Abstract
Aim
To study the use of RapidArc techniques in the treatment of prostate cancer patients with hip prosthesis.
Background
An important aspect of treatment planning is to achieve dose homogeneity inside the planning target volume (PTV). Especially for those patients presenting with hip prosthesis, it becomes a challenging task to achieve dose uniformity inside the PTV.
Materials and methods
Five prostate patients presenting with hip prosthesis who had undergone radical radiotherapy were selected for this study. Depending on the composition of prosthesis, a predefined set of Hounsfield values were assigned to each study set. RapidArc plans were generated on an Eclipse treatment planning system. Two arcs that include clockwise and counter-clockwise arcs were used in all these cases. To avoid beams passing through the prosthesis, a simple structure was defined around it with 1 cm margin and a strict dose constraint applied to the block during VMAT optimization.
Results
The mean D2/D98 ratio of PTV for all the patients was 1.06 ± 0.01. The mean percentage rectum volume receiving 50 Gy, 60 Gy, 70 Gy and 75 Gy for all the patients were 33.1 ± 5.9, 21.7 ± 5.5, 13.8 ± 4.4 and 9.5 ± 3.0, respectively.
Conclusions
This study shows that using a double arc RapidArc technique is a simple and effective treatment method of treating prostate cancer in patients presenting with a hip prosthesis. The definition of a beam avoidance structure encompassing the prosthesis and applying strict dose constraints to it reduces the beam contribution to the prosthesis
Keywords: Prostate cancer, Radiotherapy, Treatment planning, VMAT, Prosthesis
1. Background
High dose radiotherapy has been shown to be an effective treatment modality for prostate cancers.1–3 An important aspect of treatment planning is to achieve uniform dose inside the planning target volume with reduced dose to the surrounding critical structures. Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant. Prostheses are usually made of high density material such as titanium, steel, etc. High density material poses significant problem to the incident or exiting beam. These materials not only attenuate the treatment beam but also scatter the dose which may lead to dosimetric uncertainty at the soft tissue interfaces. Hence, it becomes a challenging task to achieve dose uniformity inside the PTV for these patients who undergo radiotherapy. Besides, for those patients with unknown prosthetic material, the chances of dosimetric uncertainty are imminent. Even if the electron density of the prosthesis is known, most dose calculation algorithms have been designed and tested only for lower densities, and do not accurately model the absorption and scattering properties of metals. The Treatment Planning System (TPS) is likely to overestimate dose, particularly if the prosthesis is solid and made of stainless steel or cobalt-chromium. However, several dose calculation algorithms 4–7 have been developed over the period of time that predict dose closer to Monte Carlo computed dose for high density material. Another problem with computed tomography (CT) scan images is the presence of artifacts on images caused by prosthesis due to attenuation of X-ray beam. Hence, it has become a standard practice to avoid beams passing through these prosthetic materials.8 In the midst of the above factors, there is a need to assess the dosimetry for patients presenting with hip prosthesis with advanced treatment techniques, such as volumetric modulated radiotherapy (VMAT). Hence, in this study, an effort has been made to demonstrate the use of volumetric modulated arc therapy for prostate patients presenting with hip prosthesis.
2. Aim
To study the use of RapidArc technique in the treatment of prostate cancer patients with hip prosthesis.
3. Materials and methods
Five prostate patients who had undergone radical radiotherapy were selected for this study. Of the five patients, four had unilateral hip prosthesis and one patient presented with bilateral hip prosthesis. All patients underwent CT scanning on Philips Brilliance CT scanner and the Dicom CT datasets were transferred to Eclipse treatment planning system (Varian Medical System, USA) for contouring. The regions of dark and bright streaking artifacts replacing the soft tissues present in the CT images due the effect of kilovoltage beams on the prosthesis were contoured and Hounsfield unit (HU) value of 0 was assigned. Depending on the type of prosthesis, the following Hounsfield values were used during planning: Bone: 800 HU; Titanium: 4500 HU; Co–Cr–Mo or stainless steel: 9500, respectively. To those prosthesis whose composition is unknown, 7000 HU was assigned. The PTV was generated from clinical target volume (CTV) on assigning a uniform margin of 1 cm in all directions except for 0.7 cm along the posterior direction. RapidArc plans were generated on Eclipse treatment planning system. Two arcs that include clockwise and counter clockwise arcs were used in all these cases. To avoid beams passing through prostheses, a simple structure known as beam avoidance structure was generated around the prosthesis with a 1 cm margin and a strict dose constraint (0% of the volume receives over 500 cGy) was applied to the block during volumetric modulated radiation therapy (VMAT) optimization to avoid dose to the prosthesis. A dose of 78 Gy was prescribed to all the patients with 95% of prescription dose covering 99% of the target volume. Collimator was rotated to 30° for the clockwise and 330° for the counter-clockwise arc. All RapidArc plans were generated with photon beam energy of 6 MV from a 21iX linear accelerator (Varian Medical Systems, Palo, Alto, CA) using Analytical Anisotropic Algorithm (AAA) dose calculation algorithm.
4. Results
Table 1 shows the dosimetric parameters for five prostate patients selected in this study. Figs. 1 and 2 show the dose distribution and the dose volume histogram of a bilateral hip prosthesis for a double arc VMAT technique. Fig. 1 illustrates the use of the beam avoidance structure (green color contour) around the femur defined for reducing the beam contribution to the prosthesis. Fig. 3 depicts the dose distribution of a unilateral hip prosthesis for a double arc VMAT technique. No volume of the right and left femur received dose greater than 45 Gy. The mean D2/D98 (D2: near maximum absorbed dose that covers 2% of the volume; D98: near minimum absorbed dose that covers 98% of the volume) ratio of PTV for all the patients was 1.06 ± 0.01. The mean percentage rectum volume receiving 50 Gy (V50), 60 Gy (V60), 70 Gy (V70), 75 Gy (V75) for all the patients was 33.1 ± 5.9, 21.7 ± 5.5, 13.8 ± 4.4 and 9.5 ± 3.0, respectively. The mean bladder volume receiving 70 Gy (V70) and 60 Gy (V60) for all the studied patients was 13.8 ± 9.8% and 24.4 ± 16.0%, respectively. For the right femur, the mean dose, and volume receiving 10 Gy (V10) for patients with bilateral and right hip prosthesis was 500.5 ± 112.4 cGy and 6.90 ± 8.3% respectively. Similarly for the left femur, the mean does and V10 for bilateral and patients with left hip prosthesis was 502.1 ± 19.8 cGy and 2.87 ± 2.9%, respectively.
Table 1.
Dosimetric parameters for unilateral and bilateral hip prostate patients.
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Bilateral | Rt. Lateral | Rt. Lateral | Lt. Lateral | Lt. Lateral | |
| PTV | |||||
| Max. dose (%) | 105.8 | 108.2 | 111.9 | 111.7 | 109.2 |
| D2 (cGy) | 8010.0 | 7910.0 | 7987.0 | 7951.0 | 7971.0 |
| D98 (cGy) | 7496.0 | 7549.8 | 7502.0 | 7517.0 | 7543.0 |
| Bladder | |||||
| Max. dose (%) | 105.8 | 105.6 | 107.6 | 105.5 | 104.3 |
| V70 (%) | 20.0 | 7.3 | 28.0 | 8.7 | 5.2 |
| V50 (%) | 31.1 | 14.3 | 49.7 | 13.8 | 13.1 |
| Rectum | |||||
| V50 (%) | 32.8 | 32.9 | 42.6 | 30.8 | 26.5 |
| V60 (%) | 21.4 | 24.1 | 29.3 | 19.3 | 14.6 |
| V70 (%) | 13.6 | 16.1 | 19.4 | 12.1 | 7.6 |
| V75 (%) | 9.4 | 11.7 | 12.8 | 8.5 | 5.1 |
| Rt. femur | |||||
| Mean dose (cGy) | 384.1 | 606.0 | 526.5 | 481.6 | 921.7 |
| Max. dose (cGy) | 2757.0 | 1333.0 | 3690.0 | 3472.0 | 1995.0 |
| V10 (%) | 16.1 | 0.0 | 4.6 | 5.8 | 46.0 |
| Lt. femur | |||||
| Mean dose (cGy) | 500.8 | 979.0 | 965.9 | 522.5 | 483.0 |
| Max. dose (cGy) | 1742.6 | 3254.0 | 3104.0 | 1932.0 | 973.0 |
| V10 (%) | 2.8 | 0.5 | 40.3 | 5.8 | 0.0 |
Fig. 1.
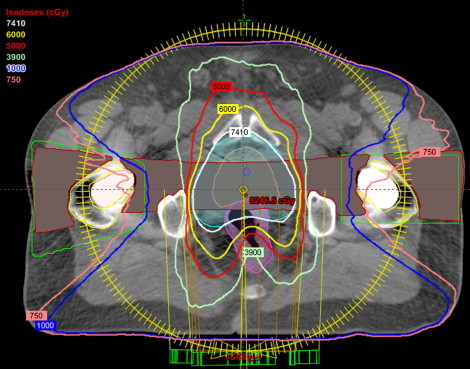
Dose distribution of a bilateral hip prosthesis for a double arc VMAT technique.
Fig. 2.
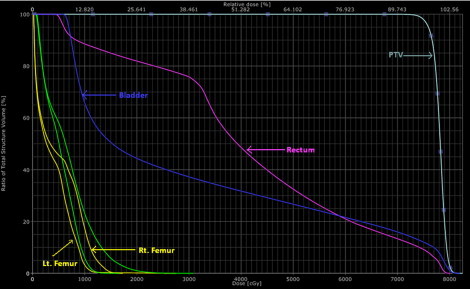
Dose volume histogram of a bilateral prosthesis.
Fig. 3.
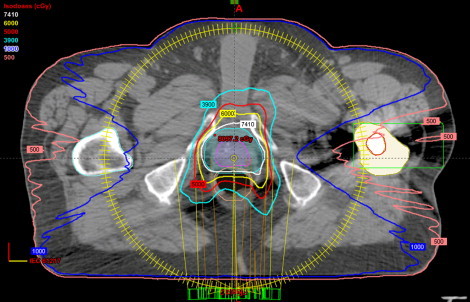
Dose distribution of a unilateral hip prosthesis for a double arc VMAT technique.
5. Discussion
Intensity modulated radiation therapy is currently the standard treatment technique used in the treatment of prostate cancer with metal hip prosthesis.9,10 Different forms of beam arrangements have been proposed for unilateral and bilateral prostheses using intensity modulated radiation therapy (IMRT) that includes coplanar11 and non-coplanar beam arrangements.12 Although, it has been found to produce acceptable target volume coverage for most of the clinical situations in prostate cancer involving prostheses, it is still regarded as a challenging task in treatment planning due to the prectal volume overlapping PTV. The presence of metal prostheses leads to artifacts on CT images and poses a challenge in defining the organs at risk and target volumes on kilovoltage CT. These artifacts have a significant impact of up to 70% on the Hounsfield values for nearby and distant structures on the transverse slice.13 The quality of CT images can also be improved with artifact-suppressing algorithms.14,15 Another simpler and elegant way of tackling these metal artifacts is the use of megavoltage CT images. The megavoltage CT scanners minimize artifacts as compared to kilovoltage CT scanners and such megavoltage images are more useful for delineating the target and critical structures.13 One such megavoltage scanner is the helical tomotherapy that provides a remarkable trend in radiotherapy by providing megavoltage images for contouring, treatment planning and treatment verification.16 Meeks et al.17 demonstrated that the uniformity and spatial resolution of megavoltage CT (MVCT) images generated by Hi-Art II tomotherapy were comparable to diagnostic CT images; however; they do not have the same performance characteristics as diagnostic CT images.
An increase in dose at the bone-metal interface may lead to bone necrosis and weakening of the implant fixation. To date, there are no quantitative data available on the effects of the increased dose at the interface between a hip prosthesis and surrounding tissue in patients undergoing radical treatment of tumors in the pelvic region. It has been shown by Keall et al.18 that the interface between the prostate and the prosthesis did not affect the dose to the target due to a sufficient gap between them. Monte Carlo treatment planning systems are shown to predict accurate doses at the tissue-prosthesis interface.19
Our study presents a simple and effective treatment method using RapidArc technique for patients presenting with hip prosthesis. It clearly illustrates that the use of a beam avoidance structure contoured around the prosthesis and assigning a strict dose constraint during the optimization process helps in restricting the dose from beams passing through prostheses resulting in a uniform dose to PTV complying with the dose to critical structures. The technique can be used for all unilateral and bilateral hip prostheses. The planning is quite simple and straightforward unlike in IMRT which requires multiple runs to achieve an acceptable dose distribution. VMAT is currently evolving as the most popular treatment modality due to shorter time20 required to deliver treatment as compared to conventional 3D conformal radiotherapy and IMRT technique. This may also reduce a possible uncertainty in dose due to a setup error. The treatment of prostate patients treated in prone position tends to reduce the dose to organs at risk21 as compared to supine position which needs further study in the case of patients presenting with hip prosthesis.
6. Conclusion
This study shows that RapidArc technique with double arcs is a simple and effective treatment method in case of patients presenting with hip prosthesis. The use of a beam avoidance structure encompassing the prosthesis and applying strict dose constraints reduces the beam contribution to the prosthesis.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Viani G.A., da Silva L.G., Stefano E.J. High-dose conformal radiotherapy reduces prostate cancer-specific mortality: results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2012;83:619–625. doi: 10.1016/j.ijrobp.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky M.J., Fuks Z., Hunt M. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166(3):876–881. [PubMed] [Google Scholar]
- 3.Pahlajani N., Ruth K.J., Buyyounouski M.K. Radiotherapy doses of 80 Gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(5):1949–1956. doi: 10.1016/j.ijrobp.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahnesj̈o A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogenous media. Med Phys. 1989;16:577–592. doi: 10.1118/1.596360. [DOI] [PubMed] [Google Scholar]
- 5.Wieslander E., Knoos T. Dose perturbation in the presence of metallic implants: treatment planning system versus Monte Carlo simulations. Phys Med Biol. 2003;48:3295–3305. doi: 10.1088/0031-9155/48/20/003. [DOI] [PubMed] [Google Scholar]
- 6.Han T., Mourtada F., Kisling K., Mikell J., Followill D., Howell R. Experimental validation of deterministic Acuros XB algorithm for IMRT and VMAT dose calculations with the Radiological Physics Center's head and neck phantom. Med Phys. 2012;39(4):2193–2202. doi: 10.1118/1.3692180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasenbalg F., Neuenschwander H., Mini1 R., Born E.J. Collapsed cone convolution and analytical anisotropic algorithm dose calculations compared to VMC++ Monte Carlo simulations in clinical cases. Phys Med Biol. 2007;52:3679. doi: 10.1088/0031-9155/52/13/002. [DOI] [PubMed] [Google Scholar]
- 8.Schild S.E., Robinow J.S., Casale H.E., Bellefontaine L.P., Buskirk S.J. Radiotherapy treatment planning for prostate cancer in patients with prosthetic hips. Med Dosim. 1992;17(2):83–86. doi: 10.1016/0958-3947(92)90018-b. [DOI] [PubMed] [Google Scholar]
- 9.Kung J.H., Reft H., Jackson W., Abdalla I. Intensity-modulated radiotherapy for a prostate patient with a metal prosthesis. Med Dosim. 2001;26(4):305–308. doi: 10.1016/s0958-3947(01)00079-6. [DOI] [PubMed] [Google Scholar]
- 10.Su A., Reft C., Rash C., Price J., Jani A.B. A case study of radiotherapy planning for a bilateral metal hip prosthesis prostate cancer patient. Med Dosim. 2005;30(3):169–175. doi: 10.1016/j.meddos.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Fattahi S., Ostapiak O.Z. An opposed matched field IMRT technique for prostate cancer patients with bilateral prosthetic hips. J Appl Clin Med Phys. 2012;13(1):3347. doi: 10.1120/jacmp.v13i1.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks C., Cheung R.M., Kudchadker R.J. Intensity-modulated radiation therapy with noncoplanar beams for treatment of prostate cancer in patients with bilateral hip prosthesis – a case study. Med Dosim. 2010;35(2):87–91. doi: 10.1016/j.meddos.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Aubin M., Morin O., Chen J. The use of megavoltage cone-beam CT to complement CT for target definition in pelvic radiotherapy in the presence of hip replacement. Br J Radiol. 2006;79(947):918–921. doi: 10.1259/bjr/19559792. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Zhang L., Zhu X.R., Lee A.K., Chambers M., Dong L. Reducing metal artefacts in cone-beam CT images by preprocessing projection data. Int J Radiat Oncol Biol Phys. 2007;67:924–932. doi: 10.1016/j.ijrobp.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Yu L., Li H., Mueller J. Metal artifact reduction from reformatted projections for hip prostheses in multislice helical computed tomography: techniques and initial clinical results. Invest Radiol. 2009;44:691–696. doi: 10.1097/RLI.0b013e3181b0a2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdi M., Gingras L., Beaulieu L. An adaptive approach to metal artifact reduction in helical computed tomography for radiation therapy treatment planning: experimental and clinical studies. Int J Radiat Oncol Biol Phys. 2005;62(4):1224–1231. doi: 10.1016/j.ijrobp.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 17.Meeks S.L., Harmon J.F., Jr., Langen K.M. Performance characterization of megavoltage computed tomography imaging on a helical tomotherapy unit. Med Phys. 2005;32(8):2673–2681. doi: 10.1118/1.1990289. [DOI] [PubMed] [Google Scholar]
- 18.Keall P.J., Siebers J.V., Jeraj R., Mohan R. Radiotherapy dose calculations in the presence of hip prostheses. Med Dosim. 2003;28(2):107–112. doi: 10.1016/S0958-3947(02)00245-5. [DOI] [PubMed] [Google Scholar]
- 19.Mesbahi A., Nejad F.S. Dose attenuation effect of hip prostheses in a 9-MV photon beam: commercial treatment planning system versus Monte Carlo calculations. Radiat Med. 2007;25(10):529–535. doi: 10.1007/s11604-007-0181-z. [DOI] [PubMed] [Google Scholar]
- 20.Leszczyński W., Ślosarek K., Szlag M. Comparison of dose distribution in IMRT and RapidArc technique in prostate radiotherapy. Rep Pract Oncol Radiother. 2012;17(6):347–351. doi: 10.1016/j.rpor.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajon T., Piotrowski T., Antczak A., Bąk B., Błasiak B., Kaźmierska J. Comparison of dose volume histograms for supine and prone position in patients irradiated for prostate cancer – a preliminary study. Rep Pract Oncol Radiother. 2011;16(2):65–70. doi: 10.1016/j.rpor.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


