Abstract
Aim
Review of main SBRT features and indications in primary tumors.
Background
Stereotactic body radiotherapy has been developed in the last few years. SBRT allows the hypofractionated treatment of extra cranial tumors, using either a single or limited number of dose fractions, and resulting in the delivery of a high biological effective dose with low toxicity.
Material and methods
SBRT requires a high level of accuracy for all phases of the treatment process: effective patient immobilization, precise target localization, highly conformed dosimetry and image guided systems for treatment verification. The implementation of SBRT in routine requires a careful considering of organ motion. Gating and tracking are effective ways to do so, and less invasive technologies “fiducials free” have been developed. Due to the hypofractionated scheme, the physician must pay attention to new dosimetric constraints in organ at risk and new radiobiological models are needed to assess the optimal fractionation and dose schemes.
Results
Currently, SBRT is safe and effective to treat primary tumors, which are otherwise untreatable with conventional radiotherapy or surgery. SBRT has quickly developed because of its excellent results in terms of tolerance and its high locoregional control rates. SBRT indications in primary tumors, such as lung primary tumors, have become a standard of care for inoperable patients. SBRT seems to be effective in many others indications in curative or palliative intent such as liver primary tumors, and novel indications and strategies are currently emerging in prostate cancer, head and neck tumor recurrences or pelvis reirradiations.
Conclusion
Currently, SBRT is mainly used when there is no other therapeutic alternative for the patient. This is due to the lack of randomized trials in these settings. However, the results shown in retrospective studies let us hope to impose SBRT as a new standard of care for many patients in the next few years.
Keywords: SBRT, Requirements, Organ motion, Early stage NCSLC, Hepatocellular carcinoma, Head and neck recurrences
1. SBRT overview and requirements
Over the last decades stereotactic cranial radiosurgery has been successfully used for the treatment of intracranial lesions as it can precisely deliver a high dose of radiation to a tumor with very low dose to surrounding critical tissues. The good results obtained in intra-cranial localizations have led to the development of extra-cranial stereotactic radiation therapy known as stereotactic body radiation therapy (SBRT). SBRT, as a precise external beam radiation technique, allows the hypofractionated treatment of extra cranial tumors, using either a single or limited number of dose fractions, and resulting in the delivery of a high biological effective dose, often above 100 Gy which could be considered as “ablative” dose (stereotactic ablative body radiotherapy “SABR” is another suggested name for this technique). As in intracranial stereotactic radiosurgery, SBRT requires a high level of accuracy for all phases of the treatment process and organ and patient motion must be considered in the treatment planning. Recommendations and treatment quality control guidelines have also been established for SBRT.1,2
Accuracy in SBRT requires effective patient immobilization, precise target localization with the integration of modern image systems (CT, MRI, PET-CT) in order to properly define the tumor area for treatment and to further minimize radiation dose to healthy surrounding tissues; treatment planning also requires highly conformed dosimetry and isotropic dose fall-off (Fig. 1). Dose calculations should be carried out with algorithms which can account for the effects of tissue heterogeneities. Therefore, in order to deliver these accurate doses, linear accelerators must be equipped with micromultileaf collimators, the possibility of using multiple non overlapping beams of radiation and even intensity modulated radiation therapy.
Fig. 1.
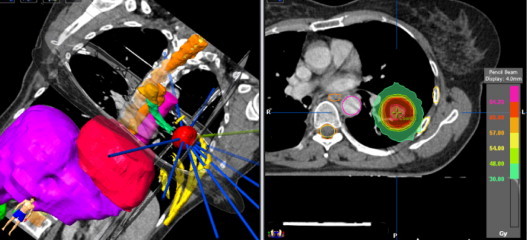
SBRT treatment for early stage peripheral NSCL cancer. Highly conformed dosimetry with multiple beams. Dose prescribed: 60 Gy in 3 fractions of 20 Gy.
One of the key challenges and requirements of SBRT is to reach the same “stereotactic accuracy” for extra cranial treatment as is achieved for cranial treatments. SBRT requires new image-guided verification techniques (IGRT), such as cone beam imaging or stereoscopic X-ray imaging, that allow a precise treatment of non-moving lesions (spine, prostate, etc.). For moving lesions such as lung and liver tumors, problems with accuracy still remain a challenge, and the assessment of tumor motion can be approached in different ways. Quantification of tumor motion can be measured and an ITV (internal target volume) can be deduced using a 4D-CT scan during the different respiratory phases. To reduce the target treatment area and spare healthy tissue more effectively, restrictive techniques, such as “dampening” with abdominal compression, can be used. Other methods called “compensating techniques” can not only quantify tumor motion but also have an intra-fraction control of tumor position and can treat the tumor exactly at the place where it is located, by visualizing its motion during the treatment. These techniques allow a precise delivery of high dose of radiation to the tumor with minimal dose to surrounding critical tissue (Fig. 1).
Novalis Exactrac Adaptive Gating® technique has been developed as a method that improves the accuracy of SBRT for lung and liver lesions, by monitoring organ tumor motion and irradiating within a selected area of the respiratory cycle. The tumor can be irradiated in this selected area by using internal markers previously implemented close to or directly into the tumor. Based on external markers, internal tumor motion is correlated with the external respiratory signal. During patient setup, infrared markers track the respiratory cycle. The Exactrac image guided system localizes the internal marker by X-rays and quantifies the tumor motion by correlating the external marker motion to the internal marker position. A selected area of this cycle is defined as the “beam on area” of irradiation. The validity of this model is verified in real time by ExacTrac X-Rays3,4 (Fig. 2).
Fig. 2.
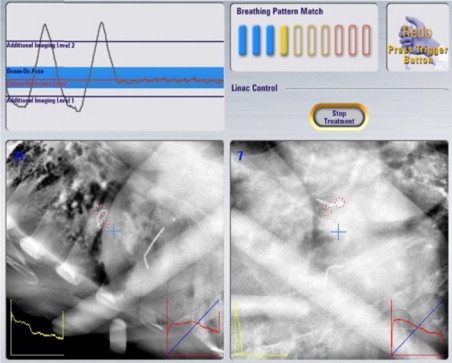
Organ motion compensating technique example: Novalis® Exactrac Adaptive Gating SBRT for lung and liver lesions. Monitoring organ tumor motion and irradiation within a selected area of the respiratory cycle with Exactrac image guide system localization of the internal marker.
“Tracking” is another compensating technique, and the tumor can be followed by “tracking” these fiducials or even by tracking certain tumors visible in the guidance X-ray image system. The CyberKnife system can track the tumor during the treatment using fiducials. However, transthoracic fiducials implantation under CT guidance can be responsible for pneumothorax (24–45%).5,6 A fiducial-free tracking system, Xsight® Lung Tracking System (XLTS), has therefore been designed. This system requires that the tumor is wider than 15 mm and that it is visible in the orthogonal X-ray images (the tumor must be distant of at least 15 mm from any major vascular structures and its projection on the spine must be different from 45°). The system also uses a correlation model between external markers and the internal tumor positions to attain a millimetric precision. Both tracking systems can be combined with the Synchrony Respiratory Tracking System in order to synchronize the beam targeting with the respiratory cycle.7
All these compensating techniques reduce the treated volume and the dose to the normal tissue. Therefore, accuracy in all phases of SBRT procedure is absolutely required in order to give effective and safe treatments with high and doses to the tumor and low risk of toxicity for the patient.
2. SBRT for primary lung tumors
Non small cell lung cancer (NSCLC) is a leading cause of cancer death in the world. Approximately 20% of patients are diagnosed with stage I, and surgery is the standard treatment for them with overall survival rates of 60–70%.8,9 Surgical lobectomy may be associated with significant morbidity and mortality, and up to 25% of the patients cannot be operated due to a poor pulmonary function or comorbidities. With the wait and see policy, the overall median survival of these patients is only 9 months. Surgery is associated with the deterioration of quality of life, and in order to reduce its morbidity and risks of mortality, video-assisted thoracic surgery (VATS) has also been proposed.10,11
Until recently, for non operable stage I NSCLC, conventional radiotherapy (3D-CRT) has been the treatment of choice reaching local recurrence rates of 40% and specific survival of 39% at 3 years.12
Other alternatives, as radiofrequency ablation (RFA), have been explored for the treatment of patients who are not candidates for surgery on the basis of age, underlying lung disease, or other medical comorbidities. RFA works by transferring radiofrequency energy from a generator through an electrode, causing coagulation and necrosis of the tumor. The results of the published studies indicate the best results are achieved in small lesions (≤3 cm). Median survival of patients receiving RFA ranged from 8.6 months to 33 months, 1 year survival rate ranged from 63 to 85% and 3 year survival rate ranged from 15 to 46%. The 1–3 year local tumor progression-free rates were 45–83% and 25–57%, respectively. The adverse effects associated with RFA include pneumothorax (28–49%), pleural effusion, hemoptysis or pain.13,14
SBRT offers the possibility to treat these patients efficiently with a minimal risk of toxicity and to escalate dose of radiotherapy. SBRT has emerged as a new cancer therapy for the treatment of early-stage NSCLC, showing excellent clinical results without high costs in terms of toxicity, which has allowed to argue that the new technique is an alternative not only to surgery in inoperable patients, but perhaps also in operable patients.
The cost-effectiveness of these alternatives, 3D-CRT, SBRT, and RFA, has been analyzed. In comparison to 3D-CRT and RFA, SBRT was the most cost-effective treatment for medically inoperable NSCLC over a wide range of treatment and disease assumptions, so on these basis, SBRT could be the primary treatment approach for this disease.15
3. Lung SBRT retrospective studies
Early experience with SBRT for early stage NSCLC come from Sweden and Japan with excellent results in local control and survival in several retrospective studies.16–20 Perhaps the most significant study was a multi-institutional Japanese study published by Onishi, including 245 patients, of whom 158 (65%) inoperable.21 The dose administered was 18–75 Gy in 1–22 fractions with an average of biological equivalent dose (DEB) > 108 Gy. With a mean follow up of 24 months, the overall response rate was 85% and overall survival at 3 and 5 years was 56% and 47%, respectively. This study showed that patients treated with SBRT with DEB > 100 Gy had better overall survival rates, and concluded that these doses are adequate to achieve good local control and survival in stage I NSCL.
4. Lung SBRT prospective studies
Indiana University is one of the first prospective studies of SBRT for early-stage NSCLC. It is a phase I study of 47 inoperable stage I (T1-T2) patients. The maximum tolerated dose was not reached for tumors smaller than 5 cm, reaching levels of 20–22 Gy dose × 3 (60–66 Gy).22 Based on this study, Timmermann performed a phase II in 70 patients using a scheme of 3 fractions of 20 Gy for T1 and 3 of 22 Gy for T2 tumors.23 With a median follow-up of 17.5 months, the rate of LC at 2 years was 95% and overall survival was 55%. Patients with central tumors had a complication rate significantly higher than patients with peripheral tumors, with grade 5 toxicity recorded in 6 patients. In the multivariate analysis, tumor size also significantly influenced the occurrence of grade 3–5 toxicity although it was not such a powerful predictor as tumor location (Table 1).
Table 1.
Selected published series of stereotactic body radiotherapy for early stage NSCLC. Retrospective and phase I–II studies.
| Author | Patients | Study | Doses | Local control | Toxicity |
|---|---|---|---|---|---|
| Onishi 200421 | 245 | Multicentric retrospective | 18–75 Gy/1–22 fx | 5 years 84% | 7.6% grade ≥ 3 |
| Baumann 200620 | 138 | Multicentric retrospective | 30–48 Gy/3 fx | 33 months 88% | 10% grade ≥ 3 |
| McGarry 200522 | 47 | Phase I | 60–66 Gy/3 fx | 15 months 79% | 15% grade ≥ 3 |
| Timmerman 200623,24 | 70 | Phase II | 60–66 Gy/3 fx | 2 years 95% | 20% grade ≥ 3 |
| Zimmerman 200518 | 68 | Retrospective | 24–40 Gy/3–5 fx | 3 years 88% | 9% grade ≥ 3 |
| Nyman 200619 | 57 | Phase II | 15 Gy/3 fx | 3 years 92% | 26% grade ≥ 3 |
| Lagerwaard 200826 | 206 | Multicentric retrospective | 60 Gy (3 × 20 Gy/5 × 12 Gy/8 × 7.5 Gy) | 3 years 93% | 6% grade ≥ 3 |
This phase II study was the basis of the RTOG 0236 phase II study performed in 59 biopsy-proven peripheral T1T2N0M0 tumors which were treated with 54 Gy in three fractions Patients with tumors ≤2 cm from the proximal bronchial tree were excluded. In the 55 evaluable patients, at a median follow-up of 34 months, there were two grade four adverse events, the 3-year primary tumor control rate was 98%, and 3-year local control rate was 91%. The 3-year disease-free and overall survival rates were 48 and 56%, respectively.24
A phase I/II study (RTOG 0813, NCT00750269) evaluated escalating doses delivered in five fractions for centrally located tumors. The goal of that trial was to determine whether a modified schedule may minimize toxicity for centrally located tumors. The starting dose was 50 Gy in five fractions (10 Gy per fraction) with escalation to 12 Gy per fraction for tumors located within the proximal bronchial tree.25
In 2008, the Dutch multi-center experience in lung SBRT was published, including a total of 206 patients who had inoperable early stage SNCLC or who refused surgery.26 Only 31% of the patients had histological confirmation of malignancy. Both central and peripheral tumors were included, using a “risk adjusted” fractionation scheme according to size and tumor location. Three fractions of 20 Gy were used for small tumors, 5 fractions of 12 Gy for large tumors or those close to chest wall and 8 fractions of 7.5 Gy for central tumors. Two years’ results were a 93% LC, 64% OS and grade 3 late toxicity ≤6%. The update of this study with 676 patients treated between 2003 and 2011 found a median overall survival of 41 months (35–47 months) and a 5 year local control of 89%27 (Table 1).
This data from literature has already shown that SBRT can be firmly established as a standard of care for early stage NSCLC inoperable patients, with very high local control and overall survival rates and relatively low morbidity.24–26
These excellent results in inoperable patients bring the challenge to show if SBRT can also be an alternative to surgical treatment in operable patients, although this question is yet to be answered.28
There are recent data of a prospective phase II trial (RTOG 0618) of SBRT for early stage non-small cell lung cancer in operable patients with peripheral T1-T3, N0, M0 tumors. The prescription dose was 54 Gy (3 fractions of 18 Gy). Of 26 evaluable patients, 16% had SBRT related grade 3 toxicity. Median follow-up was 25 months. 2-year primary tumor failure rate of 7.7%. 2-year estimates of PFS and OS are 65.4% and 84.4%.29,11 There is at least another ongoing phase II trial (Japanese Clinical Oncology group 0403) of SBRT in operable patients.30
The started prospective phase III studies (ROSEL and STARS) randomizing surgery with SBRT in operable stage IA NSCLC had difficulties recruiting patients and were closed.31,32
At this moment the only phase III study comparing SBRT and surgery is the ACOSOG Z4099-RTOG 1021 trial. This trials compares sublobar resection with or without brachytherapy (I 125 implant at the resection margin) in high risk stage IA-IB NSCLC.33
5. Lung SBRT with intrafraction control of tumor motion
Accuracy in the treatment of lung tumors with SBRT is one of the main issues, and compensating techniques with gating and tracking can add an advantage to this treatments in terms of toxicity. Early experience with Novalis Exactrac Adaptive Gating has been published for 34 patients.34 Peripheral lung lesions were treated with 3 fractions of 15–20 Gy and central lung lesions with 5 fractions of 10 Gy. Internal markers were implanted in all the patients with a 26% rate of pneumotorax. Two year local control was 93.8%. Clinical tolerance was excellent, and no acute or late lung grade 3 was observed.
Bibault35 reported 51 patients treated with SBRT with the XLTS without fiducials. The local control and overall survival rate were, respectively, 92% and 85.5% at 1 year and 86% and 79.4% at 2 years. These efficacy results are comparable with other published series using fiducials. Only 7 cases of grade 1 and one grade 2 radiation pneumonitis were reported and obviously there was no pneumothorax case. No rib fractures were observed but this study only included tumors distant of at least 15 from the ribs. Indeed, due to the hypofractionation scheme and the high dose per fraction in SBRT, the ribs must be considered as organs at risk. In the literature, rib fracture occurs approximately in 40% of patients treated with SBRT and most reported risk factors for rib fractures are chest wall/tumor distance, female gender and maximum dose to 8cc to chest wall.36
Although not the subject of this review, it is interesting to note that SBRT has also an important role to play in the treatment of patients with oligometastases. SBRT appears to be an effective and well tolerated local therapy for patients with limited metastatic disease within the lung or the liver. Prospective phase I/II studies refer to 2-year local control rates that reach 92–96%, with very low toxicity related after SBRT doses from 48 to 60 Gy in 3 fractions.37,38 Providing equivalent local control to surgical resection, SBRT may also be an alternative to surgery in patients with oligometastatic disease.
6. SBRT for liver primary tumors
Primary liver cancer is the world’ third most common cause of cancer death and hepatocellular carcinoma (HCC) accounts for 85–90% of them. Risk factors for HCC include hepatitis B or C, alcohol ingestion and any cause of cirrhosis.39 This underlying liver disease and the fact that HCC tend to be multifocal or with vascular invasion, make many patients with liver tumors poor candidates for surgery, and only 30–40% of the patients can benefit from curative treatments. In selected patients, with early stage, adequate liver function (Child–Pugh A), performance status of 0, single or 3 lesions < 3 cm, and no associated diseases, surgical resection can result in 5-year survival rates of 40–70% and liver transplantation can cure both, the cancer and the underlying liver.40 In patients with associated disease, early stage HCC and lesions < 3 cm away from large vessels, radiofrequency ablation and alcohol injection are also used as an alternative to surgery.
Cure with preserved liver function is a treatment goal and liver function is an important component of treatment decisions. Historically, radiation therapy has had a limited role in the treatment of liver tumors because of the low tolerance of the liver to radiation. The major dose-limiting concern is the risk of radiation-induced liver disease (RILD), and progression of Child–Pugh class must be considered as an end point of toxicity for those patients. However, because the functional units of the liver parenchyma obey the parallel model of radiobiology, a high dose radiation can be focally administered if sufficient normal tissue can be spared, and with SBRT it is possible to treat safely small or moderated sized hepatic tumors.
For HCC SBRT some specific considerations must be taken into account: GTV target delineation has to rely on phase arterial contrast CT or MRI imaging, the risk of microscopic extension should contemplate CTV margins of at least 5 mm, and in order to minimize toxicity it is imperative to use tight margins in PTV, therefore ITV should be reduced with restrictive or compensating techniques. Dose prescription and dosimetric constraints must take into account patient’ Child–Pugh class score, and lesions size.
Blomgren, in 1991, first used SBRT to treat liver malignancies and since then retrospective and prospective studies have demonstrated efficacy and safety with local control rates in the range of 70–90% at 1 year (Table 2). Because SBRT is relative new, prospective data are quite scarce.16
Table 2.
Selected published series of stereotactic body radiotherapy for primary liver tumors. Retrospective and phase I–II studies.
| Author | Patients | Study | Doses | Local control | Toxicity |
|---|---|---|---|---|---|
| Blomgren 199816 | 11 | Retrospective | 30 Gy 2–5 fx | 1 year 95% | 10% grade ≥ 3 HCC Child B |
| Mendez Romero 200641 | 8 | Prospective | 25–37.5 Gy/3–5 fx | 1 year 75% | 18% grade ≥ 3 HCC Child B |
| Choi 200642 | 20 | Prospective | 50 Gy/5 fx | 1 year 80% | No grade ≥ 3 |
| Tse 200843 | 31 | Prospective | 36 Gy/6 fx | 1 year 65% | 16% grade ≥ 3 HCC Child B |
| Cardenes 200844,45 | 17 | Prospective | 36–48 Gy/3fx Child A 40 Gy/3 fx Child B |
1 year 96% | 18% grade ≥ 3 HCC Child B |
| Dewas 201248 | 42 | Retrospective | 40–45 Gy/3 fx | 2 years 90–5% | 10% grade ≥ 3 |
| Andolino 201146 | 60 | Retrospective | 42 Gy/3 fx (Child A) 40 Gy/5 fx (Child B) |
2 years 90% | No grade ≥ 3 |
7. HHC SBRT prospective studies
The first prospective study came from Mendez-Romero in 2006 who treated 8 HCCs (37.5 Gy in 3 fractions of 12.5 Gy or 25 Gy in 5 fractions of 5 Gy), with 1-year LC of 75% and severe toxicity only in a Child–Pugh B patient.41 Choi in 2006 treated 20 patients with HCCs (15 Child class A), with 50 Gy in 5 fractions of 10 Gy, no episodes of severe toxicity were reported, 1-year LC of 80% and overall survival (OS) of 70%.42 Another phase I study from Canada (Tse 2008) treated 31 HCCs, with a median dose of 36 Gy (range 24–54 Gy), tumors were generally large and half of the patients had large-vessel thrombosis.43 No patients developed grade 4/5 liver toxicity. One-year LC was 65% and OS of 48%. The University of Indiana conducted a prospective study in 17 patients with HCCs.44,45 The dose was escalated in Child–Pugh A patients from 36 Gy in 3 fractions of 12 Gy to 48 Gy in 3 fractions of 16 Gy. Two Child–Pugh B patients developed grade 3 hepatic toxicity at 42 Gy in 3 fractions, so subsequently these patients were treated with 40 Gy in 5 fractions of 8 Gy. Only 12% of the patients with a Child–Pugh score ≤7 developed hematologic or hepatic dysfunction > grade I, whereas 4 of 8 patients with score ≥ 8 developed progressive liver failure.
8. HCC SBRT retrospective studies
There are also several retrospective studies of interest. Andolino, continuing with the study from the University of Indiana, treated 60 patients with HCCs (36 Child–Pugh A and 24 Child–Pugh B), with a 2-year LC of 90% and OS of 67%.46 Size of the lesions (>6 cm) and previous liver function were limiting factors (Child–Pugh score ≥ 8) for toxicity, and those patients should only be consider for SBRT as a “bridge therapy” to transplant. SBRT as definitive therapy for those ineligible for transplant should include 1–3 lesions, maximum tumor diameter ≤ 6 cm, and a Child–Pugh A–B score ≤ 7. Son also reported the experience with 36 HCC patients treated with 30–39 Gy in 3 fractions. 11% of the patients developed progressions of Child–Pugh class when V18 Gy of > 800 ml.47
Lille experience in 2012 reported a high local control rate (89% 1-year LC) in 42 patients treated for an HCC and six for a cholangiocarcinoma.48 To track the lesions, they implemented fiducials (2–6) around each lesion. A total dose of 40–45 Gy at the 80% isodose was delivered. Local control rates at one and 2 years were 89% and 81% for the HCC. Overall survival and local control rate for cholangiocarcinoma were 100% at 1 year. Toxicity consisted mainly of nausea, abdominal pain and asthenia and was correlated with the duration of treatment (p < 0.04). 12 patients presented with grade 3 or 4 gastro-intestinal side effects. Half of them were gastrointestinal toxicities involving lesions which were close to the digestive structures. Prognostic factors associated with better local control were lesion size < 50 mm (p = 0.019), PTV volume < 200cc (p = 0.014) and a delivered dose ≥ 45 Gy. Until now, SBRT appears to be a safe way to treat liver tumors with little toxicity, if lesions at immediate proximity of digestive structures are excluded.49
For cholangiocarcinomas both intra and extrahepatic surgical resections have historically been considered the only curative option. However, only about one-third of patients present with resectable disease and for unresectable patients the prognosis is particularly dismal with a median survival of only 2–6 months. Single agent or combination chemotherapy and conventionally fractionated radiotherapy seems to be largely ineffective. Improved local control and survival rates have been observed with higher radiotherapy doses suggesting dose escalation strategies as a promising therapeutic approach. SBRT experience in the treatment of unresectable cholangiocarcinoma is limited. Twenty-seven patients with unresectable cholangiocarcinoma (n = 26 Klatskin tumors and one intrahepatic cholangiocarcinoma (IHCC) were treated with SBRT.50 The dose schedule was 45 Gy in three fractions. The median progression-free survival and overall survival were 6.7 and 10.6 months, respectively. With a median follow-up of 5.4 years, 6 patients had severe duodenal/pyloric ulceration and 3 patients developed duodenal stenosis.
Early experience with Novalis Exactrac Adaptive Gating in 13 HCC and 9 cholangiocarcinoma is presented in this review and supports its accuracy, efficacy and excellent tolerance. 11 Child–Pugh A HCC patients were treated with 3 fractions of 15–16 Gy and 2 Child–Pugh B with 10 fraction of 5 Gy. No acute or late grade ≥ 3 was observed. With a medium follow up of 18 months, local control was 91.7%. In the follow up, 4 patients developed intrahepatic progression with new lesions and 2 presented distant metastases. Nine patients with Cholangiocarcinomas where also treated with SBRT and with standard concurrent capecitabine as a neoadjuvant combined treatment. The total dose was 50 Gy in 10 fractions. Tolerance was excellent and only one patient developed grade II transaminitis. Of the 9 patients, 8 underwent surgery, one patient progressed with liver metastases. All surgical margins were tumor free. One year local control was 100% and median survival was 17.67 months.
SBRT offers a safe and effective alternative for early stage HCCs and should be considered for patients who are poor candidates for surgery or transplantation, patients with compromised liver function must be treated more cautiously, and randomized studies are warranted to compare the efficacy of SBRT with other treatment modalities.
9. SBRT in prostate cancer
Prostate cancer is the most frequent male cancer in developed countries and radical prostatectomy and radiotherapy are treatment approaches for most localized tumors. Radiotherapy dose-escalation studies have shown a significantly better outcome (5-year disease control probability > 80%). Prostate cancer is characterized by an α/β ratio = 1.4 Gy, lower than that of most tumors or even of the late-responding normal tissues surrounding the tumor: the rectum and the bladder (α/β = 3–5 Gy).51 Thus, large treatment fractions (hypofractionation) may increase the tumor cell killing effect while biologically protecting the surrounding late responding normal tissues. Preliminary results of two pilot studies on extreme hypofractionation SBRT (5 daily fractions of 6.7 and 7.25 Gy over 5 and 5–9 days, respectively) have been reported, 5-year biochemical disease control rates above 90%.52,53 Therefore, there is a need to test the effect on tolerance and outcome of large fractions. A prospective multi-center phase II study in France has been coordinated by Lartigau. The aim was to demonstrate the feasibility of delivering a 3 × 6 Gy boost after IMRT or 3D delivery of 46 Gy in intermediate risk tumors. Seventy-two patients were included in less than 2 years in 4 participating centers (3 CyberKnife and 1 Linac). No acute toxicity was recorded and feasibility was excellent. Six Centers in France will participate in the new upcoming phase III PACE trial on the role of exclusive SBRT compared to radical prostatectomy.
10. SBRT in reirradiation
Most SBRT indications that have previously been described concern patients who are not amenable to surgery. For head-and-neck or pelvic tumors that were first treated with radiation therapy, SBRT enables to consider reirradiation, which was otherwise clearly not an option.54 Indeed, most of the recurrences occur in the previously irradiated field and it is impossible in traditional radiotherapy to deliver enough dose-intensity to control the disease. The surgery is often impossible due to the previous irradiation and the location of the recurrence.
11. Head and neck SBRT irradiation
Reirradiation is an alternative for patients who are not candidates for surgical salvage although has a significant challenge regarding toxicity.55 Three-year survival rates of 13–22% have been reported with external beam RT alone.56 Results from phase I/II trials have also demonstrated the benefit of reirradiation with concurrent chemotherapy over observation.57,58 Emerging data also support the safety of conformal delivery techniques, like intensity modulated radiation therapy, with potentially less treatment-related toxicity and local control improvement.59,60
SBRT is an attractive treatment modality for HNC reirradiation. The primary benefits of SBRT relative to conventional re-irradiation are shorter treatment times and decreased toxicity, which are achieved without compromising clinical outcomes.61,62
Currently an on-going phase II trial in Lille is leaded for recurrent head and neck squamous cell carcinoma, and preliminary experience was already published.63 Forty patients were treated between June 2007 and January 2010 for a median tumor size of 29 mm. A dose of 36 Gy was delivered in 6 fractions. 14 patients received a concurrent infusion of cetuximab. After 25.6 months of median follow-up, 15 patients experienced a complete response and overall objective response was 79.4%. 20 presented with disease progression but only 3 with local relapse. 4 patients experienced a grade 3 toxicity but no carotid blowout was observed. This was explained by the fact that tumors invading more than one third of the carotid were not treated. Median overall survival was 13.6 months which is a good result considering the poor prognostic of these patients.
SBRT boost technique can be also a highly efficacious dose escalation modality in terms of local control. However, high late complications have been observed and should be indicated only in selective cases because large treatment volumes treated with very high fractional doses have been related with high risk of developing late toxicities.64
12. Pelvic SBRT reirradiation
Pelvic tumor recurrence is usually treated by surgery but in case of lateral pelvic invasion recurrence or proximity of the iliac vessel, local treatment cannot be achieved. Without treatment, local progression often causes pain and impacts the quality of life. Since June 2007, 16 patients with pelvic recurrence were reirradiated with the CyberKnife.65 Primary tumors were rectal cancer (4 patients), anal canal (6 patients), uterine cervix cancer (4 patients), endometrial cancer (1 patient) and bladder carcinoma (1 patient). The prescribed dose was36 Gy in 6 fractions. Of 8 patients, who had sciatic pain before treatment, 4 patients reported an improvement. The local control rate at 1 year was 51.4%. The treatment was well tolerated and we observed no grade 3 toxicity.
Reirradiation is often a complex situation in which there is no alternative and SBRT seems to be a safe and efficient option to treat tumor recurrences even if not in a curative intent. Nevertheless, further prospective studies are needed to precise the indications and the toxicity profile for these treatments.
13. Follow-up after SBRT
Dose distribution in SBRT is extremely different from conventional radiotherapy. Early and late reactions can also be very different. Radiologic aspect after SBRT can be sometimes very difficult to analyze. Indeed, RECIST criteria, which are based on the change of lesion size, appear to be inappropriate. After SBRT, lung condensation can mimic an increase of size of the lesion although there is no recurrence. Bibault66 described the semiology that should lead to suspicion of recurrence: localization in continuation with treated lesion, pleural effusion, disappearance of initial bronchiectasis and morphological modifications of fibrosis contours. Timing of the appearance of these images is essential: early reactions occur between six and nine months late changes, after 12 months, including size or contours modifications with enlarging opacity can be considered high-risk CT changes and recurrent disease should be suspected if seen with SUVmax ≥ 5 on PET, with a high predictive value.67
The paradigms of evaluation of liver lesions have been modified in regard of radiologic aspect after novel targeted therapies. For example, modified RECIST criteria have been developed for HCC. Jarraya68 showed that RECIST criteria could lead to a misinterpretation of some lesions, especially in case of tumors that become necrotic after treatment. They suggest using lobular enhancement, total necrosis and rimming enhancement in addition to RECIST criteria (Fig. 3). Metabolic imaging like FDG-PET could also be useful but has not been evaluated in this indication. If considered, FDG-PET imaging must not be realized before a minimal delay of 6 months. Indeed, initial inflammatory reactions caused by high doses per fraction/SBRT can be responsible for false-positive results; however, some publications suggest metabolic response after SBRT could be a good prognostic factor of loco-regional relapse.69 Currently, the optimal supervision after SBRT is not known. Follow-up must be carried by radiologists who are accustomed to SBRT post-therapeutic aspects.
Fig. 3.
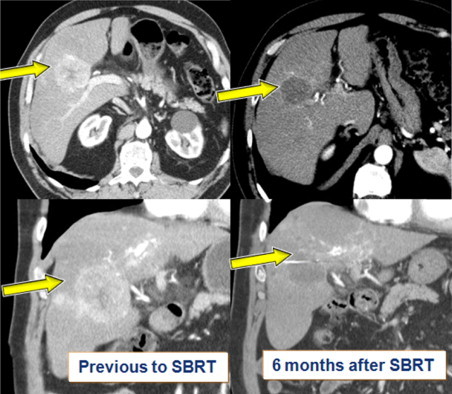
Follow up with radiological evaluation of response after SBRT treatment for Hepatocellullar carcinoma. Reduction of arterial contrast enhancement is included in the new RECIST criteria of response for HCC.
14. Conclusion
Stereotactic body radiation therapy is a recent radiotherapy technique, which has quickly developed because of its excellent results in terms of tolerance and its high loco-regional control rates. The implementation of SBRT in routine requires a careful considering of organ motion. Gating and Tracking are effective ways to do so, and the development of new technologies such as X-Sight Lung have made it become less invasive. Due to the hypofractionated scheme, the physician must pay attention to new dosimetric constraints and new radiologic post-treatment aspects. Although there seems to be a link between BED and local control, new radiobiological models are needed to assess the optimal fractionation and dose scheme. These limitations imply that SBRT should only be conducted by experienced multidisciplinary teams at the time being.
Some SBRT indications, such as lung primary tumors treatments, have become a standard of care for inoperable patients. SBRT seems to be effective in many other indications in curative or palliative intent such as primary liver tumors. Novel indications and strategies are currently emerging, for example prostate cancer treatment or associations of chemotherapy and SBRT.
Currently, SBRT is mainly used when there is no other therapeutic alternative for the patient. This is due to the lack of randomized trials in these settings. However, the results shown in retrospective studies let us hope to impose SBRT as a new standard of care for many patients in the next few years.
Conflict of interest
Not declared.
Financial disclosure
Not declared.
Contributor Information
Carmen Rubio, Email: crubio@hmhospitales.com.
Rosa Morera, Email: rmorera9@yahoo.es.
S. Eric Lartigau, Email: e-lartigau@o-lambret.fr.
References
- 1.Potters L., Steinberg M., Rose C. American Society of Therapeutic Radiology and Oncology and American College of Radiology practice guidelines for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60:1026–1032. doi: 10.1016/j.ijrobp.2004.07.701. [DOI] [PubMed] [Google Scholar]
- 2.Benedict S., Yenice K., Followill D. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 3.Wurm R.E., Gum F., Erbel S. Image guided respiratory gated hypofractionated Stererotactic Body Radiation Therapy (H-SBRT) for liver and lung tumors: initial experience. Acta Oncol. 2006;45:881–889. doi: 10.1080/02841860600919233. [DOI] [PubMed] [Google Scholar]
- 4.Verellen D., Soete G., linthout N. Quality assurance of a system for improved target localization and patient set-up that combines real-time infrared tracking and stereoscopic X-ray imaging. Radiother Oncol. 2003;67:129–134. doi: 10.1016/s0167-8140(02)00385-7. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs I.C., Loo B.W., Jr. CyberKnife stereotactic ablative radiotherapy for lung tumors. Techol Cancer Res Treat. 2010;9:589–596. doi: 10.1177/153303461000900607. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Velilla Peña M., Rubio Rodríguez C., Krauel A. Tratamiento de lesiones pulmonares mediante radioterpia estereotáxica extracraneal tras colocación guiada por tomografía computarizada de marcador Visioil®: experiencia inicial. Radiología. 2012 doi: 10.1016/j.rx.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Bibault J., Prevost B., Dansin E. Stereotactic radiotherapy for lung cancer: early efficacy and toxicity evaluation of a non-invasive real time tumor tracking system. Lung Cancer. 2011;71(2):S38–S39. [Google Scholar]
- 8.Naruke T., Goya T., Suchica R. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96:440–447. [PubMed] [Google Scholar]
- 9.Shields W. Surgical therapy for carcinoma of the lung. Clin Chest Med. 1993;14:1–7. [PubMed] [Google Scholar]
- 10.Swanson S.J., Herndon J.E., 2nd, D’Amico T.A. Video-assisted thoracic surgery lobectomy: report of CALGB 39802—a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 11.Flores R.M., Park B.J., Dycoco J. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Wisnivesky J.P., Bonomi M., Henschke C. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005;128:1461–1467. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 13.Simon C.J., Dupuy D.E., DiPetrillo T.A. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 14.Beland M.D., Wasser E.J., Mayo-Smith W.W. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology. 2010;254(1):301. doi: 10.1148/radiol.00000090174. [DOI] [PubMed] [Google Scholar]
- 15.Sher D.J., Wee J.O., Punglia R.S. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable. Early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):767–774. doi: 10.1016/j.ijrobp.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 16.Blomgren H., Goranson H. Radiosurgery for tumors in the body: clinical experience using a new method. J Radiosurg. 1998;1:63–74. [Google Scholar]
- 17.Xia T., Li H., Sun Q. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable stage I/II non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–125. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann F.B., Geinitz H., Schill S. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. 2005;48:107–114. doi: 10.1016/j.lungcan.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Nyman J., Johansson K., Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer. Mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Baumann P., Nyman J., Lax I. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45:787–795. doi: 10.1080/02841860600904862. [DOI] [PubMed] [Google Scholar]
- 21.Onishi H., Araki T., Shirato H. Stereotactic hypofractionated high-dose irradiation for stage I non-small cell lung cancer. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 22.McGarry R.C., Papiez L., Williams M., Whitford T., Timermman R. Stereotactic body radiation therapy of early-stage non-small cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010–1015. doi: 10.1016/j.ijrobp.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 23.Timermman R., McGarry R., Yiannoustsos C. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4838. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RTOG 0813 Protocol. Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients.
- 26.Lagerwaard F., Haasbeek C., Smit E. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Senthi S., Lagerwaard F.J., Haasbeek C.J. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13(8):802. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 28.Filippi A., Franco P., Ricardi U. Is stereotactic ablative radiotherapy an alternative to surgery in operable stage I non-small cell lung cancer? Review article. Rep Pract Oncol Radiother. 2013 doi: 10.1016/j.rpor.2013.05.005. [in press, 1 July 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmerman R., Paulus R., Pass H. RTOG 0618: a phase II trial of stereotactic body radiation therapy in the treatment of patients with operable stage I/II non-small cell lung cancer. 2013 ASCO annual meeting abstract. J Clin Oncol. 2013;31(Suppl.) [abstr 7523] [Google Scholar]
- 30.Hiraoka M., Ishikura S. A Japan clinical oncology group trial for stereotactic body radiation therapy of non-small cell lung cancer. J Thorac Oncol. 2007;2:S115–S117. doi: 10.1097/JTO.0b013e318074de1b. [DOI] [PubMed] [Google Scholar]
- 31.Hurkmans Recommendations for implement of stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol. 2009;4:1. doi: 10.1186/1748-717X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phase III STARS Protocol. Randomized Study to Compare CyberKnife to Surgical Resection In Stage I Non-small Cell Lung Cancer.
- 33.Surgery With or Without Internal Radiation Therapy Compared With Stereotactic Body Radiation Therapy in Treating Patients With High-Risk Stage I Non-Small Cell Lung Cancer. ACOSOG Z4099-RTOG 1021 trial. NCT01336894
- 34.Hernando O., Sánchez E., Fernández-Letón P. Institutional experience on the treatment of lung and liver lesions with stereotactic body radiotherapy with Novalis Exactrac Adaptive Gating Technique. J Radiosurg SBRT. 2011;3:231–236. [PMC free article] [PubMed] [Google Scholar]
- 35.Bibault J.-E., Prevost B., Dansin E., Mirabel X., Lacornerie T., Lartigau E. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol. 2012;7(June (1)):1. doi: 10.1186/1748-717X-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nambu A., Onishi H., Aoki S. Rib fracture after stereotactic radiotherapy for primary lung cancer: prevalence, degree of clinical symptoms, and risk factors. BMC Cancer. 2013;13:68. doi: 10.1186/1471-2407-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rusthoven K.E., Kavanagh B.D., Burri S.H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 38.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 39.Bruix J., Llovet J.M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:51. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 40.Llovet J.M., Fuster J., Bruix J. Intention to treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 41.Mendez Romero A., Wunderink W., Hussain S.M. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I–II study. Acta Oncol. 2006;45(7):831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 42.Choi B.O., Jang H.S., Kang K.M. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154–158. doi: 10.1093/jjco/hyi236. [DOI] [PubMed] [Google Scholar]
- 43.Tse R.V., Hawkins M., Lockwood G. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 44.Cárdenes H., Price T., Perkins S. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2008;72:S128–S129. [Google Scholar]
- 45.Cárdenes H.R., Price T.R., Perkins S.M. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(March (3)):218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 46.Andolino D., Johnson C.S., Maluccio M. Stereotactic Body Radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:447–453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Son H.S., Choi B.O., Ryu M.R., Kang Y.N., Jang J.S. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2011;81(4):447–453. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Dewas S., Mirabel X., Kramar A. Radiothérapie stéréotaxique hépatique par CyberKnife®: l’expérience lilloise. Cancer/Radiother. 2012;16(February (1)):58–69. doi: 10.1016/j.canrad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Dewas S., Bibault J.-E., Mirabel X. Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol. 2012;7:166. doi: 10.1186/1748-717X-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopek N., Holt M.I., Hansen A.T., Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Miralbell R., Roberts S.A., Zubizarreta E., Hendry J.H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcome of 5969 patients in seven international institutional datasets: α/β = 1.4 (09–2.2) Gy. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 52.King C.R., Brooks J.D., Gill H., Presti J.C. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;78(3):S-336. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 53.Pham H.T., Song G., Badiozamani K. Five-year outcome of stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP) for patients with low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(Suppl.):S58. [Google Scholar]
- 54.Mantel F., Flentje M., Guckenberger M. Stereotactic body radiation therapy in the re-irradiation situation--a review. Radiat Oncol. 2013;5(8):7. doi: 10.1186/1748-717X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald M.W., Lawson J., Garg M.K. ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1292. doi: 10.1016/j.ijrobp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Langendijk J.A., Kasperts N., Leemans C.R. A phase II study of primary reirradiation in squamous cell carcinoma of head and neck. Radiother Oncol. 2006;78:306. doi: 10.1016/j.radonc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Spencer S.A., Harris J., Wheeler R.H. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30:281. doi: 10.1002/hed.20697. [DOI] [PubMed] [Google Scholar]
- 58.Langer C.J., Harris J., Horwitz E.M. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25:4800. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 59.Lee N., Chan K., Bekelman J.E. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 60.Sulman E.P., Schwartz D.L., Le T.T. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys. 2009;73:399. doi: 10.1016/j.ijrobp.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 61.Rwigema J.C., Heron D.E., Ferris R.L. Fractionated stereotactic body radiation therapy in the treatment of previously-irradiated recurrent head and neck carcinoma: updated report of the University of Pittsburgh experience. Am J Clin Oncol. 2010;33:286. doi: 10.1097/COC.0b013e3181aacba5. [DOI] [PubMed] [Google Scholar]
- 62.Rwigema J.C., Heron D.E., Ferris R.L. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol. 2011;34(August (4)):372–379. doi: 10.1097/COC.0b013e3181e84dc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comet B., Kramar A., Faivre-Pierret M. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-and-neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2012;84(September (1)):203–209. doi: 10.1016/j.ijrobp.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 64.Lee D.S., Kim Y.S., Cheon J.S. Long-term outcome and toxicity of hypofractionated stereotactic body radiotherapy as a boost treatment for head and neck cancer: the importance of boost volume assessment. Radiat Oncol. 2012;June (7):85. doi: 10.1186/1748-717X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dewas S., Bibault J.-E., Mirabel X. Robotic image-guided reirradiation of lateral pelvic recurrences: preliminary results. Radiat Oncol. 2011;6(June (1)):77. doi: 10.1186/1748-717X-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bibault J.E., Ceugnart L., Prevost B. CT appearance of pulmonary carcinomas after stereotactic radiation therapy. Diagn Intervent Imaging. 2013;94(3):255–262. doi: 10.1016/j.diii.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Huang K., Dahele M., Senan S. Radiographic changes after lung stereotactic ablative radiotherapy (SABR) – can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol. 2012;(3):335–342. doi: 10.1016/j.radonc.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 68.Jarraya H., Mirabel X., Taieb S. Image-based response assessment of liver metastases following stereotactic body radiotherapy with respiratory tracking. Radiat Oncol. 2013;8(1):24. doi: 10.1186/1748-717X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solanki A.A., Weichselbaum R.R., Appelbaum D. The utility of FDG-PET for assessing outcomes in oligometastatic cancer patients treated with stereotactic body radiotherapy: a cohort study. Radiat Oncol. 2012;7(December (1)):1. doi: 10.1186/1748-717X-7-216. [DOI] [PMC free article] [PubMed] [Google Scholar]


