Abstract
CXCR3 is a chemokine receptor that is rapidly induced on naïve T cells following activation, and preferentially remains highly expressed on type-1 helper (Th1)-type CD4+ T cells, effector CD8+ T cells and innate-type lymphocytes, such as natural killer (NK) and NKT cells. CXCR3 is activated by three interferon (IFN)-γ-inducible ligands CXCL9 (monokine induced by gamma-interferon), CXCL10 (interferon-induced protein-10) and CXCL11 (interferon-inducible T-cell alpha chemoattractant). Although some studies have revealed that these ligands have redundant functions in vivo, other studies have demonstrated that the three CXCR3 ligands can also collaborate and even compete with each other. Differential regulation of the three ligands at specific times in defined anatomically restricted locations in vivo likely participates in the fine control of T-cell trafficking over the course of an immune response. Among the differences in regulation, CXCL10 is induced by a variety of innate stimuli that induce IFN-α/β as well as the adaptive immune cell cytokine IFN-γ, whereas CXCL9 induction is restricted to IFN-γ. In this review, we will discuss how the balance, timing and pattern of CXCR3 ligand expression appears to regulate the generation of effector T cells in the lymphoid compartment and subsequent migration into peripheral sites of Th1-type inflammation in which the CXCR3 ligands also then regulate the interactions and migratory behavior of effector T cells in an inflamed peripheral tissue.
Keywords: CXCR3, CXCL9, CXCL10, chemokines, T-cell trafficking
One of the fundamental questions in chemokine biology has been the apparent redundancy of chemokine ligands. We now know that there are approximately 50 chemokines and 20 chemokine receptors. Many chemokine receptors therefore have multiple chemokine ligands. In vitro assays have revealed that multiple chemokines can have similar binding affinities for the same receptor, and induce a similar signaling cascade and a similar chemotaxis profile through the same receptor. Thus, the question arises as to why have multiple ligands for the same receptor? The CXCR3 receptor and its ligands represent a complex chemokine system whereby one receptor has three interferon (IFN)-γ-inducible ligands, CXCT9, CXCT10 and CXCL11.
CXCR3 is an inflammatory chemokine receptor whose expression is associated with CD4+ Type-1 helper (Th1) and CD8+ cytotoxic lymphocytes (CTLs) (Table 1).1–4 Early studies found that T cells, recovered from inflamed peripheral tissues in human autoimmune disease, were highly enriched in CXCR3 surface expression relative to T cells found in the blood.2,5,6 This observation coupled with finding that the CXCR3 ligands were highly expressed in these same diseased tissue, indicated a specific role for CXCR3 and its ligands in the recruitment of T cells into these otherwise restricted sites (Table 2). CXCR3 is absent on naïve T cells, but is rapidly upregulated following dendritic cell (DC)-induced T-cell activation.3,7,8 CXCR3+ cells make up between 60 and 90% of CD8+ memory T cells9,10 and 40% of CD4+ memory T cells.11,12 T-bet, the master transcription factor of Th1 and CTL commitment, directly transactivates CXCR3 and other genes important for Th1 and CTL cell function, thus allowing these cells to infiltrate into the inflamed tissues.13–16 The tight correlation between CXCR3 expression and Th1 and CTL differentiation led to the hypothesis, subsequently verified in mouse models, that CXCR3 and its ligands regulate the migration of Th1 cells into sites of Th1-driven inflammation8,17,18 (Table 2).
Table 1.
CXCR3 and its ligands
| Receptor | Highly expressed |
Ligands | Activity | Ligand inducers | Promoter elements |
||
|---|---|---|---|---|---|---|---|
| CXCR3 | Effector CD4+Th1, CD8+CTL, NK and NKT cells, pDCs |
CXCL9 | Activate to induce chemotaxis, cell migration and adhesion |
IFN-γ⋙TNF | γIRE-1, NF-κB2 | ||
| CXCL10 | IFN-γ>IFN-α/β>TNF | IRSE, NF-κB1 | |||||
| CXCL11 | IFN-γ=IFN-β>IFN-α>TNF | STAT3, NF-κB2 |
Abbreviations: CTL, cytotoxic lymphocyte; DC, dendritic cell; IFN, interferon; NF, nuclear factor; NK, natural killer; NKT, natural killer T celI; Th1, type-1 helper; TNF, tumor necrosis factor.
Table 2.
CXCR3 and its ligands in human disease and murine disease models
| Disease/disease model | Receptor | Ligands |
||
|---|---|---|---|---|
| CXCR3 | CXCL9 | CXCL10 | CXCL11 | |
| Autoimmmune | ||||
| Psoriasis | 91, 92 | 91, 93 | 91 | |
| Sarcoid | 94, 95 | 95, 96 | 94, 95 | 95, 96 |
| Rheumatoid arthritis | 21, 97–100 | 98 | 99, 101 | |
| Asthma | 102, 103 | 102 | 102, 104–106 | 102 |
| Atherosclerosis | 107, 108 | 107 | 107, 109 | 107 |
| Multiple sclerosis | 110–116 | 111 | 110, 111, 117, 118 | |
| IBD | 119 | 73 | ||
| Idiopathic pulmonary fibrosis | 120, 121 | 59, 60 | 122 | |
| Type 1 diabetes mellitus | 70, 123 | 69, 123, 124 | ||
| SLE | 75, 125, 126 | 75 | 126, 127 | |
| Cigarette smoke injury/COPD | 128–131 | 131 | 128, 131 | 131 |
| Myocarditis | 132 | 96, 133 | ||
| Transplantation | ||||
| Heart transplant | 86, 134–137 | 41, 138 | 41, 139 | |
| Lung transplant | 38, 140 | 38, 89 | 38, 89, 140 | 89 |
| GVH | 141, 142 | 142 | ||
| Small bowel | 72 | 72 | ||
| Infections | ||||
| Leprosy | 143 | |||
| Tuberculosis | 144, 145 | |||
| Influenza | 146, 147 | 146, 147 | ||
| Toxoplasma gondii | 17, 148 | |||
| Malaria | 42, 78 | 42, 78 | 42, 78, 79, 149 | |
| Dengue | 66 | 66, 150 | ||
| Hepatitis B and C | 151–153 | 151, 152 | 64, 151, 153, 154 | 151 |
| Herpes simplex | 22, 80 | 22, 80 | 22, 80, 155, 156 | |
| HIV-1 | 157 | 157 | 157–159 | 158 |
| Leishmania | 160 | 161 | 161, 162 | |
| Chlamydia trachomatis | 163, 164 | 164 | ||
| Lyme | 165 | 166, 167 | 166, 167 | |
| West nile virus | 67 | 67 | ||
| Cancer | ||||
| Renal | 168 | 168 | 168 | 168 |
| Colon | 169, 170 | |||
| Melanoma | 171 | 171 | 171 | |
| Lymphoma | 172–174 | 175 | ||
| Breast | 176 | 176 | ||
Abbreviations: COPD, chronic obstructive pulmonary disease; GVH, graft-versus-host; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; SLE, systemic lupus erythematosus.
In addition to CXCR3 expression on effector CD4+ and CD8+ T cells, CXCR3 is also highly expressed on innate lymphocytes, such as natural killer (NK) cells and NKT cells in which it is thought to participate in the localization of these first-line defenders at sites of infection and inflammation.5,19 Further, CXCR3 is expressed on plasmacytoid DCs and subsets of B cells in which it may have a role in the migration of these cells in the inflamed lymph node (LN).20,21
CXCR3 through binding of its chemokine ligands has been shown to coordinate inflammation in the periphery.22 CXCR3 binds three chemokines: CXCL9 (also known as monokine induced by gamma-interferon, MIG), CXCL10 (interferon-induced protein of 10kDa, IP-10), CXCL11 (interferon-inducible T-cell alpha chemoattractant, I-TAC) to induce migration.1,2,23,24 Although CXCL9, CXCL10 and CXCL11 all bind CXCR3 and elicit migration of CXCR3-expressing cells in vitro, inflammatory models demonstrate that chemokine redundancy may not be operative in vivo. As we will discuss, CXCL9, CXC’L10 and CXCL’11 have been shown to work redundantly, synergistically collaboratively and, in some instances, apparently antagonistically in vivo. Thus, understanding the balance, timing and expression pattern of this chemokine system in vivo will be important for understanding the respective roles of the three CXCR3 ligands in the generation and delivery of an immune response.
CXCR3 LIGAND INDUCTION
As their original names suggest, IFN-γ Type II interferon mediates the induction of all three CXCR3 ligands (Table 1).23,25,26 However, unique promoters control the distinct pattern of expression of each CXCR3 ligand. The CXCL10 promoter contains a functional IRSE and nuclear factor kappa Bl (NF-κBI) element,27–29 whereas the CXCL9 promoter contains an γIRE-1 element and a weak NF-κB2 element.29–31 The CXC11 promoter is more similar to the CXCL10 promoter, and contains an IRSE site and an NK-κB2 site.32 For CXCL11 expression, a STAT3-STAT1 heterodimer, and not the classical STAT1–STAT2 heterodimer, binds to the CXCL11 promoter subsequent to IFN stimulation. In addition, the NF-κB family member p65 and IFN-regulatory factor 1 also binds to the CXCL11 promoter.33 As such, the CXCR3 ligands are differentially regulated. CXCL10 is strongly induced by IFN-γ as well as by the Type I interferons IFN-α//β, and weakly induced tumor necrosis factor, although tumor necrosis factor synergizes strongly with the IFNs for CXCL10 induction.34,35 CXCL9 is strongly induced by IFN-γ but not induced by IFN-α/β, and whereas tumor necrosis factor does not induce CXCL9 expression alone, it does synergizes with the IFN-γ in inducing CXCL9 transcription.34,36 CXCL11 is induced by IFN-γ and by IFN-β but surprisingly not by IFN-α37 As with the other two CXCR3 ligands, tumor necrosis factor synergizes with IFN-γ in inducing CXCL11 transcription. Thus, although CXCL9 is completely dependent on IFN-γ for its induction, CXC10 and CXCL11 are not.22,38 In addition, as CXCL10 is induced by IFN-α, it is also induced by innate sensors, such as the toll-like receptors and the RNA helicases (for example, RIG-I and MAD-5), through activation of IFN-regulatory factor 3 and the release of IFN-α.39 Autocrine signaling of IFN-α and activation of Stat1 activates the CXCL10 gene.40 CXCL10 is also preferentially induced by hypoxia-reperfusion injury by NF- κB activation, independent of p50, and has been shown to have an early role in the hypoxia-induced inflammation associated with solid-organ transplantation, such as the heart and lung.38,41 Thus, owing to different regulatory elements on their promoters, CXCR3 ligands have different temporal and spatial patterns of expression, regulated by different stimuli and expressed by distinct cell types during the course of an immune response.38,42 These differences undoubtedly account for the unique role of the CXCR3 ligands in regulating the fine control of T-cell trafficking in vivo.
Receptor binding
In addition to different expression profiles, CXCR3 ligands display unique binding characteristics toward their shared receptor. This may result in these ligands transducing slightly different signals to CXCR3-expressing cells, which may also contribute to the unique roles of the three CXCR3 ligands in vivo. There is a hierarchy of affinity for CXCR3, with CXCL11 binding to CXCR3 having the highest affinity followed by CXCL10 having intermediate affinity and then CXCL9 having the lowest affinity for CXCR3.43–45 Concordant with their affinity, CXCL11 induces calcium mobilization and chemotaxis at lower doses than CXCL9 and CXCL10.23,46 Different regions of the CXCR3 receptor enact binding, activation and internalization by each protein. CXCR3 is sulfated on its N-terminus and that sulfation is required for binding and activation by all three ligands, whereas the proximal 16 amino acid residues of the N terminus are required for CXCL10 and CXCL11 binding and activation but not CXCL9 activation.47 Two distinct domains control internalization of CXCR3.48 The carboxyl-terminal domain and beta-arrestin-1 binding domain are predominantly required for CXCL9- and CXCL10-directed internalization, whereas third intracellular loop is required by CXCL11.48 Structure-activity studies with CXCR3 ligands have identified unique regions in each protein, which are important for their binding to CXCR3 and to heparin, further explaining their differential effects on the receptor.49–51 These differences in protein-receptor binding and protein structure can potentially be exploited to develop new therapeutics, with enhanced functional properties, which has been demonstrated for a CXCL10-CXCL11 chimeric protein that had greater antitumor activity than the parent chemokines.52
The CXCR3 ligands also enact their functions through the binding of other chemokine receptors as well as glycosaminoglycan (GAG)-containing molecules, such as proteoglycans. At high concentrations, all three ligands are thought be natural antagonists for CCR3, a chemokine receptor normally expressed on eosinophils.53 Therefore, differential expression of CCR3 may also alter the function of these chemokines. In addition, CXCL11 but not CXCL9 and CXCL10 binds to CXCR7.54,55
The CXCR3 ligands are basic proteins that bind avidly to negatively charged GAG molecules, both on the surface of cells and in the extracellular matrix.50,51,56 GAG binding is thought to be important for the retention and presentation of chemokines to their chemokine receptors in vivo. Although the in vitro chemotactic activity of CXCL10 and CXCL11 was shown to be independent of GAG binding, the ability of these chemokines to induce CXCR3-dependent T-cell migration in vivo was shown to be dependent on their ability to bind GAGs.51,57 Although it is not the focus of this review, the ability of CXCL0 to influence the behavior of certain non-immune cells, such as endothelial cells and fibroblasts, that do not express CXCR3, is a function of the ability of these chemokines to bind to cell surface GAGs.58–63
LIGAND-DEFICIENT COMPLEXITY
A further piece of information adding to the confusion surrounding the CXCR3 ligand system is that wild-type C57BL/6 mice, which are most commonly used for mouse experimentation, contain a point mutation and a single-base deletion in the leader sequence, resulting in a reading frame shift that introduces a stop codon early within the Cxcl11 gene, making these mice deficient for CXCL11. The C57BL/6 mice are still capable of responding to injected CXCL11 in a CXCR3-dependent manner.18 This observation, further confounds the interpretation of results, as both the Cxcl9−/− and Cxcl10−/− mice were generated using 129 embryonic stem cells, and subsequently backcrossed onto the C57BL/6 background.64,65 The close linkage of these chemokine genes means that both Cxcl9−/− and Cxcl10−/− mice have an intact Cxcl11 gene, so there is a discrepancy between Cxcl9−/− and Cxcl10−/− and their wild-type controls. Owing to this added complexity, caution must be taken when interpreting results from Cxcl9−/− and Cxcl10−/− mice on a C57BL/6 background. It will be important for future studies to investigate the expression of CXCL11 in these knockout mice, to determine whether some observed affects are because of the difference in CXCL11 production. Ultimately, it will be important to generate Cxcl9−/− and cxcl10−/− mice using C57BL/6 embryonic stem cells.
LIGAND REDUNDANCY
In an in vivo model of cell recruitment, each of the CXCR3 ligands are equally efficacious at recruiting activated effector CD4+ and CD8+ T cells into the lung.18 However, this T-cell recruitment model does not consider the complexity of ligand induction as discussed above. Surprisingly, even during an immune response in which the ligands are differentially expressed and regulated, there appears to be significant chemokine redundancy in this system.38 In a murine model of obliterative bronchiolitis, CXCL9 and CXCL10 are differentially regulated and they together coordinate CXCR3+ T-cell infiltration into the airways. Early after an allogenic transplant, CXCL10 is expressed in a manner that is independent of IFN-γ, p50 and STAT1.38 This CXCL10 expression was the result of an oxidant-sensitive pathway, possibly involving other NF- κB family members. However, later in the course of transplant rejection, CXCL9 and CXCL10 were both induced in an IFN-γ- and STAT1-dependent manner. Although CXCR3 deficiency reduced airway obliteration in this model, neither Cxcl9−/− or Cxcl10−/− mice showed any decreased rejection. These results suggest that despite their different induction, the loss of one chemokine is compensated for by the presence of the other, and that either of the ligand is required for the recruitment of allograft-reactive CXCR3-expressing cells.38
LIGAND DOMINANCE
In some inflammatory models, the requirement of one CXCR3 ligand dominates, and its deficiency cannot be compensated for by the presence of the other ligands. Some infections in the brain show an importance of CXCL10 over the other CXCR3 ligands. In dengue virus infection, Cxcr3−/− mice have higher viral loads than those of wild-type mice because of reduced trafficking of CD8+ effector cells into the brain. Although CXCL9, CXCL10 and CXCL11 are all induced by dengue virus infection, the latter two could not compensate for the absence of CXCL10 in Cxcl10−/− mice.66 Similarly, in West Nile virus infection, CXCL10 is expressed by neurons and directs the migration of CD8+ T cells into the brain.67 Cxcr3−/− and Cxcl10−/− mice are protected from meningitis during central nervous system lymphocytic choriomeningitis virus infection.68 Whether the predominant importance of Cxcl10 during these infections is particular to the brain or to viral infections is unknown, as this CXCR3 ligand also appears to dominate in other non-brain viral responses and non-viral central nervous system infections.17,64
In a model that uses acute lymphocytic choriomeningitis virus infection of transgenic mice that express the glycoprotein of lymphocytic choriomeningitis virus in the cells of the islets of Langerhans, all CXCR3 ligands are upregulated in the pancreas.69 Although CXCL9 immunohistochemistry showed limited expression around the outer aspects of islet, CXCL10 stained throughout the infiltrated islet.70 Cxcr3−/− mice showed delayed onset of insulitis in this model, seemingly because of an impaired T-cell infiltration into the islet through CXCL10. This was also seen in mice treated with CXCL10-neutralizing antibodies, whereas CXCL9 neutralizing antibodies had no affect on disease progression.69,70 In a small-bowel allograft rejection model, CXCR3+ T-cell infiltration of the graft lamina propria was primarily because of CXCL10, as either using neutralizing CXCL10 or using grafts from Cxcl10−/− mice significantly delayed rejection.71,72 Further, in the interleukin-10 null inflammatory bowel disease model, CXCL10 and CXCR3 were highly expressed at sites of colitis, and CXCL10 neutralization attenuated the severity of colitis.73,74
In contrast, Cxcr3- and Cxcl9-deficient, but not Cxcl10-deficient, Murphy Roth Large/lpr mice were protected from autoimmune lupus-like inflammation of the kidney75 Interestingly in this model, Cxcr3 deficiency blocked the infiltration of not only Th1 cells but also interleukin-17-secreting cells into the kidney76 Few studies have investigated the role of Cxcl11 in vivo. However, in a model of acute skin allograft inflammation, Cxcl11 was the most highly elevated chemokine. Treatment with a CXCL11-neutralizing antibody reduced the number of CXCR3+ cells in the skin allograft and prolonged graft survival.77 Although it may appear from this review that CXCL10 is the dominant CXCR3 ligand in most immune responses, it is worth noting that this chemokine is the most highly studied, and multiple studies evaluate only this single chemokine. Further studies of these models may reveal unappreciated roles for CXCL9 and CXCL11.
LIGAND COLLABORATION
As mentioned above, several models of T-cell inflammation in the brain and spinal cord have shown a dominance of CXCL10 as the primary CXCR3 ligand responsible for ‘inviting’ effector T cells into this otherwise restricted site. In contrast, in other models, cooperation between CXCL9 and CXCL10 is required for full T-cell infiltration. CXCR3 is required on CD8+ cells for infiltration into the brain during Plasmodium berghtei ANKA infection for the development of cerebral malaria symptoms.42,78 The Cxcr3−/− mice are protected from cerebral malaria because of reduced CD8+ CTL sequestration in the brain. This protection is mediated equally by CXCL9 and CXCL10, as each mouse deficient in one of these ligands showed partial disease protection.42,79 This cooperation between CXCR3 ligands appears to be because of the differential expression of the ligands by different cell types. Immuno-histochemistry of P. berghei-infected mice revealed that CXCL9 was predominantly expressed by endothelial cells and CXCL10 was predominately expressed by neurons explaining the non-overlapping roles of these two CXCR3 ligands in the pathogenesis of cerebral malaria.42 Similarly, in a mouse model of herpes simplex virus-2 infection, expression of both CXCL9 and CXCL10 was necessary for optimal recruitment of NK cells and CTLs into the spinal cord.80 During the course of the disease in the ligand-deficient mice, the RNA levels of the alternate ligand were significantly diminished. These data suggest that each CXCR3 ligand contributes to the expression of other CXCR3 ligands in this model. This regulation is likely to be indirect because of increased IFN-γ levels in the local environment provided by the first wave of recruited NK and CTL cells into the spinal cord.22,80 Unfortunately, this study did not investigate cell type-specific expression of the CXCR3 ligands, however, it is likely that each chemokine ligand is expressed by different cells in the spinal cord and brain, as was seen in the model of cerebral malaria.42,80
Differences in temporal or tissue-specific expression of the CXCR3 ligands have also been shown to collaborate to choreograph the movement of CXCR3-expressing T cell from the lymphoid compartment to the peripheral inflamed tissue. This was demonstrated in a murine model of granulomatous liver disease induced by Propioni-bacterium acnes.81 In this model, LN DCs produced CXCL10, whereas hepatic granuloma cells in the liver parenchyma produced CXCL9. Neutralization of either CXCL9 or CXCL10 gave different results, leading to the hypothesis that CXCL10 instructs CD4+ Th1 cells to stay in the LN interacting with DCs, whereas CXCL9 expression in the periphery drives the recruitment of these cells out of the LN and into the liver.81 Therefore, the transient switch in expression of CXCL9 and CXCL10 is critical during the disease to allow the progression of a Th1 cell responses.
The collaboration of CXCR3 ligands during immune responses coordinates T-cell infiltration in the periphery. Multiple studies have demonstrated that recruitment of effector T cells and inflammation drives further recruitment and inflammation. This likely involves both CXCR3-dependent and a CXCR3-independent amplification loops. The CXCR3-dependent amplification of immune responses involves the collaboration of the different CXCR3 ligands, which are regulated differentially during the course of an inflammatory response that moves from innate stimuli (for example, IFN-α/β) to adaptive stimuli (for example, IFN-γ)22,38 (Figure 1). This CXCR3-dependent inflammatory loop potentially may not only increase recruitment of CTLs into peripheral tissues but also may enhance the generation of CTLs,82 and promote increased effector responses through STAT1 signaling.83 This CXCR3-dependent inflammatory loop also has been shown to spark CXCR3-indendent inflammation. This has been observed in the context of CXCR3-dependent inflammation whereby CXCR3-dependent T-cell recruitment permits the entry of CXCR3 negative and Cxcr3−/− T cells into immune-privileged sites.42,76
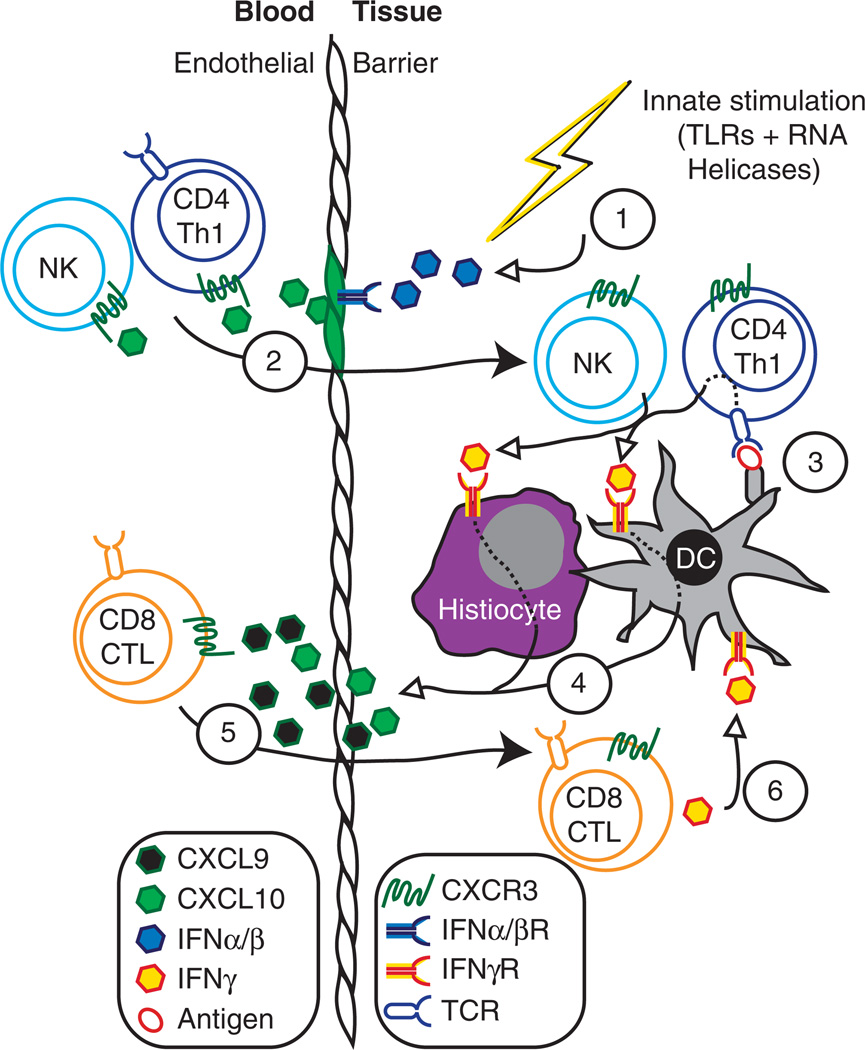
Figure 1.
Collaboration between CXCR3 ligands coordinates effector immune responses. This model describes the sequential events from top (1) to bottom (6) involving Type I and Type II interferon induction of the CXCR3 ligands, CXCL9 and CXCL10, and how this defines their respective roles in the recruitment of effector T cell and NK cell populations into inflamed tissues. CXCL11 also likely contributes to this process but currently less is known how this CXCR3 ligand contributes to this cascade. Open arrowheads indicate cytokine/chemokine secretion and closed arrowheads indicate cellular movement. (1) Innate immune activation, such as bacterial or viral infection, activates TLRs and RNA helicases leading to the release of IFN-α/β and subsequent secretion CXCL10 by tissue resident cells and endothelial cells. (2) CXCL10 recruits NK and CD4+ Th1 cells into the target tissue. (3) CD4+ cells release IFN-γ in an antigen-specific manner. (4) IFN-γ signaling in tissue resident cells, including DCs and tissue histiocytes induce the production and secretion of CXCL9 and CXCL10. (5) Secreted CXCL9 and CXCL10 recruit CD8+ CTLs into the tissue. (6) CTL-derived IFN-y further stimulate tissue resident cells to produce more CXCL9 and CXCL10, thus, amplifying the Th1-type inflammatory response.
Although the concept of an IFNγ-CXCR3-chemokine-dependent inflammatory loop is firmly established, interesting questions about the cellular sources of the ligands in different types of inflammation still remain unanswered. Whether the cells upregulating CXCR3 ligands are peripheral dendritic cell subsets and/or non-immune structural cells in the site of inflammation remains to be determined. In addition, it is not clear whether the T cells involved in this amplification loop directly contact cells that upregulate CXCL9 and CXCL10, or whether soluble chemokine gradients dispersed throughout the inflamed tissue recruit more effector cells.
APPARENT LIGAND ANTAGONISM
As was mentioned for the P. acnes model of liver granuloma above, inhibition of the individual CXCR3 chemokine ligands has on some occasion given apparent opposite results. This was recently demonstrated in a model of allograft heart transplant rejection. In this model, CXCL9 produced by allograft DCs promote the priming of host CD8+ and CD4+ cells towards CTL and Th1 IFN-γ-producing T cells.84 DC-produced CXCL9 was induced by NK cell-derived IFN-γ. Deficiency in Cxcl9, specifically in the passenger DCs from the allograft, reduced the priming of graft-reactive CTLs and Thl cells in the spleen of mice receiving heart transplants. CXCL10 was also produced in the spleen with a similar kinetics as CXCL9. Surprisingly, CXCL10 appeared to have the opposite effect on T-cell priming, in which a deficiency of Cxcl10 resulted in increased IFN-γ-producing CD8+ T cells, although this study did not dissect whether the Cxcl10 deficiency in the recipient or the allograft was responsible for this effect.84 The treatment of Cxcl10−/−-transplanted mice, with CXCL9-neutralizing antibody reduced the numbers of IFN-γ-producing CD8+ T cells to that observed in wild-type-transplanted mice, suggesting that the Cxcl10 deficiency increase in IFN-γ-producing was because of CXCL9 increased expression.84
Observations of opposing ligand function have also been detailed in a model of ocular immune response to herpes simplex virus-1 infection using Cxcl9−/− and Cxcl10−/− mice. Cxcl10−/− mice showed an increased sensitivity to ocular virus and elevated virus titer in the tear film and corneal tissue. In comparison, there was a significant reduction in CD4+ T-cell infiltration into the cornea in Cxcl9-deficient mice, illustrating opposing functions of these chemokines in vivo.85 These observations of opposing ligand function may explain studies in which Cxcr3 deficiency or antagonism results in only a modest decrease in T-cell entry, despite high expression of CXCR3 receptor and ligands in target tissue.86
Whether these findings of opposing in vivo functions represent true antagonistic function of the CXCR3 ligands or actually represent collaborative functions of these ligands that appear opposing when analyzed in different tissues at different time points, as was seen in the P. acnes model discussed above,81 remains to be determined.
Further complexity in the system is now appreciated with the finding that CXCR3 is expressed on a Th1-specific CD4+ T-regulatory cell.87 It is also therefore possible that concomitant inhibition of CXCR3-expressing T-regulatory cells may also partially explain studies in which Cxcr3 deficiency or antagonism results in only a modest decrease in T-cell entry, despite high expression of CXCR3 receptor and ligands in the target tissue. Although the trafficking requirements of CXCR3+ T-regulatory cells remain to be determined, it is possible that they may be more responsive to one CXCR3 ligand, allowing for differential trafficking of effector and T-regulatory cell CXCR3-expressing cells during T-cell priming and inflammation. It will be interesting to observe whether this direct competition between CXCR3 ligands occurs in other models. Whether these ligands have differential effects on different T-cell subsets remains to be resolved.
At present it is not clear why in some instances different results have been obtained when examining CXCR3 ligand function in similar models. For example, in various allograft models a single pattern of CXCR3 ligand function has not emerged. As discussed, CXCR3 ligands have been shown to directly antagonize each other in one model of heart transplant,84 whereas in another CXCL10 function was shown to be dominant and upstream of the other CXCR3 ligands41 and in a third model CXCL9 was shown to be the dominant CXCR3 ligand.88 In a model of lung transplant rejection, CXCL9 and CXCL10 were shown to be redundant,38 whereas in another study they were shown to be cooperative.89 In one model of skin allograft rejection, CXCL9 was shown to be the dominant CXCR3 ligand,90 whereas in another skin allograft model CXCL11 was shown to be the dominant chemokine involved in the infiltration of CXCR3+ T cells into the inflamed tissue.77 Difference in the cellular sources and the timing of CXCR3 ligand expression in these models may explain the apparent differential requirements for the three ligands in seemingly related inflammatory models, although this clearly needs to be studied in more detail.
CONCLUDING COMMENTS AND FUTURE DIRECTIONS
In the last few years, much has been learned of the involvement of the CXCR3 chemokine system during inflammation. Yet there remain many questions that still need to be answered. How precisely the chemokines are regulated, both temporally and in a cell type-specific manner, needs to be further investigated. Other factors, in addition to members of the IFN family that induce the CXCR3 ligands during the initiation of the immune response as well as during peripheral T-cell trafficking may be found. Indeed, some studies have identified ligand expression that cannot be fully explained by our current understanding of chemokine regulation.38
Despite the demonstration of their importance in multiple disease models, surprisingly few studies have investigated in detail the specific cellular sources of CXCR3 ligand production during inflammation. Much of the work in this area has been done using quantitative PCR on total inflamed organs. Although this method confirms timing and tissue expression, it obscures the fine local temporal and spatial control of CXCR3 ligands, and it offers little information about the types of cells that elicit effector T-cell recruitment, and the molecules that induce this expression. It is likely that the cell types responsible for ligand expression greatly influence the outcome of CXCR3-expressing cell responses, for instance CXCR3 ligand expression in priming or regulatory DC subsets may induce differential responses by Thl CD4+ T cells.40 In addition, investigation of the location of CXCL9 and CXCL10, by way of immunohistology staining, may not fully answer questions of which cell produce these chemokines. An interesting question in the future will be to answer whether the same cells that present these chemokines in LN and in the periphery, are the same cells that produce them, or whether CXCL9 and CXCL10 are secreted by one cell and taken up by others to present to T cells. It may be that this presentation or the activation state of the cells presenting CXCL9, CXCL10 and CXCL11 will determine the outcome of CXCR3-mediated responses.
Finally, several studies look exclusively at one ligand by expression or blocking to explain the regulation of CXCR3-dependent mechanisms. In the future, greater detail of all the ligands of CXCR3 should be investigated as, in all probability, further analysis of these systems will highlight additional circumstances of collaboration or conflicting functions of CXCR3 ligands exist. These inquiries will be particularly necessary to dissect in diseases in which CXCR3 and its ligands have been suggested as therapeutic targets.
ACKNOWLEDGEMENTS
JRG was supported by NH&MRC and Overseas Biomedical Based Fellowship and ADL was supported by NIH Grant CA69212.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, et al. Rules of chemokine receptor association with T cell polarization in vivo . J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, et al. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- 5.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of Tcells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments with in hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 7.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 9.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 10.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH, Nagata K, Butcher EC. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol. 2003;171:152–158. doi: 10.4049/jimmunol.171.1.152. [DOI] [PubMed] [Google Scholar]
- 12.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 15.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 16.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, et al. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 18.Campanella GS, Medoff BD, Manice LA, Colvin RA, Luster AD. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J Immunol Methods. 2008;331:127–139. doi: 10.1016/j.jim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, et al. CD1d–restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Jarrossay D, Facchetti F, Alebardi 0, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 21.Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A, et al. Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2009;11:R149. doi: 10.1186/ar2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (l-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Humbles A, Bota D, Gerard C, Moser B, Soler D, et al. Structure and function of the murine chemokine receptor CXCR3. Eur J Immunol. 1999;29:3804–3812. doi: 10.1002/(SICI)1521-4141(199911)29:11<3804::AID-IMMU3804>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 26.Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87:5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol. 1998;161:4736–4744. [PubMed] [Google Scholar]
- 28.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopoly-saccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677–6688. [PubMed] [Google Scholar]
- 29.Ohmori Y, Hamilton TA. IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol. 1997;159:5474–5482. [PubMed] [Google Scholar]
- 30.Wong P, Severns CW, Guyer NB, Wright TM. A unique palindromic element mediates gamma interferon induction of mig gene expression. Mol Cell Biol. 1994;14:914–922. doi: 10.1128/mcb.14.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright TM, Farber JM. 5’regulatory region of a novel cytokine gene mediates selective activation by interferon gamma. J Exp Med. 1991;173:417–422. doi: 10.1084/jem.173.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tensen CP, Flier J, Rampersad SS, Sampat-Sardjoepersad S, Scheper RJ, Boorsma DM, et al. Genomic organization, sequence and transcriptional regulation of the human CXCL 11(1) gene. Biochim Biophys Acta. 1999;1446:167–172. doi: 10.1016/s0167-4781(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 33.Yang CH, Wei L, Pfeffer SR, Du Z, Murti A, Valentine WJ, et al. Identification of CXCL11 as a STAT3-dependent gene induced by IFN. J Immunol. 2007;178:986–992. doi: 10.4049/jimmunol.178.2.986. [DOI] [PubMed] [Google Scholar]
- 34.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 35.Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton TA. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo . Am J Pathol. 1993;142:861–870. [PMC free article] [PubMed] [Google Scholar]
- 36.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 37.Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM. Characterization of beta-R1, a gene that is selectively induced by interferon beta (IFN-beta) compared with IFN-alpha. J Biol Chem. 1996;271:22878–22884. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 38.Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, et al. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. 2006;176:7087–7095. doi: 10.4049/jimmunol.176.11.7087. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian C, An H, Yu Y, Liu S, Cao X. TLR agonists induce regulatory dendritic cells to recruit Thl cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood. 2007;109:3308–3315. doi: 10.1182/blood-2006-08-040337. [DOI] [PubMed] [Google Scholar]
- 41.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campanella GS, Tager AM, EI Khoury JK, Thomas SY, Abrazinski TA, Manice LA, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, et al. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288–18291. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- 44.Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, et al. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- 45.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer M, Hensbergen PJ, van der Raaij-Helmer EM, Brandacher G, Margreiter R, Heufler C, et al. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol. 2001;31:2521–2527. doi: 10.1002/1521-4141(200108)31:8<2521::aid-immu2521>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Colvin RA, Campanella GS, Manice LA, Luster AD. CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol Cell Biol. 2006;26:5838–5849. doi: 10.1128/MCB.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 49.Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289–295. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- 50.Campanella GS, Lee EM, Sun J, Luster AD. CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10) J Biol Chem. 2003;278:17066–17074. doi: 10.1074/jbc.M212077200. [DOI] [PubMed] [Google Scholar]
- 51.Severin IC, Gaudry JP, Johnson Z, Kungl A, Jansma A, Gesslbauer B, et al. Characterization of the chemokine CXCL11-heparin interaction suggests two different affinities for glycosaminoglycans. J Biol Chem. 2010;285:17713–17724. doi: 10.1074/jbc.M109.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang P, Yang X, Xu W, Li K, Chu Y, Xiong S. Integrating individual functional moieties of CXCL10 and CXCL11 into a novel chimeric chemokine leads to synergistic antitumor effects: a strategy for chemokine-based multi-target-directed cancer therapy. Cancer Immunol Immunother. 2010;59:1715–1726. doi: 10.1007/s00262-010-0901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, Bardi G, et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- 54.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and l-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luster AD, Greenberg S, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campanella GS, Grimm J, Manice LA, Colvin RA, Medoff BD, Wojtkiewicz GR, et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity . J Immunol. 2006;177:6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 58.Campanella GS, Colvin RA, Luster AD. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS One. 2010;5:el2700. doi: 10.1371/journal.pone.0012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Pespir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 61.Luster AD, Cardiff RD, MacLean JA, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Amer Phys. 1998;110:183–196. [PubMed] [Google Scholar]
- 62.Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–3561. doi: 10.1182/blood.v98.13.3554. [DOI] [PubMed] [Google Scholar]
- 63.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo . J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 65.Park MK, Amichay D, Love P, Wick E, Liao F, Grinberg A, et al. The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo . J Immunol. 2002;169:1433–1443. doi: 10.4049/jimmunol.169.3.1433. [DOI] [PubMed] [Google Scholar]
- 66.Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, et al. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- 67.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christensen JE, de Lemos C, Moos T, Christensen JP, Thomsen AR. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J Immunol. 2006;176:4235–4243. doi: 10.4049/jimmunol.176.7.4235. [DOI] [PubMed] [Google Scholar]
- 69.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171:6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 70.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Kaptanoglu L, Haddad W, Ivancic D, Alnadjim Z, Hurst S, et al. Donor T cell activation initiates small bowel allograft rejection through an IFN-gamma-inducible protein-10-dependent mechanism. J Immunol. 2002;168:3205–3212. doi: 10.4049/jimmunol.168.7.3205. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Kaptanoglu L, Tang Y, Ivancic D, Rao SM, Luster A, et al. IP-10-induced recruitment of CXCR3 host T cells is required for small bowel allograft rejection. Gastroenterology. 2004;126:809–818. doi: 10.1053/j.gastro.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Hyun JG, Lee G, Brown JB, Grimm GR, Tang Y, Mittal N, et al. Anti-interferon-inducible chemokine CXCL10, reduces colitis by impairing Thelper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11:799–805. doi: 10.1097/01.mib.0000178263.34099.89. [DOI] [PubMed] [Google Scholar]
- 74.Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(−/−) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res. 2008;28:31–43. doi: 10.1089/jir.2007.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183:4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 77.Li B, Xu W, Xu L, Jiang Z, Wen Z, Li K, et al. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. 2010;260:83–91. doi: 10.1016/j.cellimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Miu J, Mitchell AJ, Muller M, Carter SL, Manders PM, McQuillan JA, et al. Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol. 2008;180:1217–1230. doi: 10.4049/jimmunol.180.2.1217. [DOI] [PubMed] [Google Scholar]
- 79.Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, et al. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 2009;5:el000369. doi: 10.1371/journal.ppat.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thapa M, Welner RS, Pelayo R, Carr DJ. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180:1098–1106. doi: 10.4049/jimmunol.180.2.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, et al. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon SH, Yun SO, Park JY, Won HY, Kim EK, Sohn HJ, et al. Selective addition of CXCR3(+) CCR4(−) CD4(+) Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro . Exp Mol Med. 2009;41:161–170. doi: 10.3858/emm.2009.41.3.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, et al. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenblum JM, Shimoda N, Schenk AD, Zhang H, Kish DD, Keslar K, et al. CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors. J Immunol. 2010;184:3450–3460. doi: 10.4049/jimmunol.0903831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wuest T, Farber J, Luster A, Carr DJ. CD4+ Tcell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol. 2006;243:83–89. doi: 10.1016/j.cellimm.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenblum JM, Zhang QW, Siu G, Collins TL, Sullivan T, Dairaghi DJ, et al. CXCR3 antagonism impairs the development of donor-reactive, IFN-gamma-producing effectors and prolongs allograft survival. Transplantation. 2009;87:360–369. doi: 10.1097/TP.0b013e31819574e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, et al. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167:3494–3504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 89.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Li K, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi H, Novick AC, Toma H, Fairchild RL. Chronic antagonism of Mig inhibits cellular infiltration and promotes survival of class II MHC disparate skin allografts. Transplantation. 2002;74:387–395. doi: 10.1097/00007890-200208150-00016. [DOI] [PubMed] [Google Scholar]
- 91.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 92.Goebeler M, Toksoy A, Spandau U, Engelhardt E, Brocker EB, Gillitzer R. The C-X-C chemokine Mig is highly expressed in the papillae of psoriatic lesions. J Pathol. 1998;184:89–95. doi: 10.1002/(SICI)1096-9896(199801)184:1<89::AID-PATH975>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 93.Gottlieb AB, Luster AD, Posnett DN, Carter DM. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988;168:941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agostini C, Cassatella M, Sancetta R, Zambello R, Trentin L, Gasperini S, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–6420. [PubMed] [Google Scholar]
- 95.Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, et al. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. Eur Respir J. 2009;34:676–686. doi: 10.1183/09031936.00157508. [DOI] [PubMed] [Google Scholar]
- 96.Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, et al. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clin Exp Immunol. 2007;149:317–326. doi: 10.1111/j.1365-2249.2007.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin S, Rottman JB, Myrers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T celIs associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsubaki T, Takegawa S, Hanamoto H, Arita N, Kamogawa J, Yamamoto H, et al. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol. 2005;141:363–371. doi: 10.1111/j.1365-2249.2005.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martini G, Zulian F, Calabrese F, Bortoli M, Facco M, Cabrelle A, et al. CXCR3/CXCL10 expression in the synovium of children with juvenile idiopathic arthritis. Arthritis Res Ther. 2005;7:R241–R249. doi: 10.1186/ar1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohan K, Issekutz TB. Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol. 2007;179:8463–8469. doi: 10.4049/jimmunol.179.12.8463. [DOI] [PubMed] [Google Scholar]
- 101.Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol. 2002;169:2685–2693. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- 102.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. 2007;179:1901–1912. doi: 10.4049/jimmunol.179.3.1901. [DOI] [PubMed] [Google Scholar]
- 103.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 104.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, et al. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- 105.Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31:783–789. doi: 10.1183/09031936.00111507. [DOI] [PubMed] [Google Scholar]
- 106.Bochner BS, Hudson SA, Xiao HQ, Liu MC. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol. 2003;112:930–934. doi: 10.1016/j.jaci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 107.Mach F, Sauty A, larossi AS, Sukhova GK, Neote K, Libby P, et al. The interferon-gamma inducible CXC chemokines IP-10, Mig, and I-TAC are differentially expressed by human atheroma-associated cells: implications for lymphocyte recruitment in atherogenesis. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, et al. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo . Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 109.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 110.Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 111.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balashov K, Rottman J, Weiner H, Hancock W. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1 and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sporici R, Issekutz TB. CXCR3 blockade inhibits T-cell migration into the CNS during EAE and prevents development of adoptively transferred, but not actively induced, disease. Eur J Immunol. 2010;40:2751–2761. doi: 10.1002/eji.200939975. [DOI] [PubMed] [Google Scholar]
- 114.Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I, McColl SR. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18:504–516. doi: 10.1111/j.1750-3639.2008.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 116.Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 117.Wildbaum G, Netzer N, Karin N. Plasm id DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;168:5885–5892. doi: 10.4049/jimmunol.168.11.5885. [DOI] [PubMed] [Google Scholar]
- 118.Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, et al. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol. 2004;172:550–559. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- 119.Schroepf S, Kappler R, Brand S, Prell C, Lohse P, Glas J, et al. Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1882–1890. doi: 10.1002/ibd.21312. [DOI] [PubMed] [Google Scholar]
- 120.Pignatti P, Brunetti G, Moretto D, Yacoub MR, Fiori M, Balbi B, et al. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. Am J Respir Crit Care Med. 2006;173:310–317. doi: 10.1164/rccm.200502-244OC. [DOI] [PubMed] [Google Scholar]
- 121.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burdick MD, Murray LA, Keane MP, Xue YY, Zisman DA, Belperio JA, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171:261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 123.Uno S, Imagawa A, Saisho K, Okita K, Iwahashi H, Hanafusa T, et al. Expression of chemokines CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr J. 2010;57:991–996. doi: 10.1507/endocrj.k10e-076. [DOI] [PubMed] [Google Scholar]
- 124.Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159:338–343. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Enghard P, Humrich JY, Rudolph B, Rosenberger S, Biesen R, Kuhn A, et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 2009;60:199–206. doi: 10.1002/art.24136. [DOI] [PubMed] [Google Scholar]
- 126.Kong KO, Tan AW, Thong BY, Lian TY, Cheng YK, Teh CL, et al. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin Exp Immunol. 2009;156:134–140. doi: 10.1111/j.1365-2249.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 129.Nie L, Xiang R, Zhou W, Lu B, Cheng D, Gao J. Attenuation of acute lung inflammation induced by cigarette smoke in CXCR3 knockout mice. Respir Res. 2008;9:82. doi: 10.1186/1465-9921-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nie L, Xiang RL, Liu Y, Zhou WX, Jiang L, Lu B, et al. Acute pulmonary inflammation is inhibited in CXCR3 knockout mice after short-term cigarette smoke exposure. Acta Pharmacol Sin. 2008;29:1432–1439. doi: 10.1111/j.1745-7254.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 131.Brozyna S, Ahern J, Hodge G, Nairn J, Holmes M, Reynolds PN, et al. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. COPD. 2009;6:4–16. doi: 10.1080/15412550902724164. [DOI] [PubMed] [Google Scholar]
- 132.Taqueti VR, Grabie N, Colvin R, Pang H, Jarolim P, Luster AD, et al. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177:5890–5901. doi: 10.4049/jimmunol.177.9.5890. [DOI] [PubMed] [Google Scholar]
- 133.Yuan J, Liu Z, Lim T, Zhang H, He J, Walker E, et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ Res. 2009;104:628–638. doi: 10.1161/CIRCRESAHA.108.192179. [DOI] [PubMed] [Google Scholar]
- 134.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, Lu B, et al. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86:137–147. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zerwes HG, Li J, Kovarik J, Streiff M, Hofmann M, Roth L, et al. The chemokine receptor Cxcr3 is not essential for acute cardiac allograft rejection in mice and rats. Am J Transplant. 2008;8:1604–1613. doi: 10.1111/j.1600-6143.2008.02309.x. [DOI] [PubMed] [Google Scholar]
- 137.Kwun J, Hazinedaroglu SM, Schadde E, Kayaoglu HA, Fechner J, Hu HZ, et al. Unaltered graft survival and intragraft lymphocytes infiltration in the cardiac allograft of Cxcr3−/− mouse recipients. Am J Transplant. 2008;8:1593–1603. doi: 10.1111/j.1600-6143.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 138.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, et al. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75:72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 139.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 140.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, et al. CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 142.Mapara MY, Leng C, Kim YM, Bronson R, Lokshin A, Luster A, et al. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–634. doi: 10.1016/j.bbmt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sauty A, Dziejman M, Taha RA, larossi AS, Neote K, Garcia-Zepeda EA, et al. The T cell-specific CXC chemokines IP-10, Mig, and l-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 145.Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFN gamma? PLoS One. 2008;3:e3901. doi: 10.1371/journal.pone.0003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fadel SA, Bromley SK, Medoff BD, Luster AD. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur J Immunol. 2008;38:3376–3387. doi: 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 148.Norose K, Kikumura A, Luster AD, Hunter CA, Harris TH. CXCL10 is required to maintain T cell populations and control parasite replication during chronic ocular toxoplasmosis. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5819. e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Becerra A, Warke RV, Martin K, Xhaja K, de Bosch N, Rothman AL, et al. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo . J Med Virol. 2009;81:1403–1411. doi: 10.1002/jmv.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, Vargas J, et al. Increased expression of T celI chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 153.Askarieh G, Alsio A, Pugnale P, Negro F, Ferrari C, Neumann AL, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 154.Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, et al. The T celI chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 155.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler o, et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carr DJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brainard DM, Tager AM, Misdraji J, Frahm N, Lichterfeld M, Draenert R, et al. Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T-lymphocyte dysfunction. J Virol. 2007;81:8439–8450. doi: 10.1128/JVI.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected mono-cyte-derived macrophages, dendritic cells, and lymph nodes. J Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 159.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 160.Rosas LE, Barbi J, Lu B, Fujiwara Y, Gerard C, Sanders VM, et al. CXCR3−/− mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur J Immunol. 2005;35:515–523. doi: 10.1002/eji.200425422. [DOI] [PubMed] [Google Scholar]
- 161.Hailu A, van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-gamma IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. Am J Trop Med Hyg. 2004;71:561–567. [PubMed] [Google Scholar]
- 162.Gupta G, Bhattacharjee S, Bhattacharyya S, Bhattacharya P, Adhikari A, Mukherjee A, et al. CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J Infect Dis. 2009;200:1300–1310. doi: 10.1086/605895. [DOI] [PubMed] [Google Scholar]
- 163.Olive AJ, Gondek DC, Starnbach MN. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.58. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Belay T, Eko FO, Ananaba GA, Bowers S, Moore T, Lyn D, et al. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect Immun. 2002;70:844–850. doi: 10.1128/IAI.70.2.844-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mullegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 168.Kondo T, Nakazawa H, Ito F, Hashimoto Y, Osaka Y, Futatsuyama K, et al. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci. 2006;97:780–786. doi: 10.1111/j.1349-7006.2006.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, et al. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 170.Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112–3122. doi: 10.4049/jimmunol.164.6.3112. [DOI] [PubMed] [Google Scholar]
- 171.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–632. [PubMed] [Google Scholar]
- 173.Jones D, O’Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, et al. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96:685–690. [PubMed] [Google Scholar]
- 174.Trentin L, Agostini C, Facco M, Piazza F, Perin A, Siviero M, et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest. 1999;104:115–121. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, et al. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo . Proc Natl Acad Sci USA. 1996;93:13791–13796. doi: 10.1073/pnas.93.24.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]