Abstract
Purpose
The biological process of aging encompasses a multitude of complex physiological and lifestyle changes that may alter the way typical prognostic factors affect survival among older ependymoma patients. Because very little is known about the clinical significance of traditional prognostic factors and the magnitude of their effects among older individuals, the purpose of this study was to evaluate the associations between survival and demographic and tumor characteristics among patients with ependymoma who were 60 years of age or older.
Methods
Using the 1973–2007 dataset from the Surveillance, Epidemiology and End Results (SEER) program, we evaluated the impact of several factors on both overall and ependymoma-specific survival, utilizing multivariable Cox proportional hazards regression.
Results
We identified 367 ependymoma cases who were 60 years of age or older at diagnosis and had complete data from SEER. Of these, 19 (5.2%) had anaplastic tumors; all others were low-grade tumors. Age, tumor site, extent of surgery, and tumor histology were found to be significant predictors of ependymoma prognosis. The strongest predictor of poor outcome was supratentoral tumor location (adjusted HR: 6.94, 95% CI: 3.19–15.08, compared to spinal cord tumors).
Conclusion
Our study suggests that tumor location, tumor histology, and surgical margin may be key predictors of survival among older ependymoma patients. We believe our study is one of the first to assess the prognostic value of these factors for ependymoma survival exclusively in an older patient population.
Introduction
Cancer has long been considered a disease of aging and not suprisingly, the only known risk factor common to virtually all types of cancer is increasing (or older) age [1,2]. Several factors likely contribute to the association between age and risk of cancer development, including age-related immune decline and reduction in DNA repair capacity [1,3]. Consequently, well over half of all incident cancers are diagnosed among individuals over 60 years old, who comprised an estimated 18% of the U.S. population as of 2008 [4–6]. Moreover, the proportion of older adults in the U.S. population is expected to continue increasing dramatically over the course of the next two decades [2]. Thus, the majority of cancer survivors are (and will continue to be) older adults, and yet, relatively few studies have attempted to identify predictors of cancer survival specifically among this subpopulation, especially for rare cancers.
One such rare cancer for which there is an incomplete understanding of key prognostic factors is ependymoma. Ependymomas are primary neoplasms of the central nervous system (CNS) that constitute about 3–5% of adult intracranial gliomas [7]. The annual incidence of ependymoma is approximately 2–4 new cases per million, but the incidence varies greatly by age, with a peak around age 55 among adults [8,9]. Previous studies, many of which are based on small study populations that combine pediatric and adult ependymoma cases, have been unsuccessful in establishing the importance of traditional prognostic factors, such as demographic characteristics and tumor histology, on patient survival.[7,8,10] Although some evidence supports the hypothesis that ependymoma mortality risk may be much higher among the youngest and oldest patients [8,11–13], few studies, if any, have endeavored to determine which factors may be driving this increased risk among the oldest subset of patients.
In the context of the plethora of biological processes involved in aging, the impact of traditional prognostic factors and therapies may differ. Age is essentially a surrogate measure that encompasses an extremely wide range of physiological and environmental effects, from changes at the cellular level to issues related to access to healthcare and psychosocial development [14]. Clearly, age-related effects, such as cognitive decline, endocrine changes, decreasing renal and immune function, changes in socioeconomic status, and a higher overall risk for additional comorbidities (e.g. diabetes, heart disease, etc.), may work in conjunction with typical prognostic factors to modify the probability of patient survival [14,6]. For example, some previous research indicates that complications that may be induced by certain chemotherapies (such as cardiomyopathy and acute leukemia) may be more common among older cancer patients [6,15,16]. Additionally, whether radiation therapy is significantly beneficial in treating adult ependymoma patients remains ambiguous, with studies providing evidence both for and against its use [8,11]. Thus, the potential prognostic benefits of different treatment modalities, as well as the influence of other predictive factors, need to be carefully evaluated in the milieu of older age.
Because very little is known about the clinical significance of typical prognostic factors and the magnitude of their effects among older patients with ependymoma, the purpose of this study was to evaluate the associations between clinical and patient attributes and both overall and cause-specific survival among ependymoma cases at least 60 years of age at diagnosis.
Methods
Study Population: SEER Data
We acquired the full 1973–2007 dataset from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (released in April 2010), which contains data from the following registries (with the year data collection was initiated): Arizona (1973), Connecticut (1973), Detroit (1973), Hawaii (1973), Iowa (1973), New Mexico (1973), San Francisco-Oakland (1973), Utah (1973), Seattle-Puget Sound (1974), Atlanta (1975), Rural Georgia (1978), Los Angeles (1992), San Jose-Monterey (1992), Alaska (1999), Greater California (2000), Kentucky (2000), Louisiana (2000), and New Jersey (2000). From this dataset, we isolated the low-grade (ICD-O-3 code 9391) and anaplastic ependymomas (ICD-O-3 code 9392), and obtained data on tumor location, tumor histology, month/year of diagnosis, age at diagnosis, race/ethnicity, sex, therapy, vital status, cause of death, and overall survival time. We combined available treatment information from the five different variables in the SEER dataset (containing information on surgery, extent of surgery, surgery site, radiation therapy, and sequence of surgery/radiation) into composite variables. Cases with zero or missing survival time were excluded from the analyses (n=26 among those ≥60 years of age).
Statistical Analyses
Previous studies have implied that there is a U-shaped age distribution of worsened prognosis among ependymoma patients. Initially using SEER data on ependymoma cases of all ages, we sought to determine the age at which risk increases in older adults by modeling the effect of each additional five years of age on mortality hazard, with the youngest age group (<5 years) as the reference group. We determined the age cut point (≥60 years) based on this analysis and restricted the study population to this age group for all subsequent analyses.
The distributions of population attributes were examined both overall and by tumor histology, and differences were assessed using χ2 tests. Survival probability over time was visualized with Kaplan-Meier survival curves, and the log-rank test was utilized to evaluate whether survival probability differed by characteristics of interest (α=0.05). Using Cox proportional hazards regression, a multivariable predictive model was built with a backwards stepwise selection strategy. We considered sex, race/ethnicity, age at diagnosis, tumor location, extent of surgery, radiation therapy, and tumor histology for inclusion in the model. Both overall and cause-specific survival were evaluated. For the cause-specific survival analyses, deaths unrelated to ependymoma, as determined by the SEER cause-specific death classification variable, were considered censored. All statistical analyses were conducted in STATA version 10.0 (StataCorp, College Station, TX).
Results
A total of 2802 ependymoma cases were isolated from the full 1973–2007 SEER dataset. To determine the age at which mortality risk increases in adult ependymoma patients, we modeled the effect of each additional five years of age at diagnosis on mortality hazard using Cox proportional hazards regression, and found that ages (at diagnosis) of <60 years were protective against mortality risk, compared to being diagnosed before 5 years of age. Being at least 60 years of age conferred over four times the mortality risk of being between the ages of 18 and 59, adjusting for other relevant factors (p<0.001). As a result, we restricted the rest of our analyses to the 367 ependymoma cases who were ≥60 years of age at diagnosis.
The distributions of various population and tumor attributes, both overall and by tumor histology, are provided in Table 1. Overall, the majority of cases were non-Hispanic white and more likely to be male. There were only 19 (5.2%) anaplastic ependymoma cases. While most low-grade ependymomas were mostly located in the spinal cord, the supratentorial region of the brain was the most common site for anaplastic tumors. Individuals with anaplastic tumors were significantly less likely to undergo complete surgical resection and more likely to receive radiation therapy, compared to patients with low-grade tumors (p=0.01 and p=0.03, respectively).
Table 1.
Population characteristics of adult ependymoma cases age ≥60 years from the SEER dataset 1973–2007, both overall and by tumor histology
| Characteristic | Overall (n=367) n (%) |
Ependymoma (n=348) n (%) |
Anaplastic (n=19) n (%) |
p-value |
|---|---|---|---|---|
| Age | 0.98 | |||
| 60 to 68 (median split) | 194 (52.9) | 184 (52.9) | 10 (52.6) | |
| >68 | 173 (47.1) | 164 (47.1) | 9 (47.4) | |
| Sex | 0.89 | |||
| Male | 199 (54.2) | 189 (54.3) | 10 (52.6) | |
| Female | 168 (45.8) | 159 (45.7) | 9 (47.4) | |
| Race | 0.87 | |||
| Non-Hispanic White | 311 (84.7) | 296 (85.1) | ||
| Black | 12 (3.3) | 11 (3.2) | 15 (78.9) | |
| Hispanic | 25 (6.8) | 23 (6.6) | ||
| Asian and other | 19 (5.2) | 18 (5.2) | <5 (<26.3)a | |
| Tumor Location | <.001* | |||
| Spinal Cord | 193 (52.6) | 190 (54.6) | ||
| Infratentorial | 73 (19.9) | 72 (20.7) | <5 (<26.3)a | |
| Supratentorial | 29 (7.9) | 21 (6.0) | 8 (42.1) | |
| Other/NOS sites | 72 (19.6) | 65 (18.7) | 7 (36.8) | |
| Extent of Surgeryb | 0.01* | |||
| Debulking (Gross total or radical) | 118 (32.2) | 116 (33.3) | <5 (<26.3)a | |
| Less than gross total resection | 195 (53.1) | 178 (51.2) | 17 (89.5) | |
| Radiation Therapyb | 0.03* | |||
| Yes | 132 (36.0) | 121 (34.8) | 11 (57.9) | |
| No | 226 (61.6) | 219 (62.9) | 7 (36.8) | |
Due to the SEER user agreement, we cannot disclose the exact number of cases when <5, in order to protect patient privacy.
Numbers do not add up to the column totals due to missing values.
Statistically significant difference.
Approximately half of the 189 total recorded deaths were ependymoma-related, according to SEER cause-specific death classifications. Kaplan-Meier survival curves provided a visualization of the crude relationships between ependymoma-specific survival over time and each of three prognostic factors: tumor site, tumor histology, and surgical margin (Fig. 1–3, respectively). Fig. 1 clearly illustrates that individuals with supratentorial tumors had the worst ependymoma-related survival over time, whereas those with spinal cord tumors had the best prognosis over time (logrank p<0.001). Additionally, patients with anaplastic tumors had worse survival over time than those with low-grade tumors (logrank p<0.001, Fig. 2), and individuals who underwent complete surgical resection had significantly better survival than those who had less than gross total resection (logrank p=0.001, Fig. 3).
Fig. 1.
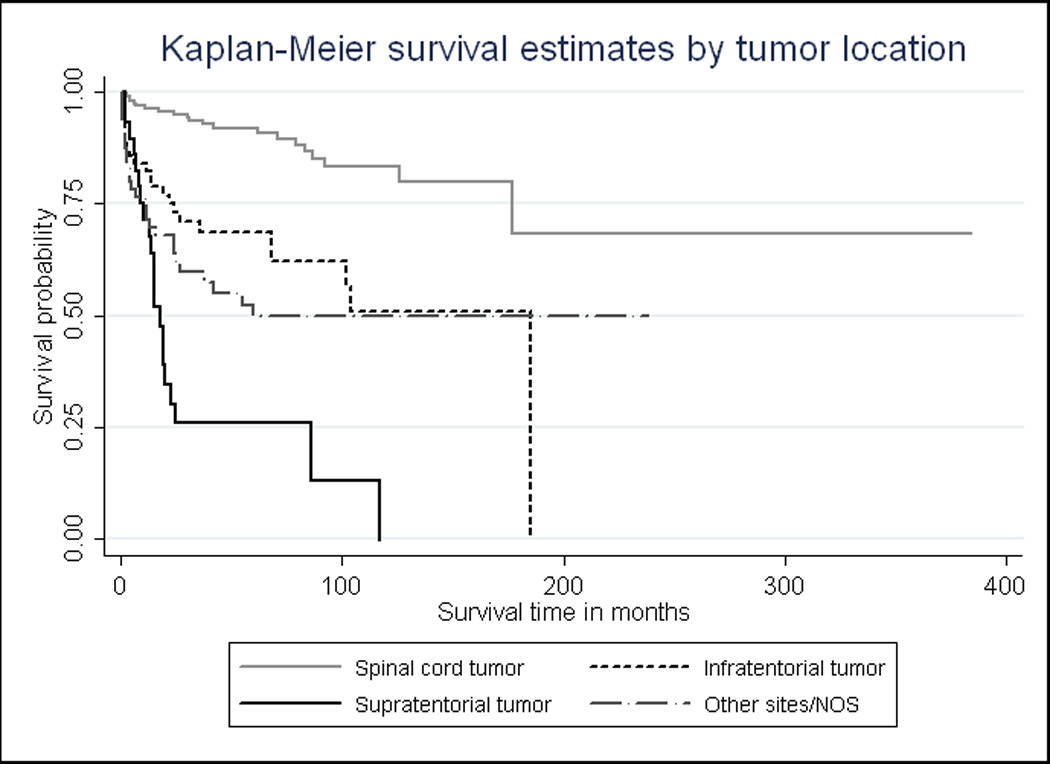
Cause-specific Kaplan-Meier survival curves by ependymoma site. Among ependymoma patients over the age of 60, those with spinal cord tumors have consistently higher survival probabilities over time, compared to those who have tumors in the supratentorial region (p<0.001)
Fig. 3.
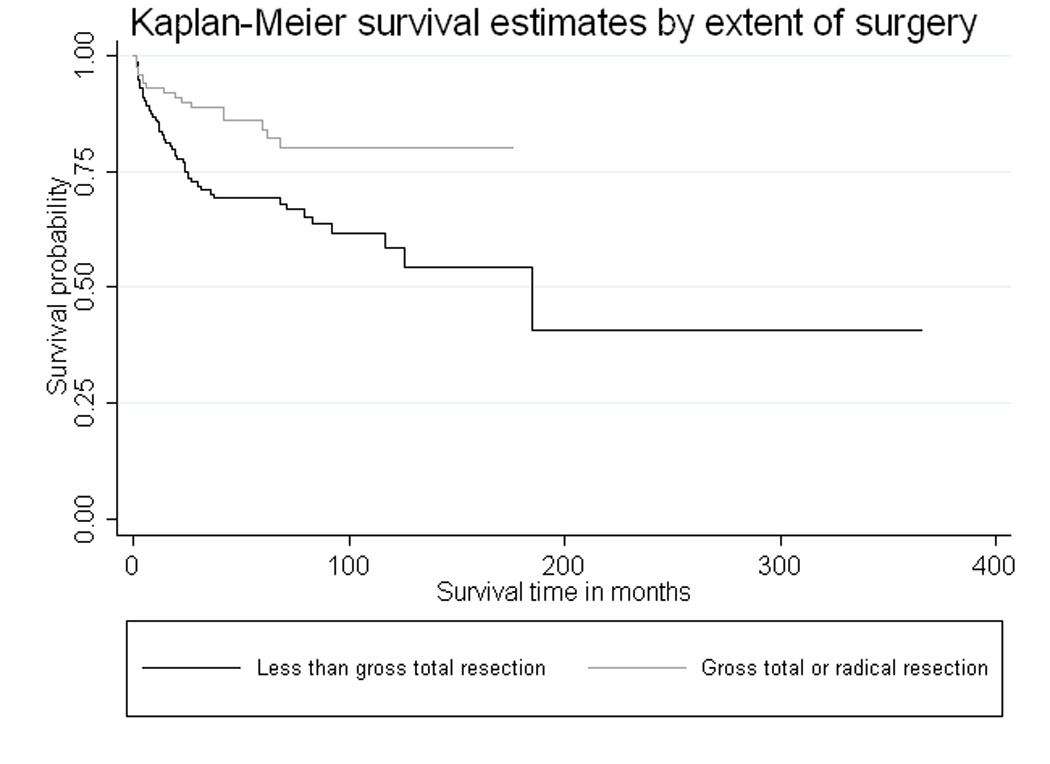
Cause-specific Kaplan-Meier survival curves by surgical margin. Patients over the age of 60 who undergo gross total (or radical) resection have better ependymoma survival over time than those who undergo incomplete resections (p=0.001)
Fig. 2.
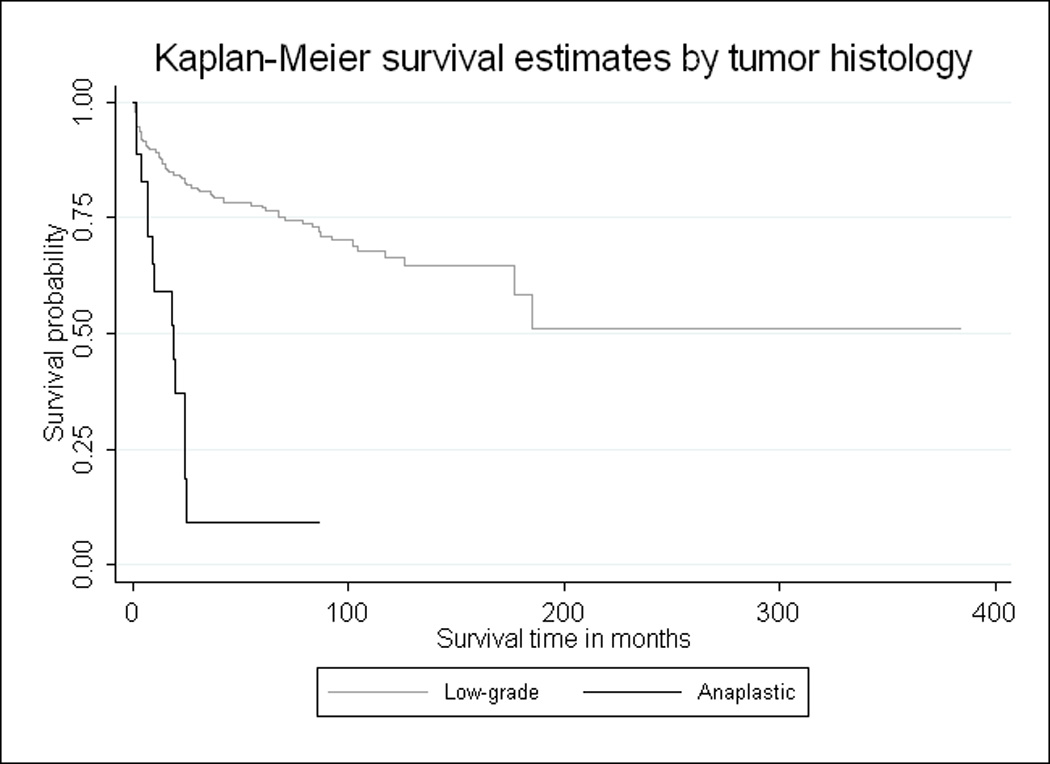
Cause-specific Kaplan-Meier survival curves by ependymoma histology. Patients over the age of 60 with low-grade tumors have consistently better ependymoma survival over time compared to those with anaplastic tumors (p<0.001)
Full and final models for both overall and cause-specific survival are provided in Tables 2 and 3, respectively. Sex, race, tumor histology, and radiation therapy were not significantly associated with overall survival in the fully-adjusted model (Table 2). Hazard ratios (HR) of significant variables (age, tumor location, and extent of surgical resection) in the final model were similar to those in the fully-adjusted model, indicating that the impact of these factors is relatively independent from that of the excluded variables. The strongest predictor of overall mortality risk, regardless of adjustment for tumor histology and other factors, was supratentorial tumor location.
Table 2.
Fully-adjusted and final predictive models for all-cause mortality among patients ≥60 years of age
| Predictor | Fully-Adjusted Model |
Final Predictive Model |
|---|---|---|
| Age | ||
| 60 to 68 (median) | Ref | Ref |
| >68 | 2.10 (1.46–3.04) | 2.15 (1.51–3.05) |
| Sex | ||
| Female | Ref | |
| Male | 1.36 (0.96–1.94) | |
| Race | ||
| Non-Hispanic White | Ref | |
| Black | 1.34 (0.67–2.69) | |
| Hispanic | 0.68 (0.33–1.41) | |
| Asian and other | 1.11 (0.52–2.36) | |
| Tumor Location | ||
| Spinal Cord | Ref | Ref |
| Infratentorial | 2.13 (1.32–3.42) | 2.50 (1.61–3.88) |
| Supratentorial | 4.72 (2.51–8.87) | 4.85 (2.86–8.23) |
| Other/NOS* | 3.29 (2.11–5.15) | 3.46 (2.27–5.27) |
| Extent of Surgery | ||
| Less than Gross Total | Ref | Ref |
| Debulking (Gross total or radical) | 0.54 (0.36–0.81) | 0.54 (0.37–0.79) |
| Histology | ||
| Low-grade Ependymoma | Ref | |
| Anaplastic Ependymoma | 1.61 (0.83–3.13) | |
| Radiation Therapy | ||
| No | Ref | |
| Yes | 0.83 (0.58–1.19) | |
Includes C715 (ventricle NOS), C718 (brain: overlap), and C719 (brain NOS).
Table 3.
Full and final models for ependymoma-related mortality among patients ≥60 years of age
| Predictor | Fully-Adjusted Model |
Final Predictive Model |
|---|---|---|
| Age | ||
| 60 to 68 (median) | Ref | Ref |
| >68 | 1.83 (1.09–3.01) | 1.75 (1.08–2.84) |
| Sex | ||
| Female | Ref | |
| Male | 1.06 (0.65–1.73) | |
| Race | ||
| Non-Hispanic White | Ref | |
| Black | 0.96 (0.29–3.13) | |
| Hispanic | 1.18 (0.51–2.75) | |
| Asian and other | 1.22 (0.42–3.50) | |
| Tumor Location | ||
| Spinal Cord | Ref | Ref |
| Infratentorial | 3.13 (1.55–6.35) | 3.70 (1.91–2.84) |
| Supratentorial | 6.46 (2.88–14.50) | 6.94 (3.19–15.08) |
| Other/NOSa | 4.46 (2.31–8.63) | 4.77 (2.52–9.03) |
| Extent of Surgery | ||
| Less than Gross Total | Ref | Ref |
| Debulking (Gross total or radical) | 0.58 (0.32–1.05) | 0.57 (0.33–1.01)b |
| Histology | ||
| Low-grade Ependymoma | Ref | |
| Anaplastic Ependymoma | 2.21 (1.04–4.66) | 2.38 (1.19–4.75) |
| Radiation Therapy | ||
| No | Ref | |
| Yes | 1.23 (0.75–2.01) | |
Includes C715 (ventricle NOS), C718 (brain: overlap), and C719 (brain NOS).
Of borderline significance (p=0.052).
The results from the cause-specific regression models are relatively similar to those from the overall survival models, with a few exceptions. While tumor histology was not a significant predictor of overall mortality hazard, having an anaplastic tumor is significantly associated with ependymoma-specific mortality hazard (HR: 2.38, 95% CI: 1.19–2.75). Another difference between the overall and cause-specific models is the magnitude of the point estimates with age and tumor location (although the 95% confidence intervals are largely overlapping). Compared to having a spinal cord tumor, having an ependymal tumor located in any of the other three sites (infratentorial, supratentorial, or other) is more strongly associated with cause-specific than overall mortality risk. However, the strongest predictor, supratentorial tumor location, is the same between both analyses.
Discussion
In this study, we examined the associations between various potential prognosticators and both overall and ependymoma-related mortality risk among a population of patients at least 60 years of age at diagnosis, using data from the SEER program. Our results suggest that age, tumor location, surgical margin, and tumor histology may be important predictors of survival among older ependymoma patients. While these attributes are established prognostic factors for many other types of cancer, their role in ependymoma prognosis continues to be controversial [8,7,10] and has not previously, to our knowledge, been evaluated exclusively among a subset of older patients.
In our study, supratentorial tumor location was the strongest predictor of both overall and ependymoma-specific survival among patients ≥60 years of age. Most studies agree that spinal cord tumors confer better long-term prognoses compared to intracranial ependymomas in general, but the impact of having a tumor in the supratentorial region, rather than the infratentorial region, of the brain is less clear [11,17,8,18,19]. Some have argued that the association between supratentorial tumors and worse survival is biologically plausible because supratentorial tumors usually have higher mitotic activity than infratentorial tumors and tend to be more difficult to completely resect [20,11,8]. Although we adjusted for extent of surgery in our models, we cannot discount the possibility that the increased mortality hazard associated with supratentorial tumors may actually be due to the fact that only about 7% (n<5) of the patients with supratentorial tumors actually underwent complete surgical resection. By contrast, approximately 27% of patients with infratentorial tumors and 39% of patients with spinal cord tumors underwent gross total (or radical) resection. Therefore, the observed association between supratentorial tumor location and mortality risk may be somewhat inflated due to residual confounding by extent of surgical resection, although the low rate of surgical resection in patients with supratentorial tumors may also reflect a difference in tumor biology.
We also found that complete surgical resection was protective against overall and ependymoma-specific mortality hazard. Patients with gross total (or radical) resections had significantly better ependymoma survival over time compared to those with incomplete resections (Fig. 3), and adjustment for tumor location, age, and tumor histology did not attenuate the magnitude of the association between surgical margin and prognosis (Table 3). Previous studies have also indicated that gross total resection may be beneficial for ependymoma patients, both among adult and pediatric case groups [17,13,21,22]. Unfortunately, it has been shown that older patients are less likely to pursue (or receive) aggressive cancer therapies [5,23], and yet, our analyses imply that attempts at complete surgical resection should not be dismissed without consideration.
Because older individuals generally have a higher all-cause mortality risk (compared to younger adults), examining predictors of cause-specific survival is especially important among older ependymoma patients. Interestingly, the final overall and ependymoma-specific models proved to be relatively similar, with the exception that tumor histology was a significant predictor of cause-specific, but not overall, mortality hazard. The cause-specific Kaplan-Meier curves illustrate the dramatic difference in ependymoma survival over time by tumor histology. However, this finding should be validated in a larger study population, because there were few anaplastic cases in this analysis and previous studies on the prognostic value of tumor histology have been fairly inconsistent [8].
Similarly, the prognostic significance of radiation therapy has been a divisive topic in the previous literature [11,8,24]. Here we did not find radiation therapy to be significantly associated with overall or ependymoma-specific survival. It is possible that we did not detect a significant association due to inadequate statistical power. Regardless, extent of field and radiation dose information are not provided in SEER, which further restricts our ability to ascertain the impact of radiation therapy, especially given that the optimal dose has not yet been established [11]. Therefore, clinical trials on this topic are warranted.
Despite the use of the most robust tumor registry, the analyses presented here were limited by a small sample size, which is not unexpected given that ependymoma is a rare disease. In order to further clarify the prognostic significance of tumor site, larger future studies should attempt to evaluate the effect of supratentorial tumor location among a group of patients who have all undergone complete surgical resections. Additionally, future studies could also attempt to clarify the impact of chemotherapy among older ependymoma cases. Unfortunately, we were unable to examine this treatment factor in the current study, as information on chemotherapy use and response is not available through SEER. Furthermore, future research on older patients with ependymoma should incorporate data on common age-related comorbidities, such as diabetes and heart disease, into the model and could use such information to better assess the impact of competing risks on survival.
In the context of older age, the impact of prognostic factors could differ considerably from what is expected. Without examining the influence of these factors specifically among older patients, we would be unable to determine whether it is appropriate to generalize inferences made from studies conducted mostly among younger adults (or among all ages) to older patient populations. This is particularly problematic for studies of rare brain tumors, such as ependymomas, which often analyze adult and pediatric cases together in an effort to boost statistical power. While some studies have previously implied that tumor location, surgical margin, and tumor histology may be important prognostic determinants among ependymoma patients, others have not found these variables to be associated with ependymoma survival [7,8,10]. Even among the studies that have found significant associations with these factors, the magnitudes of the effect estimates, and consequently the purported importance of these variables as prognosticators, vary widely. Perhaps one reason why there is so much discordance in the literature about which factors are actually associated with ependymoma prognosis may be that pediatric, adult, and geriatric patient populations are truly heterogenous with regard to what predicts ependymoma survival over time. If so, studies that combine cases of all ages into a single analysis would be unable to provide a clear picture of the prognostic significance of such factors. Thus, by being one of the first studies to evaluate predictors of ependymoma survival specifically in an older population, our study has provided a crucial first step toward potentially uncovering the enigmatic set of factors that determine patient prognosis.
Acknowledgements
We acknowledge the Collaborative Ependymoma Research Network for their support. This work was partially funded by a grant from the National Cancer Institute (K07CA131505 to M.E.S.).
Footnotes
Disclosure: M.R. Gilbert served on advisory boards for Genentech and Schering-Plough, from which he has also received research support. No other conflicts of interest relevant to these analyses were declared.
Contributor Information
E. Susan Amirian, Dan L Duncan Cancer Center, Baylor College of Medicine, Houston, Texas.
Terri S. Armstrong, Department of Integrative Nursing Care, UTHSC-SON, Houston, Texas
Mark R. Gilbert, Department of Neuro-Oncology, UT-MD Anderson Cancer Center, Houston, Texas
Michael E. Scheurer, Dan L Duncan Cancer Center, Baylor College of Medicine, Houston, Texas; Department of Pediatrics, Baylor College of Medicine, Houston, Texas.
References
- 1.Miller RA. Gerontology as oncology. Research on aging as the key to the understanding of cancer. Cancer. 1991;68(11 Suppl):2496–2501. doi: 10.1002/1097-0142(19911201)68:11+<2496::aid-cncr2820681503>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Rowland JH, Yancik R. Cancer survivorship: the interface of aging, comorbidity, and quality care. J Natl Cancer Inst. 2006;98(8):504–505. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- 4.Bureau USC General Demographic Characteristics. [Accessed January 25, 2011];Population Estimates. 2008 http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml.
- 5.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113(12 Suppl):3519–3529. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
- 6.Bellizzi KM, Mustian KM, Palesh OG, Diefenbach M. Cancer survivorship and aging : moving the science forward. Cancer. 2008;113(12 Suppl):3530–3539. doi: 10.1002/cncr.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010;10(3):240–247. doi: 10.1007/s11910-010-0109-3. [DOI] [PubMed] [Google Scholar]
- 8.Reni M, Gatta G, Mazza E, Vecht C. Ependymoma. Crit Rev Oncol Hematol. 2007;63(1):81–89. doi: 10.1016/j.critrevonc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg. 2009;110(4):725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: prognosis and the M. D. Anderson Cancer Center experience. Neuro Oncol. 2010;12(8):862–870. doi: 10.1093/neuonc/noq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reni M, Brandes AA, Vavassori V, Cavallo G, Casagrande F, Vastola F, Magli A, Franzin A, Basso U, Villa E. A multicenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer. 2004;100(6):1221–1229. doi: 10.1002/cncr.20074. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005) J Surg Res. 2009;156(2):340–351. doi: 10.1016/j.jss.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metellus P, Figarella-Branger D, Guyotat J, Barrie M, Giorgi R, Jouvet A, Chinot O. Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113(1):175–185. doi: 10.1002/cncr.23530. [DOI] [PubMed] [Google Scholar]
- 14.Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434–440. [PMC free article] [PubMed] [Google Scholar]
- 15.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, Wood WC, Henderson IC, Hudis C, Winer E, Cohen H, Wheeler J, Norton L. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 16.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 17.Metellus P, Guyotat J, Chinot O, Durand A, Barrie M, Giorgi R, Jouvet A, Figarella-Branger D. Adult intracranial WHO grade II ependymomas: long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol. 2010;12(9):976–984. doi: 10.1093/neuonc/noq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw EG, Evans RG, Scheithauer BW, Ilstrup DM, Earle JD. Postoperative radiotherapy of intracranial ependymoma in pediatric and adult patients. Int J Radiat Oncol Biol Phys. 1987;13(10):1457–1462. doi: 10.1016/0360-3016(87)90310-5. [DOI] [PubMed] [Google Scholar]
- 19.Kovalic JJ, Flaris N, Grigsby PW, Pirkowski M, Simpson JR, Roth KA. Intracranial ependymoma long term outcome, patterns of failure. J Neurooncol. 1993;15(2):125–131. doi: 10.1007/BF01053933. [DOI] [PubMed] [Google Scholar]
- 20.Goldwein JW, Leahy JM, Packer RJ, Sutton LN, Curran WJ, Rorke LB, Schut L, Littman PS, D'Angio GJ. Intracranial ependymomas in children. Int J Radiat Oncol Biol Phys. 1990;19(6):1497–1502. doi: 10.1016/0360-3016(90)90362-n. [DOI] [PubMed] [Google Scholar]
- 21.Metellus P, Barrie M, Figarella-Branger D, Chinot O, Giorgi R, Gouvernet J, Jouvet A, Guyotat J. Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130(Pt 5):1338–1349. doi: 10.1093/brain/awm046. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau P, Habrand JL, Sarrazin D, Kalifa C, Terrier-Lacombe MJ, Rekacewicz C, Rey A. Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys. 1994;28(2):381–386. doi: 10.1016/0360-3016(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 23.Crivellari D, Aapro M, Leonard R, von Minckwitz G, Brain E, Goldhirsch A, Veronesi A, Muss H. Breast cancer in the elderly. J Clin Oncol. 2007;25(14):1882–1890. doi: 10.1200/JCO.2006.10.2079. [DOI] [PubMed] [Google Scholar]
- 24.Vitanovics D, Balint K, Hanzely Z, Banczerowski P, Afra D. Ependymoma in adults: surgery, reoperation and radiotherapy for survival. Pathol Oncol Res. 2010;16(1):93–99. doi: 10.1007/s12253-009-9194-5. [DOI] [PubMed] [Google Scholar]


