Abstract
We have examined the ability of single-stranded DNA to participate in homologous recombination reactions in mammalian cells and nuclear extracts derived from them. We have inserted a fragment of the neo gene into the single-stranded DNA phage vector M13 mp11. The neo fragment was derived from a deletion derivative of the prokaryotic-eukaryotic shuttle vector pSV2neo. The resulting single-stranded DNA was mixed with a double-stranded deletion derivative of pSV2neo and tested for recombination in human cells, monkey cells, and nuclear extracts obtained from human cells. We were able to obtain recombinant molecules containing wild-type neo genes in all three systems. Examination of the products of recombination indicated that they resulted from correction of the deletion in the double-stranded DNA substrate. We were unable to detect any extensive conversion of single-stranded DNA into its double-stranded counterpart before it participated in the recombination reaction. We have also tested the ability of single-stranded DNA to yield transfectants. When a single-stranded DNA derivative of the herpes simplex virus thymidine kinase (TK) gene was introduced into mouse L-M(TK-) cells, we were able to obtain TK+ colonies. From these results, we conclude that single-stranded DNA can participate in transfection as well as homologous recombination reactions in mammalian cells.
Full text
PDF
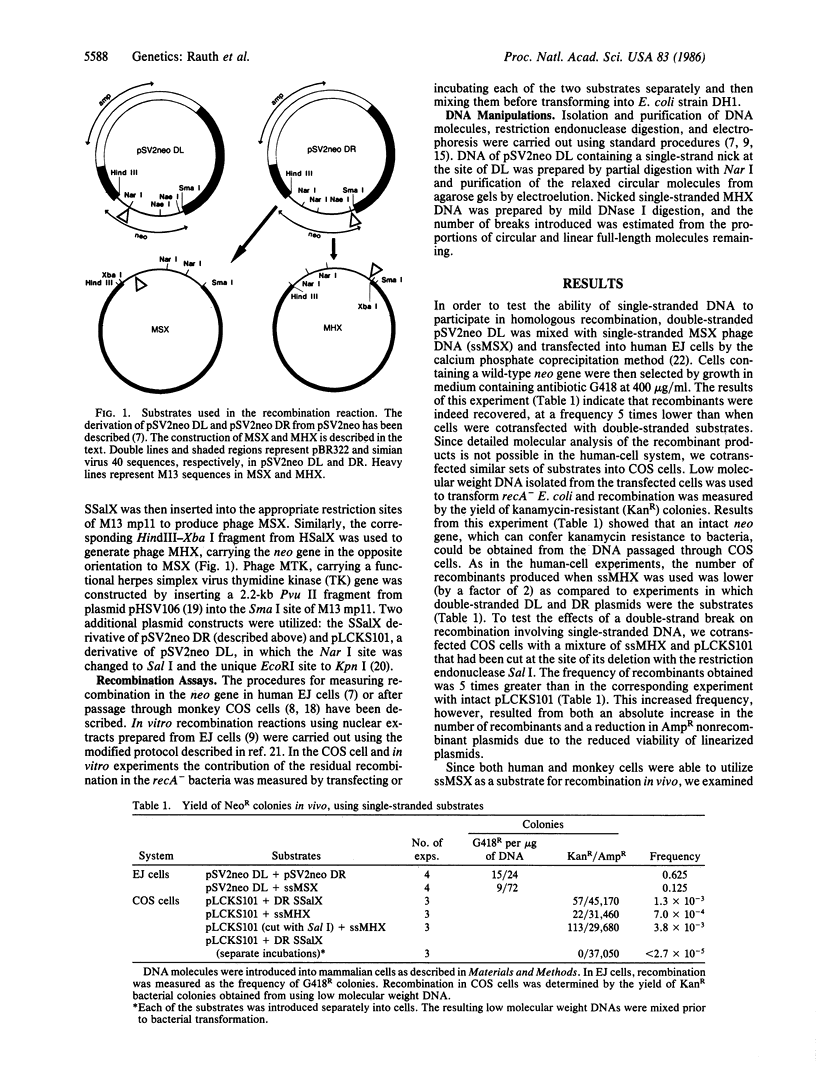
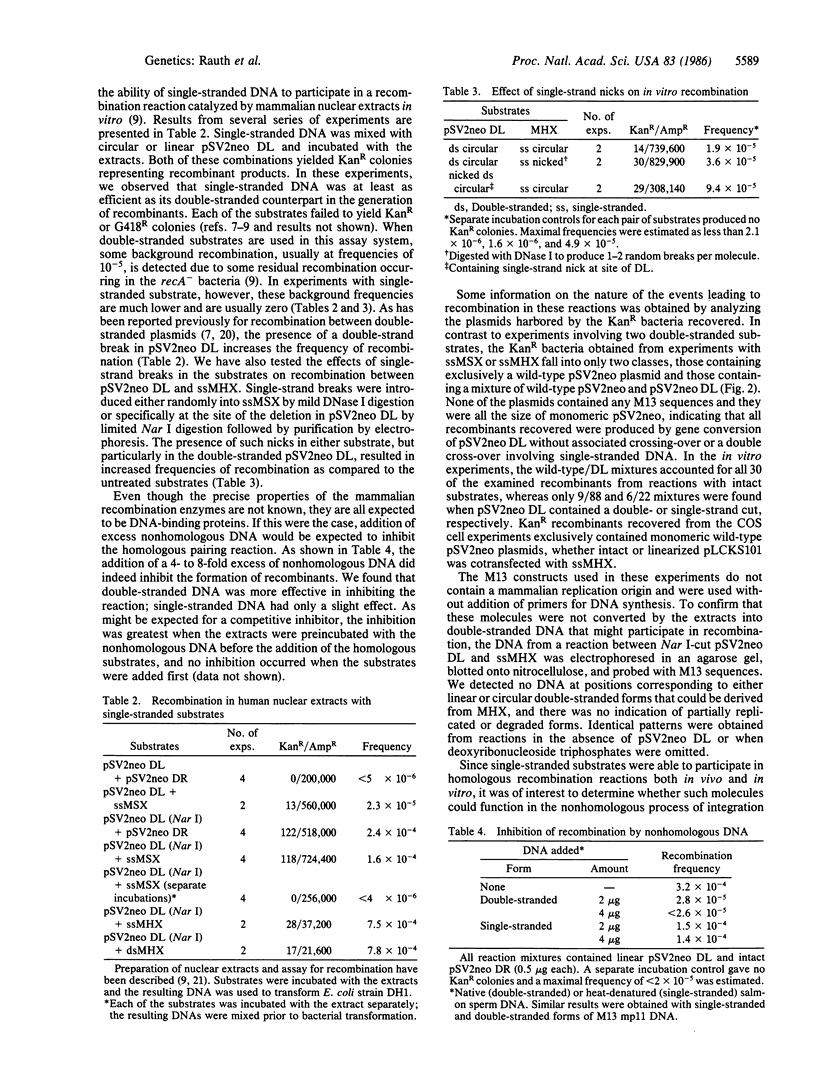

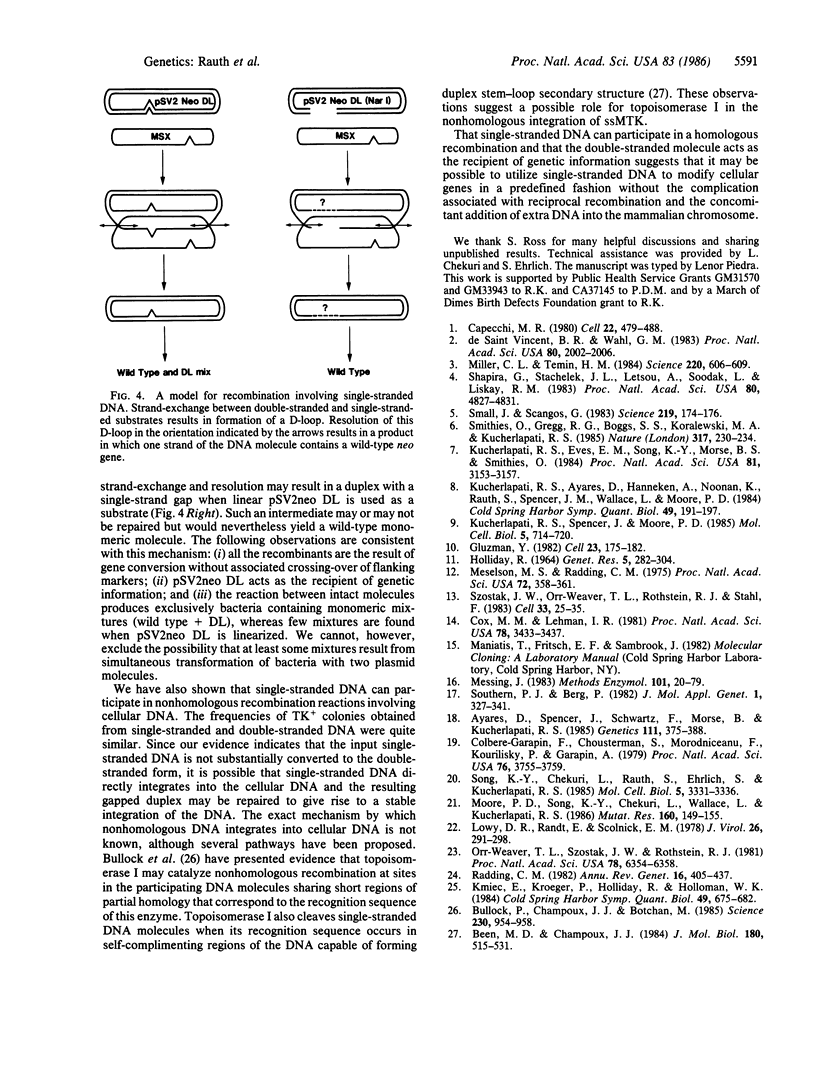
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayares D., Spencer J., Schwartz F., Morse B., Kucherlapati R. Homologous recombination between autonomously replicating plasmids in mammalian cells. Genetics. 1985 Oct;111(2):375–388. doi: 10.1093/genetics/111.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. Breakage of single-stranded DNA by eukaryotic type 1 topoisomerase occurs only at regions with the potential for base-pairing. J Mol Biol. 1984 Dec 15;180(3):515–531. doi: 10.1016/0022-2836(84)90025-1. [DOI] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Kmiec E., Kroeger P., Holliday R., Holloman W. Homologous pairing promoted by Ustilago RecI protein. Cold Spring Harb Symp Quant Biol. 1984;49:675–682. doi: 10.1101/sqb.1984.049.01.076. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Ayares D., Hanneken A., Noonan K., Rauth S., Spencer J. M., Wallace L., Moore P. D. Homologous recombination in monkey cells and human cell-free extracts. Cold Spring Harb Symp Quant Biol. 1984;49:191–197. doi: 10.1101/sqb.1984.049.01.022. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Eves E. M., Song K. Y., Morse B. S., Smithies O. Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3153–3157. doi: 10.1073/pnas.81.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R. S., Spencer J., Moore P. D. Homologous recombination catalyzed by human cell extracts. Mol Cell Biol. 1985 Apr;5(4):714–720. doi: 10.1128/mcb.5.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. High-efficiency ligation and recombination of DNA fragments by vertebrate cells. Science. 1983 May 6;220(4597):606–609. doi: 10.1126/science.6301012. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Song K. Y., Chekuri L., Wallace L., Kucherlapati R. S. Homologous recombination in a Chinese hamster X-ray-sensitive mutant. Mutat Res. 1986 Apr;160(2):149–155. doi: 10.1016/0027-5107(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Shapira G., Stachelek J. L., Letsou A., Soodak L. K., Liskay R. M. Novel use of synthetic oligonucleotide insertion mutants for the study of homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4827–4831. doi: 10.1073/pnas.80.15.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J., Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Chekuri L., Rauth S., Ehrlich S., Kucherlapati R. Effect of double-strand breaks on homologous recombination in mammalian cells and extracts. Mol Cell Biol. 1985 Dec;5(12):3331–3336. doi: 10.1128/mcb.5.12.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Wahl G. M. Homologous recombination in mammalian cells mediates formation of a functional gene from two overlapping gene fragments. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2002–2006. doi: 10.1073/pnas.80.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]