Abstract
Background
Intensity modulated radiotherapy (IMRT) is being used to treat carcinoma of cervix (Ca Cx). Integral dose to normal tissue and increased leakage are the concern about IMRT. 6 MV photon beam is a good choice of energy for Ca Cx IMRT treatment.
Aim
The objective of this study was to compare intensity modulated radiotherapy (IMRT) plans generated by 6 MV and 15 MV photon energies for carcinoma of cervix (Ca Cx) with regards to dosimetric parameters of planning target volume (PTV) and organs at risk (OAR), homogeneity index (HI), conformity index at 98% level (CI 98%), integral dose to normal tissue (NTID) and total number of monitor units (MUs).
Material and methods
A cohort of 16 patients was selected for this study. All patients were to receive a dose of 50 Gy in 25 fractions. IMRT plans were generated for both energies using same dose–volume constraints.
Results
Our results show a comparable coverage of planning target volume (PTV) for both energies. Volume of PTV receiving a prescription dose is 97.8 ± 0.5% and 98.8 ± 0.4% for the 6 MV and the 15 MV plans. Volume of PTV receiving a dose of 107% is 4.4 ± 7.8% and 16.1 ± 22.2%. Bladder and rectum mean doses for the 6 MV and the 15 MV photon plans were 39.8 ± 3.0 Gy and 40.0 ± 3.2 Gy, and 35.8 ± 3.1 Gy and 36.0 ± 3.1 Gy, respectively. Homogeneity index (HI) for both energies was 1.04. The conformity indices at 98% isodose (CI 98%) were 1.3 ± 0.1 and 1.4 ± 0.1 for 6 MV and 15 MV photon plans, respectively.
Conclusions
We conclude that a 6 MV photon is a good choice for Ca Cx IMRT as it produces a highly conformal, homogeneous plan with superior target coverage and better OAR sparing.
Keywords: Carcinoma cervix, IMRT, Photon energy, Conformity index, Integral dose
1. Introduction
Carcinoma of cervix (Ca Cx) is a common gynecological cancer among women around the world.1 Radiation therapy has a long history in the treatment of Ca Cx. Three-dimensional conformal radiotherapy (3DCRT) for Ca Cx had been commonly delivered through a four-field beam arrangement untill intensity modulated radiotherapy (IMRT) came into practice. 3DCRT for carcinoma of cervix is most commonly delivered with high-energy photons. IMRT is an advanced form of a conformal radiation therapy. It conforms the prescription radiation dose to the shape of target tissue in three dimensions resulting in the sparing of normal surrounding tissues.2 IMRT involves multiple beams from different directions having nonuniform fluences. These beams are optimized to deliver a high dose to the target volume and an acceptably low dose to the surrounding normal structures. Sparing the surrounding normal tissue may reduce the risk of toxicity.
It has been shown that IMRT significantly reduces the volume of normal tissues irradiated to high doses in patients with gynecologic tumors.3,4 IMRT is increasingly being used nowadays in cervical cancer since several studies have reported its dosimetric and clinical benefit over a conventional whole pelvis external beam radiotherapy.5–7 In IMRT, total numbers of monitor units (MUs) are two to three times higher than in the conventional radiotherapy. That raises the concern about leakage radiation and secondary malignancy.8,9 Huq et al.10 showed a 40% increase in leakage between the leaves with high-energy photons (25 MV) compared to low energy (6 MV); the measured average leakage was 2.5% and 3.5% for 6 MV and 25 MV, respectively. Higher leakage may lead to a higher dose to the patient outside the irradiated volume. The concern about leakage and secondary malignancy makes photon beam energy an important parameter to be selected during IMRT planning.11 We have generated IMRT plans using low energy photons as well as high-energy photons. In this paper, we have studied the effect of beam energy on the quality of IMRT plans for Ca Cx. This paper investigates whether 15 MV beam IMRT offers a better target coverage and normal tissue sparing than 6 MV. Dosimetric parameters of target and OAR used for the comparison were mean dose, maximum dose, homogeneity index (HI), conformity index at 98% isodose level (CI 98%), integral dose to normal tissue (NTID), dose outside the target and total number of MUs for the plans generated for both energies.
2. Materials and methods
2.1. Patients characteristics
In this study we compared and evaluated the treatment plans in terms of dosimetric parameters using 6 MV and 15 MV photons for Ca Cx patients of different stages (II to III B). A cohort of 16 patients was selected retrospectively, who received treatment with 3DCRT or IMRT for Ca Cx. The median anterior-posterior and right–left separation, of the patient body, for the cohort was 21.0 ± 2.9 cm (ranging from 15 cm to 27.0 cm) and 34.0 ± 4.1 cm (ranging from 28.6 cm to 41.9 cm), respectively. Median PTV volume was 981.8 ± 290.3 cm3 (ranging from 648.6 cm3 to 1804.7 cm3). The rectum and bladder volumes were 83.5 ± 37.6 cm3 (ranging from 37.0 cm3 to 152 cm3) and 144.7 ± 80.9 cm3 (ranging from 66.1 cm3 to 332.5 cm3) respectively. We have found that a part of bladder and rectum is overlapping with PTV. We have calculated the non-overlapping volume of bladder (Bladder minus PTV) and rectum (Rectum minus PTV). The Bladder minus PTV volume was 98.1 ± 58.6 cm3 varying from 26.9 cm3 to 226.6 cm3. Rectum minus PTV volume was 60.8 ± 30.7 cm3 varying from 20 cm3 to 117 cm3.
2.2. Simulation, target and OAR delineation
CT simulation was done for all the patients in supine position. All patients were immobilized with a thermoplastic cast (Orfit Industry, Belgium). CT scans of each patient were obtained in the treatment position using LightSpeed VCT 64 slice CT scan (GE Medical Systems, LLC, Waukesha, WI, USA). CT scans were obtained at 2.5 mm slice thickness. The CT scans were obtained from the L2 vertebral body to 5 cm below the ischial tuberosities, which is consistent with other researchers.6 All patients were CT scanned with full bladder. All patients were simulated, planned and treated in a similar manner. All structures, gross target volume (GTV), clinical target volume (CTV), planning target volumes (PTV) were marked by radiation oncologist using ICRU recommendations.12 Inter-observer variability of contouring was not considered in this study. A uniform margin of 0.5 cm was used to create PTV from CTV expansion. Li et al.13 recommended the use of 0.83 cm as a CTV–PTV margin for pelvic tumors (including prostate and gynecologic malignancies) based on mega voltage cone beam CT (MVCT) imaging. Santanam et al.14 used a margin of 0.7 cm for gynecological malignancy. Mundt et al.7 used 1 cm PTV margin which greatly increased the volume of normal tissue irradiated. They found that upper and lower body immobilization may allow smaller PTV expansion resulting in a less normal tissue irradiation. Reduction in CTV to PTV expansion can be accomplished through an improved immobilization and the use of online imaging.15 As we use pelvic immobilization and kilovoltage (kV) imaging and cone beam computed tomography (CBCT) for our patient's setup, and it is a known fact that the kV imaging has superior soft tissue contrast,16 we have taken a 0.5 cm margin to create PTV. Rectum, bladder, small bowl and both femoral heads were marked as organs at risk (OAR). A non-overlapping structure for bladder (Bladder minus PTV) and rectum (Rectum minus PTV) was created using Boolean operation during structure delineation to optimize the dosimetry. To evaluate the dose to normal tissues (NT), a structure NT, consisting of non-PTV tissue, was created by contouring all the tissue within the external skin contour of the patient.
2.3. Treatment objective and planning
All patients were planned to a total dose of 50 Gy in 25 fractions. Our goal was to cover 98% PTV volume to 98% of the prescription dose. Dose to rectum and bladder was restricted in such a way that <40% volume should receive a dose of 40 Gy.6 Dose to both femoral heads were restricted, the maximum dose is <50 Gy and V45 < 10%, “i.e.” not more than 10% of either femoral head volume should receive a dose of 45 Gy. Clinically acceptable plans were generated on Eclipse (Version 8.5) inverse treatment planning system (Varian Medical Systems, Concord, Palo Alto, USA) using a pencil beam convolution dose calculation algorithm, for both energy levels. Fields were selected so that all the entrance and exit beams were evenly spaced around the patients. We have used seven evenly spaced beams at a gantry angle of 0, 51, 102, 153, 204, 255 and 304. Beam arrangement and dose constraints were the same for all the plans so that we can elucidate the impact of using different energies. During optimization of the 15 MV plan it was obvious that the modifications of the objective, constraints and respective weights might have resulted for particular patients in a clinically improved plan, but we did not change any parameters in order to maintain the optimum plan comparability with 6 MV. Normal tissue objective was used during optimization to reduce hotspots outside PTV. We have not tried to spare bone marrow. Due to the retrospective nature of this study none of the IMRT plan was actually delivered. The delivery of the treatment plan was simulated using a dynamic multileaf collimator (DMLC) of actual beam data of a Trilogy Tx accelerator equipped with 120 millenium MLC.
2.4. Dose reporting and evaluation
We have evaluated PTV coverage by computing V47.5, V49, V50, V53.5 and V55, which is the volume of PTV receiving 47.5 Gy, 49 Gy, 50 Gy, 53.5 Gy and 55 Gy of dose.
We have evaluated the homogeneity index (HI), and it was defined as the ratio of dose received by 5% volume of PTV (lower index) to 95% volume (upper index), “i.e.” D5/D95, where D5 (lower index) and D95 (upper index) are the doses received by the 5% and 95% volume of the PTV.
Conformality of high dose around the target was evaluated by calculating the conformity index (CI) at a given isodose level, e.g. 98% (CI 98%). CI was defined as the ratio of the volume of total tissue receiving the reference dose to the volume of PTV.
| (1) |
Rectum and bladder were evaluated for mean dose and V40, where V40 is the volume of rectum and bladder which is receiving a dose of 40 Gy. Femoral heads were evaluated for maximum dose and V45, “i.e.” volume of femoral head receiving 45 Gy dose.
To find the dose to normal tissues outside the PTV, integral dose to normal tissue (NTID) was calculated. NTID was calculated manually and defined as a mean dose times the volume of the structure.
| (2) |
NTID has no significance during optimization. NTID was calculated to evaluate the quality of the plan. A two-tailed t-test was used to compare the data of the two energies. A value of p < 0.05 was considered statistically significant.
3. Results
Comparison of plans using 6 MV and 15 MV photon intensity modulated beam were carried out for a cohort of 16 patients.
3.1. Isodose distribution
Virtual inspection of isodose surrounding the target was comparable. The 6 MV plans have shown a slightly sharper dose gradient than the 15 MV plans. To reduce the risk of sever toxicity, no plan was accepted with a hot spot along the bladder and rectal walls as these areas will get a considerable amount of radiation dose during intracavitary brachytherapy. The dose distribution for a patient along axial, coronal and sagittal planes are shown in Fig. 1 for the 6 MV and the 15 MV plans respectively. The dose volume histograms (DVH) for PTV and other critical structures are shown in Fig. 2 for both the 6 MV and the 15 MV photon beams of the same patient.
Fig. 1.
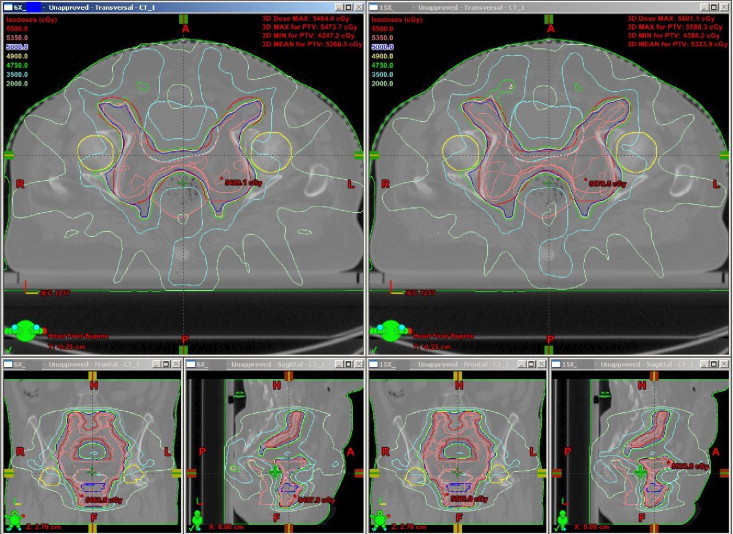
Dose distribution on axial, coronal and sagittal views for 6 MV and 15 MV plans.
Fig. 2.
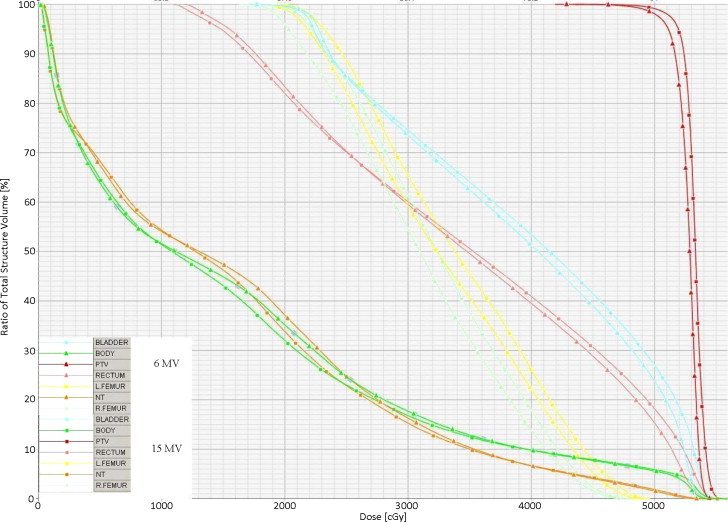
DVH for PTV and other critical structures are shown for both 6 MV and 15 MV photon beams of the same patient.
3.2. Dose volume histogram analysis of PTV and OAR
DVH were calculated for target volume and organ at risk. The small differences indicate that the plans are nearly identical in their conformality of the radiation dose. PTV dosimetry data are given in Table 1. Bladder, rectum and femoral head dosimetric data regarding mean dose, V40 and V45 are given in Table 2.
Table 1.
PTV dosimetry parameters.
| PTV | 6 MV |
15 MV |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Mean dose (Gy) | 52.24 | 0.35 | 52.59 | 0.51 |
| V47.5 | 99.66 | 0.15 | 99.79 | 0.14 |
| V49 | 99.02 | 0.25 | 99.44 | 0.26 |
| V50 | 97.82 | 0.50 | 98.8 | 0.43 |
| V53.5 | 4.45 | 7.81 | 16.1 | 22.18 |
| V55 | 0 | 0 | 0.53 | 1.81 |
Table 2.
Dosimetric data of bladder, rectum and both femoral heads.
| Structures | 6 MV |
15 MV |
p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Bladder | |||||
| Mean dose (Gy) | 39.84 | 3.06 | 40.07 | 3.17 | 0.837 |
| V40 (%) | 55.71 | 11.54 | 56.85 | 11.20 | 0.779 |
| Rectum | |||||
| Mean dose (Gy) | 35.80 | 3.15 | 36.01 | 3.15 | 0.851 |
| V40 (%) | 47.09 | 8.06 | 47.97 | 7.83 | 0.754 |
| Left femoral head | |||||
| Dmax (Gy) | 47.65 | 1.88 | 48.37 | 1.63 | 0.257 |
| V45 (%) | 1.91 | 2.66 | 2.70 | 3.38 | 0.470 |
| Right femoral head | |||||
| Dmax (Gy) | 46.04 | 4.90 | 46.78 | 4.78 | 0.669 |
| V45 (%) | 1.64 | 2.04 | 2.29 | 2.65 | 0.443 |
3.3. Homogeneity, conformity index and integral dose
Homogeneity index (HI) for both energies is 1.04. The target dose conformity is determined by comparing the volume of the PTV with the volume encompassed by the 98% isodoses surface. Our results show that the CI 98% for the 6 MV photon is slightly better than for 15 MV, “i.e.” the 6 MV photon plans are able to produce sharper and tight dose distribution around the PTV. The 15 MV photon plan delivers 11.3 ± 2.1% less MUs than the 6 MV photon plans. NTID is calculated using Eq. (2). Results are shown in Table 3. We found that the 6 MV photon plans deliver 3.1 ± 1.9 higher NTID in comparison to the 15 MV photon. The mean CI 98% was 1.3 ± 0.1 and 1.4 ± 0.1 for the 6 MV and the 15 MV photon plans, respectively, which is not statistically significant.
Table 3.
Plan comparison parameters.
| Parameters | 6 MV |
15 MV |
p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HI | 1.047 | 0.006 | 1.042 | 0.007 | 0.05 |
| MUs | 1573 | 327.43 | 1393.5 | 280.41 | 0.106 |
| CI 98% | 1.29 | 0.10 | 1.35 | 0.11 | 0.168 |
| NTID | 261.45 | 61.74 | 253.21 | 57.69 | 0.699 |
4. Discussion
The present study does not show any significant difference between the 6 MV and 15 MV photon energies for any of the evaluated parameters. We have found that the 6 MV and 15 MV plans are comparable in terms of target coverage and critical structure sparing. ICRU 50 recommends a uniform dose to the target volume within −5% to 7% of the dose prescribed.12 However, a ±10% variation from the prescription is an acceptable norm in most clinical practice and is widely used in IMRT.17 Roeske et al.5 and Mundt et al.7 reported that 110% and 115% of the prescription dose should be <20% and 2% respectively. Mell et al.18 described a tighter dose limit to PTV where 110% and 115% of the prescription dose should be <10% and <1%, respectively. We have not evaluated 115% isodose in either plan. There is no isodose of 110% of the prescription dose for the 6 MV photon, however the 15 MV photon does deliver 110% dose in 19% patients. Volume of PTV receiving a dose of 53.5 Gy (V53.5) is 4.5 ± 7.8% and 16.1 ± 22.9% for the 6 MV and 15 MV photon plans, respectively, which is not statistically significant (p-value 0.056). Volume of PTV receiving a dose of 49 Gy (V49) is 99.0 ± 0.3% and 99.4 ± 0.3% for 6 MV and 15 MV photon plans, respectively, and it is not statistically significant. Bladder mean dose is 39.8 ± 3.1 Gy and 40.1 ± 3.2 Gy for 6 MV and 15 MV photon plans, respectively. Volume of bladder receiving a dose of 40 Gy (V40) is 55.8 ± 11.5% and 56.9 ± 11.2% for the 6 MV and 15 MV beams, respectively. It is higher than reported by Mundt et al.,5 however, they prescribed 45 Gy dose to PTV and we prescribed 50 Gy. Rectum mean dose is 35.8 ± 3.1 Gy and 36.0 ± 3.2 Gy for the 6 MV and 15 MV, respectively. Volume of rectum receiving a dose of 40 Gy (V40) is 47.1 ± 8.1% and 48.0 ± 7.9% for the 6 MV and 15 MV photon plans, respectively. It is higher than reported by Mundt et al.,6 due to aforementioned reason. Bladder and rectum doses are not statistically significant for the 6 MV and 15 MV photon energies.
HI for both energies is 1.04. The mean conformity index (CI 98%) was 1.3 ± 0.1 and 1.4 ± 0.1 for the 6 MV and 15 MV, respectively, and it was not statistically significant. These small differences indicate that the plans are nearly identical in their conformity of dose to the target, however, the 6 MV photon plans are slightly superior to the 15 MV photon plans, “i.e.” spillage of high dose outside PTV is lower in the 6 MV photon plans.
D'Souza and Rosen19 calculated NTID for prostate case considering that it has uniform density. We have also assumed that the NT has uniform density for the ID calculation. We found that a low entrance dose for the 15 MV beam was almost compensated by a high exit dose even for a very large patient. Aoyama et al.20 had similar findings and they concluded that a reduced integral dose from the buildup portion is limited by a higher exit dose and there is a need for a larger beam area to accommodate the wider penumbra of the high-energy beam. It has been reported that the increase in NTID, due to multiple beam radiation therapy, is a potential risk factor for the development of secondary malignancies.9,21 Followill et al.22 made an estimation of the whole body dose equivalent resulting from IMRT. They concluded that, compared with conventional radiotherapy, IMRT may more than double the risk of secondary cancers from 0.4% to 1%. These figures are applied to a 6 MV photon beam while estimates were much higher for 18 MV and for tomotherapy. IMRT is likely to double the incidence of second malignancies compared with conventional radiotherapy from about 1% to 1.75% for patients surviving 10 years. The incidence of radiation induced secondary malignancy are rare though. There are multiple sources of leakage of radiation dose to patient, secondary neutron dose to patient, leakage through primary collimator, leakage through MLC. Leakage through MLC will add substantially to NTID.20 This risk becomes more pronounced at higher photon energies where there is neutron production. We found in our study that 6 MV delivers 3.1 ± 1.9% higher NTID. Our data is consistent with D'Souza and Rosen,19 Aoyama et al.20 and Pirzkall et al.23
Das and Kenneth24 demonstrated that there is no significant improvement in the dose distribution and integral dose ratio for photon energy above 15 MV. Sternick et al.25 showed that in treating prostate with rotational IMRT there was no difference in dose distribution for energies ranging from 4 MV to 18 MV. Soderstrom et al.26 demonstrated that the use of an optimized intensity modulated photon beam significantly reduce the need of beam energy selection. Solaiappan et al.27 reported that the percentage dose received by the 15% volume of rectum and bladder were higher for 10 MV photon energy. Sun and Ma28 reported that 6 MV plans deliver 18% more MU than 18 MV plans. In our study, the 6 MV photon plans delivered 11.3 ± 2.1% more MUs than 15 MV photon plans, which is slightly better than reported by Sun and Ma28 In theory, the increased treatment time can be compensated by increasing the dose rate and the number of MUs can be reduced by smoothing the fluence without compromising the quality of plan,29,30,31 although the ratio of MUs remains the same for 6 MV and 15 MV plans. New modalities, such as the volumetric intensity modulated radiotherapy, have the potential to reduce the MUs and treatment time to less than 2 min.32
5. Conclusion
The study was a comparative dosimetric evaluation of 6 MV and 15 MV photon beam IMRT plans for Ca Cx. We found that 6 MV plans produce relatively less hot spots than 15 MV plans, although the clinical impact of these dosimetric improvements remain unanswered. Our results revealed that, there is no clinical advantage of 15 MV over 6 MV in terms of target coverage and normal tissue sparing. Our study has shown the feasibility of achieving the desired dose distribution with 6 MV. We conclude that 6 MV photon energy is a good choice for Ca Cx IMRT.
Conflict of interest
There is no conflict of interest.
Disclosure
A part of this work has been accepted for poster viewing presentation in 52nd annual meeting, October 31–November 4, 2010, San Diego, CA, USA.
Acknowledgements
We thank Drs. S. Hukku and S. Halder, Department of Radiation Oncology, BLK Memorial Hospital, New Delhi, India and Dr. Kundan S. Chufal, Department of Oncology, Batra Hospital, New Delhi, India for their continued help and support to complete this work. We acknowledge both the unknown reviewer for their suggestion to improve the quality of the manuscript.
References
- 1.Jemal A., Tiwari R.C., Murray T. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51(November (4)):880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 3.Mundt A.J., Roeske J.C., Lujan A.E. Initial clinical experience with intensity-modulated whole-pelvis radiation therapy in women with gynecologic malignancies. Gynecol Oncol. 2001;82(3):456–463. doi: 10.1006/gyno.2001.6250. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q., Izadifar N., King S. Comparison of IMRT with 3-D CRT for gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2001;51(3 (Suppl. 1)):332. [Google Scholar]
- 5.Roeske J.C., Lujan A., Rotmensch J., Waggoner S.E., Yamada D., Mundt A.J. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48(5):1613–1621. doi: 10.1016/s0360-3016(00)00771-9. [DOI] [PubMed] [Google Scholar]
- 6.Mundt A.J., Lujan A.E., Rotmensch J. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52(5):1330–1337. doi: 10.1016/s0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 7.Mundt A.J., Roeske J.C., Lujan A.E. Intensity-modulated radiation therapy in gynecologic malignancies. Med Dosim. 2002;27(2):131–136. doi: 10.1016/s0958-3947(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 8.Hall E.J., Wuu C.S. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 9.Hall E.J. Intensity modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(May (1)):1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Huq M.S., Yu Y., Chen Z.P., Suntharalingam N. Dosimetric characteristics of a commercial multileaf collimator. Med Phys. 1995;22(February (2)):241–247. doi: 10.1118/1.597461. [DOI] [PubMed] [Google Scholar]
- 11.Welsh J.S., Mackie T.R., Limmer J.P. High-energy photons in IMRT: uncertainties and risks for questionable gain. Technol Cancer Res Treat. 2007;6(April (2)):147–149. doi: 10.1177/153303460700600212. [DOI] [PubMed] [Google Scholar]
- 12.International Commission on Radiation Units and Measurements . International Commission on Radiation Units and Measurements; Bethesda, MD: 1993. Prescribing, recording, and reporting photon beam therapy, report number 50. [Google Scholar]
- 13.Li X.A., Qi X.S., Pitterle M. Interfractional variations in patient setup and anatomic change assessed by daily computed tomography. Int J Radiat Oncol Biol Phys. 2007;68(2):581–591. doi: 10.1016/j.ijrobp.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Santanam L., Esthappan J., Mutic S. Estimation of setup uncertainty using planar and MVCT imaging for gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2008;71(5):1511–1517. doi: 10.1016/j.ijrobp.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 15.Roeske J.C., Bonta D., Mell L.K., Lujan A.E., Mundt A.J. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69(2):201–207. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Xing L., Chang J., Orton C.G. Point/counterpoint. Kilovoltage imaging is more suitable than megavoltage imaging for guiding radiation therapy. Med Phys. 2007;34(December (12)):4563–4566. doi: 10.1118/1.2799489. [DOI] [PubMed] [Google Scholar]
- 17.Das I.J., Cheng C.-W., Chopra K.L., Mitra R.K., Srivastava S.P., Glatstein E. Intensity-modulated radiation therapy dose prescription, recording, and delivery: patterns of variability among institutions and treatment planning systems. J Natl Cancer Inst. 2008;100(5):300–307. doi: 10.1093/jnci/djn020. [DOI] [PubMed] [Google Scholar]
- 18.Mell L.K., Roeske J.C., Mehta N., Mundt A.J. In: Intensity modulated radiation therapy: a clinical perspective. 1st ed. Mundt A.J., Roeske J.C., editors. PMPH; USA: 2005. pp. 492–505. (Chapter 23) [Google Scholar]
- 19.D'Souza W.D., Rosen I.I. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med Phys. 2003;30(8):2065–2071. doi: 10.1118/1.1591991. [DOI] [PubMed] [Google Scholar]
- 20.Aoyama H., Westerley D.C., Mackie T.R. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys. 2006;64(3):962–967. doi: 10.1016/j.ijrobp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Kry S.F., Salehpour M., Followill D.S. The calculated risk of fatal secondary malignancies from intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62(4):1195–1203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Followill D., Geis P., Boyer A. Estimates of whole body dose equivalent produced by beam intensity modulated conformal therapy. Int J Radiat Oncol Biol Phys. 1997;38(3):667–672. doi: 10.1016/s0360-3016(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 23.Pirzkall A., Carol M.P., Pickett B., Xia P., Roach M., 3rd, Verhey L.J. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int J Radiat Oncol Biol Phys. 2002;53(2):434–442. doi: 10.1016/s0360-3016(02)02750-5. [DOI] [PubMed] [Google Scholar]
- 24.Das I.J., Kenneth R.K. Higher energy: is it necessary, is it worth the cost for radiation oncology? Med Phys. 1992;19(4):917–925. doi: 10.1118/1.596779. [DOI] [PubMed] [Google Scholar]
- 25.Sternick ES, Bleier AR, Carol MP, et al. Intensity modulated radiation therapy: what photon energy is best? In: Leavitt DD, editor. Presentation at The XII international conference on the use of computers in radiation therapy (ICCR), Salt Lake City, UT, 27–30 May 1997. Medical Physics Publishing; 1997, p. 418–9.
- 26.Soderstrom S., Eklof A., Brahme A. Aspects on the optimal photon beam energy for radiation therapy. Acta Oncol. 1999;38(2):179–187. doi: 10.1080/028418699431591. [DOI] [PubMed] [Google Scholar]
- 27.Solaiappan G., Singaravelu G., Prakasarao A., Rabbani B., Supe S.S. Influence of photon beam energy on IMRT plan quality for radiotherapy of prostate cancer. Rep Pract Oncol Radiother. 2009;14(1):18–31. [Google Scholar]
- 28.Sun M., Ma L. Treatment of exceptionally large prostate cancer patients with low-energy intensity-modulated photons. J Appl Clin Med Phys. 2006;7(4):43–49. doi: 10.1120/jacmp.v7i4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matuszak M.M., Larsen E.W., Jee K.W., McShan D.L., Fraass B.A. Adaptive diffusion smoothing: a diffusion-based method to reduce IMRT field complexity. Med Phys. 2008;35(April (4)):1532–1546. doi: 10.1118/1.2889703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spirou S.V., Fournier-Bidoz N., Yang J., Chui C.S., Ling C.C. Smoothing intensity-modulated beam profiles to improve the efficiency of delivery. Med Phys. 2001;28(October (10)):2105–2112. doi: 10.1118/1.1406522. [DOI] [PubMed] [Google Scholar]
- 31.Giorgia N., Antonella F., Eugenio V., Alessandro C., Filippo A., Luca C. What is an acceptably smoothed fluence? Dosimetric and delivery considerations for dynamic sliding window IMRT. Radiat Oncol. 2007;2(November):42. doi: 10.1186/1748-717X-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cozzi L., Dinshaw K.A., Shrivastava S.K. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol. 2008;89(2):180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]


