary lymph node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Onol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U., Paganelli G., Viale G. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Eng J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano A.E., Kirgan D.M., Guenther J.M., Morton D.L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton D.L., Chan A.D. The concept of sentinel node localization: how it started. Semin Nucl Med. 2000;30:4–10. doi: 10.1016/s0001-2998(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 6.Krag D.N., Weaver D.L., Alex J.C., Fairbank J.T. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 7.Belacumaraswami L., Abu-Omar Y., Anastasiadis K. Does off-pump total arteria grafting increase the incidence of intraoperative graft failure? J Thorac Cardiovasc Surg. 2004;128:238–244. doi: 10.1016/j.jtcvs.2003.11.072. [DOI] [PubMed] [Google Scholar]
- 8.Sekijima M., Toijmbara T., Sato S. An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc. 2004;36:2188–2190. doi: 10.1016/j.transproceed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Parungo C.P., Ohnishi S., Kim S.W. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J Thorac Cardiovasc Surg. 2005;129:844–850. doi: 10.1016/j.jtcvs.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen J.C., Tan I.C., Marshal M.V. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20:74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitai T., Inomoto T., Miwa M., Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12(3):211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 12.Sevick-Muraca E.M., Sharma R., Rasmussen J.C. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246(3):734–741. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tagaya N., Yamazaki R., Nakagawa A. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195(6):850–853. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Murawa D., Hirche C., Dresel S., Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96(11):1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 15.Troyan S.L., Kianzad V., Gibbs-Strauss S.L. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16(10):2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirche C., Murawa D., Mohr Z. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121(2):373–378. doi: 10.1007/s10549-010-0760-z. [DOI] [PubMed] [Google Scholar]
- 17.Hojo T., Nagao T., Kikuyama M. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19(3):210–213. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa M., Regino C.A., Choyke P.L., Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8(1):232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen T.W., Lim J.I., Capone A., Jr. Anaphylactic shock following indocyanine green angiography. Arch Ophthalmol. 1996;114(1):97. doi: 10.1001/archopht.1996.01100130093018. [DOI] [PubMed] [Google Scholar]
- 20.Alford R., Simpson H.M., Duberman J. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging. 2009;8(6):341–354. [PubMed] [Google Scholar]
- 21.Leong S.P., Donegan E., Heffernon W. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7(5):361–366. doi: 10.1007/s10434-000-0361-x. [DOI] [PubMed] [Google Scholar]
- 22.Albo D., Wayne J.D., Hunt K.K. Anaphylactic reactions to isosulfan blue dye during sentinel lymph node biopsy for breast cancer. Am J Surg. 2001;182(4):393–398. doi: 10.1016/s0002-9610(01)00734-6. [DOI] [PubMed] [Google Scholar]
- 23.Hunting A.S., Nopp A., Johansson S.G. Anaphylaxis to Patent Blue V. I. Clinical aspects. Allergy. 2010;65(1):117–123. doi: 10.1111/j.1398-9995.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 24.East J.M., Valentine C.S., Kanchev E., Blake G.O. Sentinel lymph node biopsy for breast cancer using blue dye manifests a short learning curve among experienced surgeons: a prospective tabular cumulative sum (CUSUM) analysis. BMC Surg. 2009;9:2. doi: 10.1186/1471-2482-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


