Abstract
Aim
When investigating radiation accidents, it is very important to determine the exposition dose to the individuals. In the case of exposures over 1 Gy, clinicians may expect deterministic effects arising the following weeks and months, in these cases dose estimation will help physicians in the planning of therapy. Nevertheless, for doses below 1 Gy, biodosimetry data are important due to the risk of developing late stochastic effects. Finally, some accidental overexposures are lack of physical measurements and the only way of quantifying dose is by biological dosimetry.
Background
The analysis of chromosomal aberrations by different techniques is the most developed method of quantifying dose to individuals exposed to ionising radiations.1,2 Furthermore, the analysis of dicentric chromosomes observed in metaphases from peripheral lymphocytes is the routine technique used in case of acute exposures to assess radiation doses.
Materials and methods
Solid stain of chromosomes is used to determine dicentric yields for dose estimation. Fluorescence in situ hybridization (FISH) for translocations analysis is used when delayed sampling or suspected chronically irradiation dose assessment. Recommendations in technical considerations are based mainly in the IAEA Technical Report No. 405.2
Results
Experience in biological dosimetry at Gregorio Marañón General Hospital is described, including own calibration curves used for dose estimation, background studies and real cases of overexposition.
Conclusion
Dose assessment by biological dosimeters requires a large previous standardization work and a continuous update. Individual dose assessment involves high qualification professionals and its long time consuming, therefore requires specific Centres. For large mass casualties cooperation among specialized Institutions is needed.
Keywords: Biodosimetry, Dicentrics, Translocations, FISH
1. Background
The Gregorio Marañón University General Hospital has the Spanish National Reference Centre for the attention of irradiated and/or contaminated people. This Centre works coordinating the medical needs of any person suspected of being exposed to ionising radiations from external sources or from internal contamination, includes immediate care, and follow up when necessary. The only specific facility of the Centre is the Biological Dosimetry Laboratory, which is in charge of individual estimation of dose when there is a suspected over exposition to ionising radiations.
The Biological Dosimetry Laboratory has an experience of more than 20 years. It has standardized the dicentrics technique, holding own calibrations curves for neutrons, gamma and X rays, and has performed calibration curves for X and gamma rays, using the fluorescence in situ hybridization (FISH) technique for the identification of translocations.
It is well known that ionising radiations produce several biological effects, some of them are quantifiable and thus can be used to estimate the radiation dose, but when looking for a biological dosimeter it is necessary to take into account more characteristics like reproducibility, sensibility, low cost, nonaggressive…
As the biological target for ionisations is the DNA, any method analysing lesions to cellular DNA could potentially be a biological dosimeter when meet also the other properties.
Ionising radiations produce double and single strand breaks, base damage and DNA protein cross-links, this damage can be repaired giving apparently normal chromosomes, but if the primary lesion caused by ionising radiations in cellular DNA, mainly double strand breaks, is misrepaired could produce exchanges that would generate chromosomal aberrations observed at metaphase.3,4
Human peripheral lymphocytes are mainly in a resting phase of the cell cycle called G0, but can be stimulated in vitro, to undergo mitosis, by phytohaemagglutinin (PHA).5 All chromosomal aberrations induced in vivo would be chromosome type ones, both chromatids of the chromosome are involved, because lymphocytes are in the pre-synthetic phase, G0.6
2. Materials and methods
In this section general procedures from the laboratory are described, could be slight specific modifications for each work that will be described in the correspondent section.
A venipuncture blood sample of 10 ml is collected as soon as possible after irradiation occurs, to avoid several consequences derived from delayed sampling.7 Cells cultures established based on modified Moorhead et al.8 technique, at least two different cultures per sample are set up both for 48 h at 37 °C using RPMI1640 supplemented with foetal calf serum, l-glutamine and antibiotics. We use to separate lymphocytes from the total blood to improve growth, but when small blood sample (1–2 ml) is available, a culture of whole blood is established. Add PHA (phytohaemagglutinin) for lymphocyte cell cycle induction, and Bromo deoxi uridin (BrDU) to monitored second division metaphases. Last two hours incubation with colchicine provoke mitotic arrest.
Once finished the culturing time, samples are centrifuged to remove culture medium and additives. Cells are treated with a hypotonic solution of potassium chloride 0.075 M during 10–15 min at 37 °C, after centrifugation at 200 × g during 10 min to remove ClK solution, freshly prepared fixative of 3:1 methanol: acetic acid, is added. Cells are spun down and resuspended with fixative three times. Depending on the laboratory routine work, at this point, tubes may be stored at −20 °C.
Using clean and grease free slides, drop down 3–4 drops of sample per slide (depends on cell density). At this stage, slides with metaphases are evaluated under inverted microscope and stored according to the type of analysis designated for.
2.1. Dicentrics analysis
For dicentrics analysis, two stain methods are used: giemsa solid stain (3 min in a giemsa 2% diluted in distilled water) and Fluorescence plus giemsa stain (FPG) described by Perry and Wolff 1974.9 When staining by FPG, slides are previously aged for two days at 56 °C. This type of stain allows the differentiation between first and second division metaphases, based on the addition of BrDU to the cell cultures for its incorporation to the replicating DNA during the synthesis phase, as a second division metaphase has undergone two DNA synthesis, one of the chromatids of each chromosome has both DNA chains with BrDU incorporated. FPG protocol includes incubation of the slides in 2% Hoechst during 30 min, placing them on a hot plate at 56 °C, adding McIlrens buffer, and illuminate them under a 20 W UV lamp at 5 cm distance during 40 min, staining with 3% giemsa solution.
If the number of second division metaphases is under 5%, giemsa stained slides are used to complete the analysis (Fig. 1).
Fig. 1.
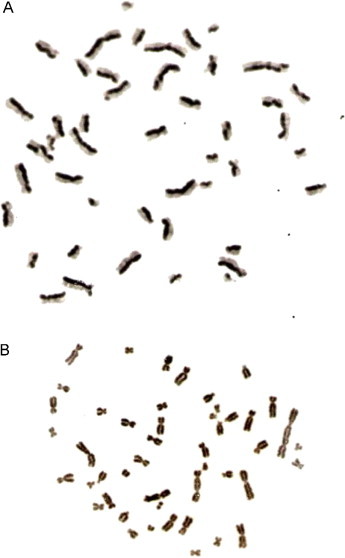
microscope photography (×1000) of two metaphases stained by FPG technique. (A) Second metaphase after 48 h of BrDU culture initiation. (B) First metaphase after 48 h of BrDU culture initiation, showing a dicentric and an acentric fragment.
2.2. Translocations analysis
For translocations analysis, FISH technique is used. When there is a sampling delay longer than a month, as dicentrics are unstable, its frequency will decay causing a great uncertainty in any estimation of radiation dose, in these situations, the analysis of translocations is utilized. Commercially (Chrombios) available red painting probes for chromosomes one and two mixed with a green pancentromeric probe are used, translocations are observed as a monocentric bicolour red-blue chromosome (Fig. 2). Hybridization is performed after slide incubation at 60 °C for 1 h as follows: high temperature denaturalization of pancentromeric probe at 95 °C for 8 min and of painting probes at 80 °C for 8 min followed by its re-annealing at 37 °C for 30 min. Slide metaphases denaturalization in 70% formamide 2 SSC at 70 °C is done. Mixed pancentromeric and painting probes are placed over the slide, covered with a plastic cover slide and incubation over night in a humidified chamber at 37 °C. Astringency washes with 2 SSC for 7 min and a 0.4 SSC 1% NP40 solution at 70 °C for 1 min. Counterstained with DAPI.
Fig. 2.
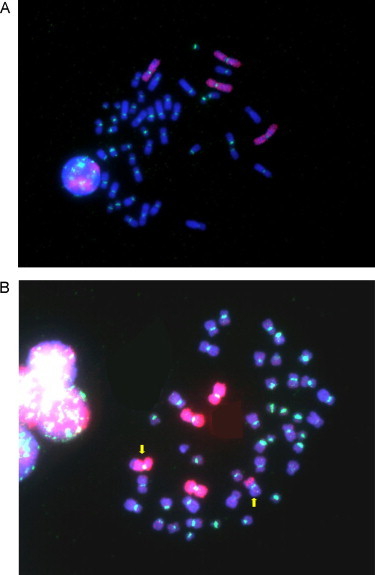
Fluorescence microscopy (×1000) triple filter, FISH technique performed in our laboratory, red painted chromosomes 1 and 2 and a green pancentromeric probe. (A) Normal metaphase. (B) Metaphase with a reciprocal translocation.
Slides are scanned completely at low magnification (100–200×) looking for good quality metaphases, chromosomal analysis is done at high magnification (1000×) a minimum of 500 complete metaphases, with 46 or more chromosomes, are analyzed for dose estimation, if cells has unstable aberrations, only balanced ones are recorded.
For FISH testing specific fluorescence filters are used. When analysing translocations by FISH, only exchanges affecting painted chromosomes will be observed. Therefore for dose estimation, extrapolation to the whole genome, is done to obtain the genomic translocation frequency, the Lucas and Deng formula10 is used, this equation is based in the assumption that the probability of a painted chromosome to be involved in a translocation is proportional to its DNA content. Using this equation, an efficiency of about 28% in detecting translocations is obtained.
There are two important considerations for dose estimation assessment: First is the background level of chromosome aberrations, and second, the dose respond calibration curves.
2.3. Chromosome aberrations background
A known background level obtained from population's studies11 is used when estimating individual doses. For the dicentric technique, our laboratory has background data obtained from two population's studies, one performed for people of the geographical area of Madrid, and the other for the same geographical area but occupationally exposed to ionising radiations.
For the first study, 72 healthy persons born or, living longer than 30 years in the geographical area of Madrid, were randomly selected and grouped in six age intervals from 18 to 65. In each group, there were the same amount of male and female, and smokers and non-smokers. There were analyzed 500 metaphases per individual (36,000 metaphases) to obtain a background data for dicentrics.
The other population study performed in our laboratory included 64 occupationally exposed to low doses of ionising radiations individuals, working at the radio-diagnostic Department of Gregorio Marañón General Hospital. This sub-population was selected among the 138 workers of this department and grouped in four groups related to the time working in this department: 1) less than five years, 2) 5–10 years, 3) 11–15 years, and 4) more than 15 years. All people have physical doses records without any event of overexposure or readings over limits. There were analyzed 500 metaphases per individual (32,000 metaphases) to obtain a background data for dicentrics.
When analysing translocations, as they are stable aberrations, they will accumulate along life, therefore, a high background frequency due to age has been observed, for individual dose estimation, translocations frequencies published for different age intervals are used.12
Our laboratory has performed a translocations study by FISH in an aircrew population compared to a control group. Compare number of translocations founded in an aircrew population matched for age, gender, and smoking habits with a control population. This work includes Aircrew members of intercontinental flights above 9000 m, with the following requirements:
Active, at least 10 years of continuous service with full schedules, at least 5 years of continuous service in intercontinental flights, preferably A-340.
The comparison group was Iberia staff. We have analyzed 136,987 metaphases from blood samples of 80 persons included in the study. We sent data to the Health Protection Agency (HPA, Chilton Didcot Oxford, UK) for statistical analysis.
2.4. Dose response calibration curves
The chromosomal aberrations scored in the lymphocytes metaphases, are interpreted in terms of absorbed dose by reference to a dose response calibration curve. These curves have been produced previously by exposure of blood samples in vitro, to known doses of the adequate quality of radiation.
The biological effectiveness of radiations depends on the radiation quality, therefore it is necessary to perform calibrations curves for different energies defined by Linear Energy Transfer (LET) in general classifying neutrons as high LET radiations and X and gamma rays as low LET radiations.13–15
For Low LET radiations, dose response curve fits a linear quadratic equation
For high LET radiations, dose response curve fits a linear equation:
where, Y is the yield of dicentrics, A is the background frequency, D is dose, and α and β are the linear and dose squared coefficients.
Our laboratory has
-
1.
Neutron calibration curve for dicentrics: Two people from our laboratory were at the Oak Ridge National laboratory ORNL, USA. Blood irradiation, culturing and processing of samples was done by us at Oak Ridge laboratory. The dicentrics analysis was done in our laboratory. We used a californium 252 source with a dose rate of 0.002795 Gy/h, 1 m distance from source to sample and 4 dose points at 0.05 Gy, 0.1 Gy, 0.15 Gy and 0.2 Gy, we analyzed 500 metaphases each dose and the control sample.
-
2.
X-rays calibration curve for dicentrics: Two people from our laboratory were at the Oak Ridge National laboratory ORNL, USA. Blood irradiation, culturing and processing of samples was done by us at Oak Ridge laboratory. The dicentrics analysis was done in our laboratory. We used an X rays generator of 300 Kvp at a dose rate of 96.4 cGy/min. We irradiated samples at seven dose points: 0.25, 0.5, 0.75, 1, 1.5, 3 and 4 Gy. We analyzed 2000 metaphases from the control sample, 1800 metaphases for the 0.25 dose point and 500 from each of the others dose points.
-
3.
Gamma rays calibration curve for dicentrics: was performed at our Centre. We used Co 60 radiotherapy equipment for the irradiation of blood samples with a dose rate of 23.85 cGy/min and the following dose points: 0.1, 0.2, 0.4, 0.6, 1, 2, 3 and 4 Gy. Cytogenetic data were sent to HPA, UK for statistics analysis.
-
4.
FISH calibration curves: We have two calibration curves (for gamma and X-rays) analyzing translocations by the FISH technique described in the methodology section. Cytogenetics data were sent to ICRP for statistics analysis. For the X calibration curve a Philips radiotherapy equipment (MULLER TU1) was used, with next irradiation conditions: 19 mA, 100 keV, 37.06 cGy/min, distance from source to sample 50 cm, exposure time at all dose points was less than 10 min. For de gamma rays calibration curve the radiotherapy equipment ALCYON II was used, at a dose rate of 0.677 Gy/min, less than 6 min of exposure for each dose. Dose points for both were: 10, 20, 40, 60, 100, 200, 300, 400 cGy.
For all calibration curves, irradiation conditions and blood samples treatment carry out following IAEA reports1,2 recommendations.
3. Results
3.1. Population studies
As part of laboratory standardization, there are two population studies performed for dicentrics background
-
•
Cytogenetic study in peripheral blood lymphocytes of a population sample from the geographic area of Madrid. The estimated dicentric frequency is about 0.7 dic/1000 cells analyzed. We observed 1 dicentric in 15 individuals, 2 dicentrics in 4 individuals and 3 dicentrics in one person. Cytogenetic results were sent to HPA to complete statistical analysis. To see how factors like age, gender and smoking, influence dicentric frequency, an analysis of variance of the six possibilities was performed. See Table 1.
Table 1.
Statistical results of the analysis of dicentrics in a population sample from the geographic area of Madrid.
| Parameter | S.D. | Re. D.F. | P |
|---|---|---|---|
| 1 | 40.02 | 23 | 0.015 |
| 2 | 38.63 | 22 | 0.016 |
| 3 | 34.27 | 22 | 0.046 |
| 4 | 35.31 | 22 | 0.036 |
| 5 | 29.56 | 21 | 0.100 |
| 6 | 21.90 | 17 | 0.190 |
Scaled deviance (SD) is obtained by equation 2 log I0/Im which corresponds with a X2 distribution where, I0 is desired observations for a perfect fit and Im is desired observations for a particular fit.
Following parameters were analyzed:
-
1
All population data, including all factors: age, gender and smoking habits.
-
2
Evaluating number of dicentrics related to gender no statistical differences were found.
-
3
Statistically significant differences between smokers and non-smokers were found.
-
4
Analyzing age as a lineal regression parameter, statistically significant differences were found.
-
5
Taking together smoking and age as a linear parameter, statistically significant differences were found.
-
6Age groups and smoking habits present statistically significant differences.
-
•Cytogenetic study in peripheral blood lymphocytes of a population occupationally exposed to low dose of ionizing radiations. The estimated dicentric frequency is 0.8 dic/1000 cells.
-
•Translocations study by FISH in an aircrew population compared to a control group. Compare number of translocations founded in an aircrew population matched for age, gender, and smoking habits with a control population. This work includes Aircrew members of intercontinental flights above 9000 m, with the following requirements:
-
•
Active, at least 10 years of continuous service with full schedules, at least 5 years of continuous service in intercontinental flights, preferably A-340.
The comparison group was Iberia staff. We have analyzed 136,987 metaphases from blood samples of 80 persons included in the study. We sent data to the Health Protection Agency (HPA, Chilton Didcot Oxford, UK) for statistical analysis. No statistical differences between both populations were found.
3.2. Calibration curves
We have calibration curves performed with techniques, giemsa and FISH, and for different radiation qualities, neutrons, X rays and gamma rays.
Neutron calibration curve for dicentrics: the curve coefficient calculated by sigma plot program was 1.352 × 10−2.
-
•
X-rays dicentric calibration curve: the α and β values of the curve obtained by sigma plot program are 0.0617 × 10−2 and 0.0568 × 10−2.
-
•
Gamma rays dicentric calibration curve: the α and β values of the curve obtained are 1.0134 × 10−2 and 7.1954 × 10−2 respectively, represented in Fig. 3.
-
•
We performed gamma and X rays calibration curves using the FISH technique for the analysis of chromosomal aberrations, Fig. 4. All microscope observations were recorded in an excel file, therefore all possibilities of statistical analysis are possible to obtain different types of calibration curves, all translocations in all cells, all translocations in stable cells, reciprocal translocations in stable cell or in all cells. Results are shown in Table 2 for X rays and in Table 3 for gamma rays.
Fig. 3.
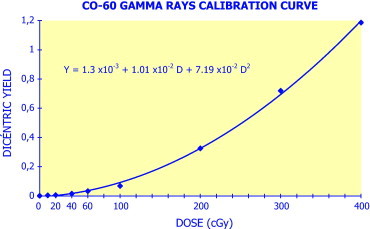
Graphical representation of a gamma-rays dicentric calibration curve.
Fig. 4.
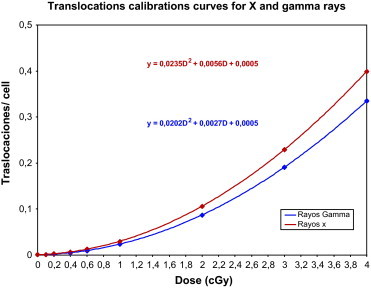
graphical representation of all translocations in all cells calibration curves obtained by FISH technique, for X and gamma rays.
Table 2.
Citogenetical results of the analysis of translocations, after X irradiation of blood samples at known dose points, by FISH, using painting probes for chromosomes one and two and a pancentromeric probe.
| X rays | |||||
|---|---|---|---|---|---|
| Dose (cGy) | Cells | Rt in SC | Rt in AC | At in SC | At in AC |
| 0 | 2651 | 0 | 0 | 1 | 1 |
| 10.7 | 2017 | 2 | 2 | 2 | 3 |
| 21.3 | 1855 | 4 | 4 | 4 | 8 |
| 42.17 | 1477 | 6 | 6 | 8 | 9 |
| 63.05 | 1497 | 12 | 13 | 16 | 19 |
| 104.6 | 550 | 9 | 9 | 13 | 15 |
| 210.6 | 400 | 14 | 27 | 19 | 44 |
| 315.2 | 168 | 7 | 23 | 10 | 38 |
| 420.9 | 124 | 8 | 29 | 11 | 64 |
Rt: Reciprocal translocations, At: all translocations, SC: stable cells, AC: all cells.
Table 3.
Citogenetical results of the analysis of translocations, after gamma irradiation of blood samples at known dose points, by FISH, using painting probes for chromosomes one and two and a pancentromeric probe.
| Gamma rays | |||||
|---|---|---|---|---|---|
| Dose (cGy) | Cells | Rt in SC | Rt in AC | At in SC | At in AC |
| 0 | 2759 | 1 | 1 | 1 | 1 |
| 10 | 2206 | 1 | 1 | 2 | 2 |
| 20 | 1680 | 3 | 4 | 4 | 6 |
| 40 | 1750 | 4 | 4 | 8 | 9 |
| 60 | 1500 | 5 | 5 | 10 | 10 |
| 100 | 1000 | 13 | 14 | 19 | 22 |
| 200 | 400 | 14 | 19 | 22 | 32 |
| 300 | 200 | 16 | 28 | 17 | 38 |
| 400 | 150 | 18 | 38 | 21 | 56 |
Rt: Reciprocal translocations, At: all translocations, SC: stable cells, AC: all cells.
3.3. Over exposition; cases experience
We have analyzed 107 people suspected over exposition to ionizing radiations coming from different working sectors as research and sanitary institutions, companies of industrial radiology, some from nuclear power plants.
There are included, as well, people who are not occupationally exposed to ionizing radiations called as general public who come because a suspicion of an incident of over exposition occurs. There were 11 positives, but only one of them with a dose estimation over 1 Gy; a 35 years old male, he was working accidentally, during six hours at a variable distance from a source of 70Ci Ir-192, there were no physical measurements, 19 days after this incident a dose of 1.3 Gy was estimated by biological dosimetry.
Most of people are coming from sanitary institutions, but the highest frequency of positives are workers of industrial radiology.
Dose assessment by biological dosimeters requires a large preceding standardization effort and a permanent update. Individual dose assessment involves high qualification professionals and its time-consuming, therefore requires specialised Centres.
4. Discussion
This paper describes the basic work needed for the establishment of a biological dosimetry laboratory. The analysis of dicentrics from peripheral blood lymphocytes is a well-established methodology for dose estimation with a very good approximation for acute whole body exposures, partial body expositions are suspected when over-dispersion is observed, and mathematical approximations like Contaminated Poisson are used. For protracted expositions to ionising radiations or delayed sampling, the, analysis of stable chromosomal aberrations like translocations, is better used.
Background studies and calibration curves are needed to use these methodologies for dose estimation, and even do there are many published papers, each laboratory must obtain its own data because some discrepancies among centres have been published.
The data provided in this document has been obtained in our Centre, most of them have been reviewed in other Institutions especially for statistical evaluation.
Calibration curves are made in line with other already published, gamma and X-ray for the analysis of dicentrics have been validated with cases that have been analyzed.
Dicentrics and translocations studies of different Spanish populations, are the only nationwide, the observed frequencies in all of them conform to expectations and are consistent with the literature on other populations.
The use of translocation analysis for dose estimation is technically easy and it does not require highly qualified cytogenetist, but technique is more expensive and presents greater difficulties of interpretation. Because of its stable nature accumulate over time and has seen their number increases with age significantly, there are also data showing that not all chromosomes have the same radiosensitivity, some chromosomes present different translocations yields than expected, therefore further research is needed.
5. Conclusions
Dose assessment by biological dosimeters requires a large preceding standardization effort and a permanent update. It is important to establish some cooperation between different laboratories at national and international level, especially when a elevated number of people are to be analyzed. For large mass casualties cooperation among specialized Institutions is needed, there are several international cooperation projects in progress.
Our Centre has a large experience in dose estimation by biological dosimetry procedures, especially using the analysis of dicentrics from peripheral blood lymphocytes. For protracted over expositions to ionising radiations translocations is better used but it has more limitations due to background frequencies and age dependence.
Acknowledgement
This work was supported in part by the Spanish Nuclear Safety Council.
References
- 1.International Atomic Energy Agency. Biological dosimetry: chromosomal aberration. Analysis for dose assessment. Technical Reports Series. No. 260. IAEA, Viena; 1986.
- 2.International Atomic Energy Agency. Cytogenetic Analysis for radiation dose assessment, a manual. Technical Reports Series. No. 405, IAEA, Viena; 2001.
- 3.Natarajan A.T., Obe G. Molecular mechanisms involved in the production of chromosome aberrations. 1. Utilization of neurospora endonuclease for the study of aberrations production in G2 stage of the cell cycle. Mutat Res. 1978;52:137–149. doi: 10.1016/0027-5107(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 4.Darroudi F., Natarajan A.T., Van der Schans G.P., Van Loon A.A.W.M. Biochemical and cytogenetic characterization of Chinese hamster ovary x-ray-sensitive mutant cells xrs. Mutat Res. 1989;213:190–197. [Google Scholar]
- 5.Nowell P.C. Phytohemagglutinin – an inhibitor of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–466. [PubMed] [Google Scholar]
- 6.Evans H.J. Chromosome aberrations induced by ionizing radiations. Int Rev Cytol. 1962;13:221–321. [Google Scholar]
- 7.Brewen L.G., Preston R.J., Littlefield L.G. Radiation induced human chromosome aberration yields following an accidental whole-body exposure to 60-Co gamma-rays. Radiat Res. 1972;49:647–656. [PubMed] [Google Scholar]
- 8.Moorhead P.S., Nowell P.C., Mellmann W.J., Battips D.M., Hungerford D.A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 9.Perry P., Wolf S. New giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- 10.Lucas J.N., Deng W. Views on issuees in radiation biodosimetry based on chromosome translocations measured by FISH. Radiat Prot Dosimetry. 2000;88:77–86. [Google Scholar]
- 11.Lloyd D.C., Purrott R.J., Reeder E.J. The incidence of unstable chromosome aberrations in peripheral blood lymphocytes from unirradiated and occupationally exposed people. Mutat Res. 1980;72:523–532. doi: 10.1016/0027-5107(80)90123-2. [DOI] [PubMed] [Google Scholar]
- 12.Whitehouse C.A., Edwards A.A., Tawn E.J. Translocations yields in peripheral blood lymphocytes from control populations. Int J Radiat Biol. 2005;81:139–145. doi: 10.1080/09553000500103082. [DOI] [PubMed] [Google Scholar]
- 13.Hall E.J. 5th ed. Lippincott, Williams and Wilkins; Philadelphia, USA: 2000. Radiobiology for the radiologist. [Google Scholar]
- 14.Edwards A.A., Lloyd D.C., Purrot R.J. Proc 7th Symp Osford 1980, vol. 2. Harwood Academic Publishers; London, UK: 1980. Dicentric chromosome aberration yield in human lymphocytes and radiation quality. A resume including recent results using alfa particles, microdosimetry; pp. 1263–1273. [Google Scholar]
- 15.Lloyd D.C., Edwards A.A. Chromosome aberrations in human lymphocytes: effect of radiation quality, dose and dose-rate. In: Ishihara, Sasaki, editors. Radiation-induced chromosome damage in man. Alan R. Liss; New York, USA: 1983. pp. 23–49. [Google Scholar]


