Abstract
Ionizing radiation, like a variety of other cellular stress factors, can activate or down-regulate multiple signaling pathways, leading to either increased cell death or increased cell proliferation. Modulation of the signaling process, however, depends on the cell type, radiation dose, and culture conditions. The mitogen-activated protein kinase (MAPK) pathway transduces signals from the cell membrane to the nucleus in response to a variety of different stimuli and participates in various intracellular signaling pathways that control a wide spectrum of cellular processes, including growth, differentiation, and stress responses, and is known to have a key role in cancer progression. Multiple signal transduction pathways stimulated by ionizing radiation are mediated by the MAPK superfamily including the extracellular signal–regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. The ERK pathway, activated by mitogenic stimuli such as growth factors, cytokines, and phorbol esters, plays a major role in regulating cell growth, survival, and differentiation. In contrast, JNK and p38 MAPK are weakly activated by growth factors but respond strongly to stress signals including tumor necrosis factor (TNF), interleukin-1, ionizing and ultraviolet radiation, hyperosmotic stress, and chemotherapeutic drugs. Activation of JNK and p38 MAPK by stress stimuli is strongly associated with apoptotic cell death. MAPK signaling is also known to potentially influence tumor cell radiosensitivity because of their activity associated with radiation-induced DNA damage response. This review will discuss the MAPK signaling pathways and their roles in cellular radiation responses.
Keywords: MAP kinases, radiation, signaling pathways
Introduction
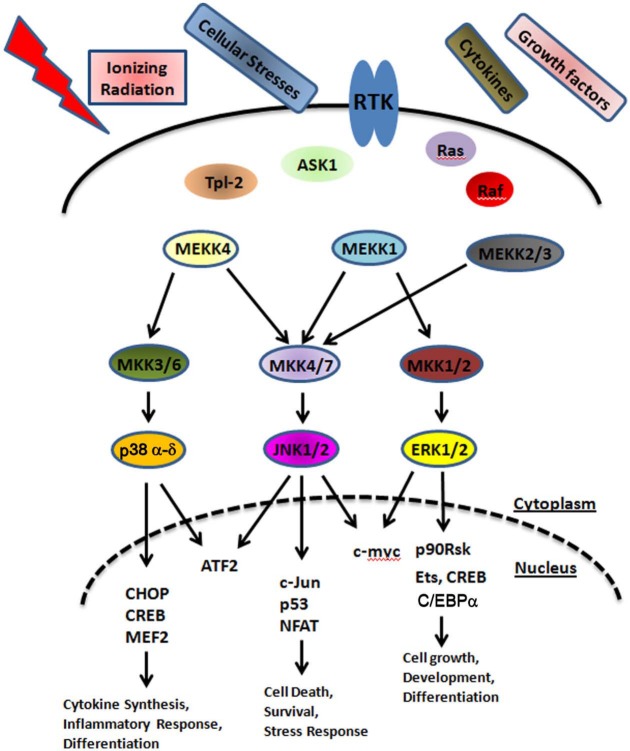
The mitogen-activated protein kinase (MAPK) signaling pathway plays an important role in regulating gene expression in eukaryotic cells and links extracellular signals to the machinery that controls fundamental cellular processes such as growth, proliferation, differentiation, migration, and apoptosis.1,2 Three major groups of distinctly regulated MAPK cascades are known in humans that lead to altered gene expression: extracellular signal–regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK (Fig. 1). MAPKs are components of signaling pathways that feature a core triple kinase cascade.3 The kinase nearest to the signal source is referred as a MAP kinase kinase kinase (MAPKKK, MEKK), which is activated by an upstream signaling protein (e.g., small GTPases) and phosphorylates a MAP kinase kinase (MAPKK, MEK). The activated MAPKK then phosphorylates the third layer of the cascade, MAPK.4,5 ERKs are activated by MAP kinase kinase (MKK) and MKK2, JNK by MKK4 and MKK7, and p38 MAP kinase by MKK3, MKK4, and MKK6.6,7 Upon activation of the MAP kinases, transcription factors present in the cytoplasm or nucleus are phosphorylated and activated, leading to the expression of target genes and resulting in a biological response (Fig. 1). In addition, MAPKs can phosphorylate and activate downstream protein kinases. MAP kinases also have overlapping substrate specificities, and phosphorylation of regulatory sites is shared among multiple protein kinases, indicating that the transcriptional regulation in response to MAPK signaling is extremely complex.8 These multiple interactions between the different MAP kinase cascades serve to integrate the responses and activate a separate set of genes.6 Changes in MAPK regulation resulting from mutations or changes in the expression of proteins regulating MAPK signaling, such as epidermal growth factor receptor (EGFR) overexpression or activating mutations of Ras or Raf, can contribute to inflammation, cancer, and other diseases as well.
Figure 1.
Mammalian MAPK modules: simplified diagram depicting the MAPK signaling network.
MAPKs are activated by a variety of stimuli including osmotic shock, exposure to ultraviolet (UV) irradiation, cytokines, growth factors, antigens, toxins, and pharmacological drugs.5,9,10 Studies have also shown that the exposure of tumor cells to ionizing radiation results in the activation or down-regulation of multiple signaling pathways, which are mediated by the MAPK superfamily and which play critical roles in cell type–specific control of survival or death in response to ionizing radiation.9-12 Ionizing radiation has been shown to activate all 3 MAPKs, although with different intensities and in a cell type–dependent context.11,13 These intracellular signaling molecules play an important role in determining the intrinsic radiosensitivity of tumor cells. Although it has been shown for many years that radiation causes cell death, there is now evidence that ionizing radiation can enhance the proliferation of surviving tumor cells and promote long-term resistance to multiple cytotoxic stresses. In this review, we will describe the role of the MAPK pathways in response to ionizing radiation and complexities associated with their activation in cancer.
The JNK Pathway
JNK1 and JNK2 were described to be stress-induced protein kinases that phosphorylated the NH2-terminus of the transcription factor c-Jun; hence, the pathway is often called the stress- activated protein kinase (SAPK) pathway. Multiple stresses strongly activate JNK activity including cytokines, UV and γ-irradiation, cytotoxic drugs, DNA-damaging agents, and reactive oxygen species.14 The diversity of upstream activators of MKK4 and MKK7 (upstream activators for the JNK pathway) allows JNK pathway activation by a large number of external stimuli. JNK activation may thereby influence important cellular consequences, such as alterations in gene expression, cell death, or altered cellular proliferation.15 Ionizing radiation activates the JNK pathway by very distinct mechanisms.16
Studies have demonstrated that radiation treatment resulted in the generation of ceramide and clustering of death receptors on the plasma membrane of cells that played an important role in JNK activation.17 This molecular mechanism of JNK activation and signaling following irradiation was linked to a proapoptotic role for JNK signaling. The kinetics and magnitude of SAPK/JNK activation also demonstrate a close correlation with the induction of apoptotic nuclear changes. Cells that are sensitive to the effects of radiation display robust SAPK/JNK activation, whereas resistant cells fail to activate SAPK/JNK.16 Studies have demonstrated that programmed cell death occurring after irradiation is associated with selective c-Jun expression.18,19 It has also been reported that the exposure of bovine aortic endothelial cells and human tumor cells to low-dose ionizing radiation induces apoptosis in a time- and dose-dependent fashion.20,21 In these studies, the authors observed SAPK/JNK activation following exposure to radiation along with morphological features of apoptosis. A critical role for the SAPK/JNK signaling cascade has been assessed in radiation-induced apoptosis using dominant- negative mutants of c-Jun.22 Radiation- induced apoptosis was significantly inhibited in cells overexpressing a dominant-negative c-Jun mutant compared to wild-type and vector- transfected cells,20,21 suggesting that radiation-induced apoptosis in human tumor cells requires a functional SAPK/JNK pathway.
Additional evidence that JNK can function as a proapoptotic kinase in response to a variety of different stimuli, including tumor necrosis factor (TNF), UV irradiation, cytokines, ceramide, and chemotherapeutic drugs, has been documented.14 These studies showed that the JNK pathway activates caspases and regulates proteins implicated in apoptosis regulation, including p53, Bcl-2, and Bax. Furthermore, JNK influences the mitochondrial death pathway because its inhibition leads to a failure in cytochrome c and apoptosis-inducing factor release and subsequent caspase activation triggered by ionizing radiation. This could be attributed to JNK-mediated Bax and Bak activation and Bcl-2 down-regulation in response to ionizing radiation.23-26 Other studies in the literature have argued that radiation-induced JNK activation was dependent on the ATM and c-Abl proteins.27,28 Activation of JNK by low-dose radiation in these studies has been shown to be biphasic in cancer cells. The first wave of JNK activation was dependent on the activation of the TNF-α receptor, whereas the second wave was dependent on EGFR and TGF-α. It has also been suggested that radiation-induced JNK activation could be a secondary event to the activation of effector procaspases; cleavage of the upstream activator MEKK1 can lead to constitutive activation of this enzyme and the downstream JNK pathway, which in some cell types plays a key role in the commitment to apoptotic cell death.
A number of studies have implicated the JNK signaling pathway acting via c-Jun or the related transcription factor ATF-2 in the modulation of DNA repair and/or cell survival in response to various forms of genotoxic damage.29-31 Consistent with these observations, MacLaren et al.32 found that mutant forms of c-Jun, which are deficient for DNA binding or transactivation or which are nonresponsive to JNK regulation, are unable to restore the normal regulation of basal or induced DNA damage in c-Jun–/– mouse embryo fibroblasts. c-Jun is involved in regulating the activity of many individual gene promoters through activator protein–1 (AP-1) response elements, and although there is limited evidence that some of these might influence DNA repair,31,33,34 a direct link between specific c-Jun target genes and repair processes remains to be established. Studies also point to a role for ATM in regulating JNK signaling, and keeping with this notion, it has been shown that c-Jun co-localizes with ATM and γ-H2AX in nuclear foci.32 Ataxia telangiectasia cells have deregulated JNK/c-Jun signaling, and that can phosphorylate ATF-2, which forms the AP-1 transcription factor together with c-Jun.29,32,35,36 c-Jun also interacts physically or functionally with 2 proteins involved in DNA repair: redox factor 1 (also known as APEX), an endonuclease specific for abasic sites,37 and thymine DNA glycosylase,38 both of which are involved in base excision repair, which is responsible for the repair of modified lesions on DNA, including those produced by oxidative damage.39
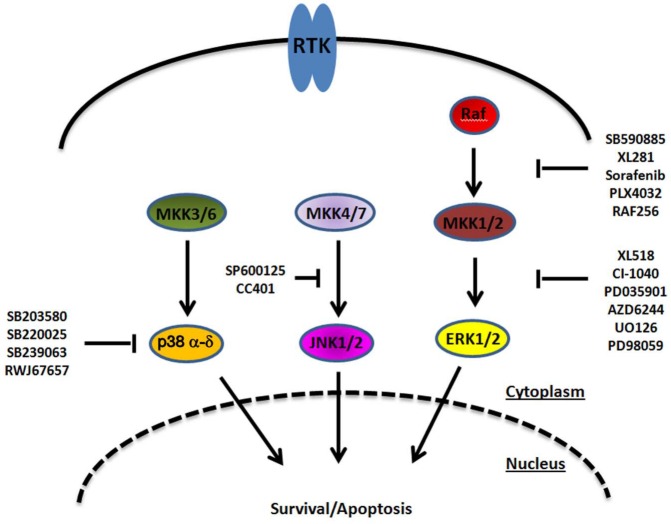
Studies have also analyzed the role of JNK in response to radiation following the use of specific JNK inhibitors. JNK inhibition was found to significantly reduce radiation sensitivity in human cancer cells, as evidenced by increased cell survival and reduced apoptotic cell death.23,40 Only a handful of JNK inhibitors have been developed and are being considered for the treatment of cancer, as well as inflammatory, vascular, neurodegenerative, and metabolic disorders.41 Preclinical observations made with a JNK inhibitor (SP600125) are likely to be less informative regarding the biological roles of JNK due to the considerable off-target activity of this compound.42 One JNK inhibitor, CC-401, is however in phase II evaluations in acute myelogenous leukemia and has also been considered for the treatment of respiratory diseases (Fig. 2). The outcomes of these studies are yet to be known.
Figure 2.
MAPK signal transduction and its pharmacological modulation at different levels.
The ERK1/2 Pathway
The ERK1/2 pathway plays an important role in cell proliferation and transduces signals that promote growth and differentiation. A growing body of evidence indicates that activation of the ERK pathway is involved in the pathogenesis, progression, and oncogenic behavior of human cancer including non–small cell lung cancer (NSCLC), breast cancer, head and neck squamous cell carcinoma, and colorectal cancer.43-46 ERK is activated very rapidly in tumor cells in response to radiation, hypoxia, and chemical signals by the ERK kinase (MEK), with Ras/Raf being its upstream activators.47-49 Mutations occurring in the Ras/Raf pathway further result in enhanced tumor cell proliferation and enhanced survival after irradiation.50-52 Furthermore, inhibition of Ras and Raf in cell lines with activating Ras mutations results in sensitization to ionizing radiation. Additionally, radiation-induced MAPK pathway activation has been shown to regulate the production of vascular endothelial growth factor–A,53 which promotes tumor angiogenesis and neovascular survival,54 and is implicated in endothelial radiosensitization, considered by some to be one of the primary determinants of tumor radiosensitivity.55,56 These observations suggest that signaling through the Ras-Raf-MEK-ERK pathway is important in radiation response and radiation resistance,50-52 and inhibition of this cascade may be an attractive means to sensitize tumor cells to ionizing radiation.57-59
Recently, there has been an explosion of interest in the components of MAPK pathways as attractive therapeutic targets for the treatment of human diseases including cancer. Inhibitors of the kinase function of Raf and MEK represent the most studied and advanced approaches for blocking ERK signaling, with several inhibitors under evaluation in clinical trials and additional inhibitors in preclinical analyses. MEK1/2, the upstream component of ERK, has been considered to play a central role in the integration of mitogenic signals into the ERK pathway, with ERK1/2 being the only substrates for MEK displaying tight selectivity.60,61 Small molecule inhibitors of MEK have been evaluated for their radiosensitizing potential both in in vitro as well as in vivo studies and provide a means to test the role of the ERK pathway in radiation response. MEK inhibitors as single agents possess radiosensitizing properties in a broad spectrum of human tumors.62-67 Several investigators have demonstrated that radiation results in the time-dependent activation of ERK in vitro and in vivo and that upstream MEK inhibition results in significant radiosensitization in cancer cell lines of various origins. Treatment with MEK inhibitors results in the inhibition of not only constitutively activated ERK1/2 but also radiation-induced ERK activation, along with a robust radiosensitizing effect as observed by clonogenic cell survival assays. A great deal of effort has been directed at understanding the molecular mechanisms involved in mediating these radiosensitizing effects. Some investigators attribute this radiosensitizing effect of MEK/ERK inhibition to an abrogation in the G2 checkpoint and an increase in the number of cells undergoing a mitotic catastrophe.68-70 Activation of the G2 checkpoint is considered protective from radiation-induced cell death. This is likely due to a reduction in phosphorylated Chk1, which is a contributor to the abrogated G2 checkpoint. Prolonged G2 arrest after genotoxic stress allows DNA damage repair before progression through mitosis.71,72 Cells that escape the initial G2 checkpoint delay after irradiation may continue through mitosis with incomplete cytokinesis with cell death or continued progression through the cell cycle73 with eventual death by a mitotic catastrophe.74 Inhibition of Chk1 after exposure to ionizing radiation results in an increased incidence of mitotic catastrophes and an impaired activation of cell cycle checkpoints.75 Other studies suggest that the mechanism of radiosensitization may be related to the inability to promote or repair DNA damage.
Studies carried out on NSCLC and glioma cells provide evidence for a common role of MEK/ERK in modulating the levels of radiation-induced double-strand breaks by stimulating nonhomologous end joining (NHEJ) by activating PARP-1, which has a role in NHEJ.76,77 ERK1/2 signaling has also been shown to be a positive ATM-dependent regulator of homologous recombination repair (HRR), and phosphorylated ATM foci formation in response to ionizing radiation critically depends on MEK/ERK signaling. Inhibition of ERK1/2 signaling reduced HRR levels. Inhibiting the ATM kinase with an ATM-specific inhibitor reduced ERK phosphorylation, suggesting that ATM controls signaling through the ERK pathway.78 A highly specific inhibitor of MEK/ERK signaling has been shown to compromise ATM kinase activity and severely attenuate the phosphorylation and localization of ATM to foci, suggesting that ERK signaling affects repair protein complex formation or stability and/or localization of ATM, which is necessary for efficient HRR. Thus, ATM and ERK signaling could be under the control of a regulatory feedback loop.
Several preclinical studies have been conducted to determine the effectiveness of MEK/ERK inhibitors in combination with radiation. Combined treatment of MEK/ERK inhibitors with radiation was found to impart a significantly greater antitumor activity than either therapy alone both in vitro and in vivo.67,79-82 A small molecule inhibitor of MEK (PD0325901) was evaluated for its radiosensitizing properties in human pancreatic carcinoma.80 Phosphorylated levels of ERK1/2 increased in response to radiation treatment but were blocked upon treatment with PD032590. MEK inhibitor–induced radiosensitization was observed both in vitro and in vivo, as evidenced by an enhanced therapeutic gain and tumor cell death. Similar studies were conducted using U0126, another MEK/ERK inhibitor, in a rhabdomyosarcoma model.81 These investigators observed a down-regulation of phosphorylated ERK, cyclin D1, and DNA-PKcs in vitro and a synergistic inhibitory effect on the growth of tumors upon combined U0126 and radiation therapy. Shannon et al.82 evaluated the potential benefit of combining AZD6244, a potent and selective inhibitor of the MEK/ERK pathway, with fractioned radiotherapy using human colon and lung carcinoma xenograft models. AZD6244 was found to reduce ERK phosphorylation in Calu-6 lung cancer cells in vitro, and in vivo administration of AZD6244 inhibited the tumor growth of Calu-6 xenografts, with regrowth occurring upon cessation of the drug treatment. The combination of fractionated tumor–localized radiotherapy with AZD2264 treatment enhanced tumor growth delay significantly when compared with either modality alone, and this effect was also observed in a colon model. A reduction in HIF-1α, GLUT-1, and VEGF levels was also observed following treatment with AZD6244, and there was a significant decrease in vascular perfusion in the tumors given combination treatment when compared with other treatment groups. Hamed et al.79 showed that 48-hour pretreatment of mammary tumor cells with the MEK inhibitor PD184352 and anticancer staurosporine analog UCN-01, followed by 24-hour drug removal before irradiation, enhanced cell death compared with either drug or radiation alone. Additionally, AZD6244 given 16 hours before radiation treatment enhanced the radiosensitivity of lung (A549), pancreatic (MiaPaCa), and prostate (DU145) cells in vitro.67 The mechanistic basis for this sensitization does not seem to involve enhanced apoptosis, with no change in PARP cleavage observed between cells treated with radiation alone or in combination with AZD6244. When AZD6244 was combined with fractionated radiotherapy in vivo, a significant increase in tumor growth delay was observed compared with either monotherapy, regardless of whether radiation was given during the first or last 5 days of the 10-day AZD6244 treatment. This shows that extended MEK inhibition before radiotherapy is unnecessary, which differs from the work of Hamed et al.,79 who showed that irradiation of PD184352- and UCN-01–treated tumors 24 hours after cessation of the drug treatment significantly enhanced the therapeutic outcome compared with concomitant therapy. Chung et al.67 demonstrated an enhanced radiotherapeutic response in vivo when a single dose of AZD6244 (50 mg/kg) was administered 4 hours before a single dose of radiotherapy (3 Gy). These studies provide evidence that the combination of a MEK inhibitor with radiation produces the greatest degree of therapeutic effect, as measured by an increased dose enhancement factor in vitro and tumor reduction in vivo.
A growing number of MEK/ERK inhibitors have now entered clinical testing against a variety of solid tumors83-88 (Fig. 2). Although several potent inhibitors of the ERK pathway have been reported, they did not prove themselves as resoundingly successful in clinical trials. The reason for this might be that these compounds are more cytostatic than cytotoxic anticancer drugs, and the effect of many of these inhibitors is reversible, allowing tumor cells to reproliferate after removal of the drug. Since irradiation by itself can stimulate the survival of single tumor cell clones by the activation of signal transduction components, and many tumor cells are not dying under the influence of these inhibitors, teaming irradiation together with specific inhibitors might prove particularly useful for cancer therapy by overcoming resistance mechanisms against irradiation. Collectively, data from the literature are indicative of enhanced cell killing by irradiation when ERK function is blocked.
The p38 MAPK Pathway
The p38 MAPK pathway was originally described as a mammalian homolog to a yeast osmolarity-sensing pathway.89 It was soon discovered that many cellular stresses activated the p38 MAPK pathway in a manner not dissimilar to that described for the JNK pathway.90 Rho family GTPases appear to play an important role as upstream activators of the p38 MAPK pathway, a role facilitated via several MAP3K enzymes, for example, the PAK family,91 which regulate the MAP2K enzymes MKK3 and MKK6.92 At least 4 isoforms of p38 MAPK exist; these are termed p38α, p38β, p38γ, and p38δ.5 There are several protein kinases downstream of p38 MAPK enzymes that are activated following phosphorylation by p38 isoforms including p90 RSK93 and MSK1/2.94 p90 RSK phosphorylates and activates heat-shock protein 27 (HSP27), while MSK1/2 can phosphorylate and activate transcription factors that regulate survival, such as CREB.95 The role of p38 MAPK signaling in cellular responses is diverse, depending on the cell type and stimulus, and has been shown to promote both cell death as well as enhance cell growth and survival.96,97 A range of proinflammatory cytokines and environmental stresses can trigger the p38 MAPK cascade. The ability of ionizing radiation to regulate p38 MAPK activity appears to be highly variable, with different groups reporting either no activation,98 weak activation,99 or strong activation.100,101 This is in contrast to the classic ERK and JNK pathways in which radiation-induced activation has been observed by many groups, in diverse cell types, and in response to low and high radiation doses.
A role for p38 in the checkpoint governing the G2-M transition has been demonstrated by Wang et al.102 in mammalian cells. The authors demonstrated that a MAPKK-p38γ cascade is required for γ-radiation–induced G2 arrest. Radiation was found to activate all 4 p38 isoforms as well as the upstream kinase MKK6 (MKK6 appears to be the main MAPKK involved, but some activation of MKK3 was detected). Only activation of the p38γ isoform was required for the G2 arrest; however, the other p38 isoforms could be involved in additional checkpoints. Activated MKK6 inhibited the cell cycle, arresting cells in G2 phase. This G2 arrest occurred independently of p53. Importantly, inhibition of MKK6 or p38γ disrupted this checkpoint. p38γ activation was dependent upon ATM and resulted in the activation of Chk2.102 The authors propose that MKK6 and p38γ function between ATM and Chk2 in the pathway. Activated Chk2 can phosphorylate Cdc25C, thus stimulating 14-3-3–dependent sequestration of Cdc25C away from Cdk1 (Cdc2). Further evidence for a close link between p38 MAPK and checkpoints is provided by Bulavin et al.,103 who examined the role of p38 in the G2 checkpoint in response to UV radiation. Again, p38 was found to have a crucial role in the initiation of a G2 delay. However, in the case of the UV radiation checkpoint response, the p38α and p38β isoforms were involved rather than the p38γ isoform. Addition of an inhibitor specific for p38α and p38β (SB202190) abrogated the cell cycle checkpoint response to UV radiation. The UV-induced G2/M checkpoint arrest appears to be achieved through p38-dependent phosphorylation of Cdc25B, resulting in 14-3-3 binding. The association of 14-3-3 with Cdc25B results in its exclusion from the nucleus and failure to dephosphorylate Cdk1 (Cdc2–cyclin B). Consistent with this model, mutating these Cdc25B phosphorylation sites resulted in checkpoint abrogation. These data support an important interplay between the p38 pathway and G2 cell cycle checkpoint control. Choi et al.,104 in their study, showed that activation of c-Abl–PKCδ–Rac1–p38 MAPK signaling in response to ionizing radiation signals conformational changes of Bak and Bax, resulting in mitochondrial activation–mediated apoptotic cell death in human NSCLC cells. Since it has been shown that p38 MAPK acts as an early step prior to the dysfunction of mitochondria and caspase activation during apoptotic cell death in several model systems, they investigated whether p38 MAPK is involved in radiation-induced mitochondrial dysfunction and cytochrome c release. Inhibition of p38 MAPK by treatment with the p38 MAPK inhibitor, SB203580, or by overexpression of a dominant-negative form of p38 effectively blocked the loss of mitochondrial membrane potential, cytochrome c release to the cytosol, and caspase activation seen after irradiation. Inhibition of p38 MAPK also suppressed the radiation-induced conformational changes of both Bax and Bak and subsequent mitochondrial translocation of Bax, indicating that p38 MAPK is an important mediator of apoptotic conformation of Bak/Bax and subsequent mitochondrial dysfunction during radiation-induced apoptotic cell death.
An extensive array of potent and specific inhibitors of p38 are being evaluated in phase I and II clinical trials105-107 (Fig. 2). They have been developed primarily for the treatment of chronic inflammatory diseases, although some trials are also evaluating possible applications in cancer.
Conclusion
Several studies demonstrate that activation of the MAPK pathway is a central feature in radioresistance mechanisms, and molecularly targeted approaches that enhance the effectiveness of radiation are particularly attractive for the treatment of cancer. Radiation therapy significantly improves local control and is considered a standard of care for patients with locally advanced cancer. As approximately half of all patients with cancer undergo radiotherapy as part of their treatment, enhancing therapeutic outcomes for even a small percentage of these patients has the potential to translate into significant clinical effects. Evidence from some of the studies outlined above supports a need for further investigations of combined modality therapy that involves radiation and MAPK pathway inhibitors as a promising strategy for improving the treatment of patients with cancer. The potential to combine such inhibitors of MAPK signaling pathways with localized radiation is considerable, although it is necessary to evaluate whether such approaches could act cooperatively with radiotherapy and to examine any potential scheduling interactions to optimize therapeutic outcome.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mordret G. MAP kinase kinase: a node connecting multiple pathways. Biol Cell. 1993;79:193-207 [DOI] [PubMed] [Google Scholar]
- 2. L’Allemain G. Deciphering the MAP kinase pathway. Prog Growth Factor Res. 1994;5:291-334 [DOI] [PubMed] [Google Scholar]
- 3. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726-35 [PubMed] [Google Scholar]
- 4. Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;7:49-139 [DOI] [PubMed] [Google Scholar]
- 5. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807-69 [DOI] [PubMed] [Google Scholar]
- 6. Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-83 [DOI] [PubMed] [Google Scholar]
- 7. Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen- activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402-11 [DOI] [PubMed] [Google Scholar]
- 9. Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283-300 [DOI] [PubMed] [Google Scholar]
- 10. Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153:245-57 [DOI] [PubMed] [Google Scholar]
- 12. Haimovitz-Friedman A. Radiation-induced signal transduction and stress response. Radiat Res. 1998;150(5 Suppl):S102-8 [PubMed] [Google Scholar]
- 13. Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-96 [DOI] [PubMed] [Google Scholar]
- 14. Ouwens DM, Gomes de, Mesquita DS, Dekker J, et al. Hyperosmotic stress activates the insulin receptor in CHO cells. Biochim Biophys Acta. 2001;1540:97-106 [DOI] [PubMed] [Google Scholar]
- 15. Stadheim TA, Kucera GL. c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone- and anisomycin-induced apoptosis in HL-60 cells. Leuk Res. 2002;26:55-65 [DOI] [PubMed] [Google Scholar]
- 16. Chen YR, Meyer CF, Tan TH. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J Biol Chem. 1996;271:631-4 [DOI] [PubMed] [Google Scholar]
- 17. Adam D, Ruff A, Strelow A, Wiegmann K, Krönke M. Induction of stress-activated protein kinases/c-Jun N-terminal kinases by the p55 tumor necrosis factor receptor does not require sphingomyelinases. Biochem J. 1998;333:343-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrer I, Olive M, Ribera J, Planas AM. Naturally occurring (programmed) and radiation-induced apoptosis are associated with selective c-Jun expression in the developing rat brain. Eur J Neurosci. 1996;8:1286-98 [DOI] [PubMed] [Google Scholar]
- 19. Ferrer I, Olivé M, Blanco R, Cinós C, Planas AM. Selective c-Jun overexpression is associated with ionizing radiation-induced apoptosis in the developing cerebellum of the rat. Brain Res Mol Brain Res. 1996;38:91-100 [DOI] [PubMed] [Google Scholar]
- 20. Verheij M, Ruiter GA, Zerp SF, et al. The role of the stress-activated protein kinase (SAPK/JNK) signaling pathway in radiation-induced apoptosis. Radiother Oncol. 1998;47:225-32 [DOI] [PubMed] [Google Scholar]
- 21. Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75-9 [DOI] [PubMed] [Google Scholar]
- 22. Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-Jun. Oncogene. 1993;8:877-86 [PubMed] [Google Scholar]
- 23. Kim MJ, Lee KH, Lee SJ. Ionizing radiation utilizes c-Jun N-terminal kinase for amplification of mitochondrial apoptotic cell death in human cervical cancer cells. FEBS J. 2008;275:2096-108 [DOI] [PubMed] [Google Scholar]
- 24. Viktorsson K, Ekedahl J, Lindebro MC, et al. Defective stress kinase and Bak activation in response to ionizing radiation but not cisplatin in a non-small cell lung carcinoma cell line. Exp Cell Res. 2003;289:256-64 [DOI] [PubMed] [Google Scholar]
- 25. Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lei K, Nimnual A, Zong WX, et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kharbanda S, Yuan ZM, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309-18 [DOI] [PubMed] [Google Scholar]
- 28. Lee SA, Dritschilo A, Jung M. Role of ATM in oxidative stress-mediated c-Jun phosphorylation in response to ionizing radiation and CdCl2. J Biol Chem. 2001;276:11783-90 [DOI] [PubMed] [Google Scholar]
- 29. Hayakawa J, Depatie C, Ohmichi M, Mercola D. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J Biol Chem. 2003;278:20582-92 [DOI] [PubMed] [Google Scholar]
- 30. Potapova O, Basu S, Mercola D, Holbrook NJ. Protective role for c-Jun in the cellular response to DNA damage. J Biol Chem. 2001;276:28546-53 [DOI] [PubMed] [Google Scholar]
- 31. Potapova O, Haghighi A, Bost F, et al. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J Biol Chem. 1997;272:14041-4 [DOI] [PubMed] [Google Scholar]
- 32. MacLaren A, Black EJ, Clark W, Gillespie DAF. c-Jun-deficient cells undergo premature senescence as a result of spontaneous DNA damage accumulation. Mol Cell Biol. 2004;24:9006-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gjerset RA, Lebedeva S, Haghighi A, Turla ST, Mercola D. Inhibition of the Jun kinase pathway blocks DNA repair, enhances p53-mediated apoptosis and promotes gene amplification. Cell Growth Differ. 1999;10:545-54 [PubMed] [Google Scholar]
- 34. Scherer SJ, Maier SM, Seifert M, et al. p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J Biol Chem. 2000;275:37469-73 [DOI] [PubMed] [Google Scholar]
- 35. Weizman N, Shiloh Y, Barzilai A. Contribution of the ATM protein to maintaining cellular homeostasis evidenced by continuous activation of the AP-1 pathway in ATM-deficient brains. J Biol Chem. 2003;278:6741-7 [DOI] [PubMed] [Google Scholar]
- 36. Bhoumik A, Takahashi S, Breitweiser W, et al. ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol Cell. 2005;18:577-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11:653-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chevray PM, Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci U S A. 1992;89:5789-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246-9 [DOI] [PubMed] [Google Scholar]
- 40. Kim MJ, Choi SY, Park IC, et al. Opposing roles of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase in the cellular response to ionizing radiation in human cervical cancer cells. Mol Cancer Res. 2008;6:1718-31 [DOI] [PubMed] [Google Scholar]
- 41. Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554-65 [DOI] [PubMed] [Google Scholar]
- 42. Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santen RJ, Song RX, McPherson R, et al. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239-56 [DOI] [PubMed] [Google Scholar]
- 44. Adjei AA. The role of mitogen-activated ERK-kinase inhibitors in lung cancer therapy. Clin Lung Cancer. 2005;7:221-3 [DOI] [PubMed] [Google Scholar]
- 45. De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16 Suppl 2:S17-27 [DOI] [PubMed] [Google Scholar]
- 46. de Bono JS, Rowinsky EK. Therapeutics targeting signal transduction for patients with colorectal carcinoma. Br Med Bull. 2002;64: 227-54 [DOI] [PubMed] [Google Scholar]
- 47. Bonner JA, Vroman BT, Christianson TJ, Karnitz LM. Ionizing radiation-induced MEK and Erk activation does not enhance survival of irradiated human squamous carcinoma cells. Int J Radiat Oncol Biol Phys. 1998;42:921-5 [DOI] [PubMed] [Google Scholar]
- 48. Kasid U, Suy S, Dent P, Ray S, Whiteside TL, Sturgill TW. Activation of Raf by ionizing radiation. Nature. 1996;382:813-6 [DOI] [PubMed] [Google Scholar]
- 49. Kharbanda S, Saleem A, Shafman T, Emoto Y, Weichselbaum R, Kufe D. Activation of the pp90 Rsk and mitogen-activated serine/threonine protein kinases by ionizing radiation. Proc Natl Acad Sci U S A. 1994;91:5416-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sklar MD. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science. 1988;239:645-7 [DOI] [PubMed] [Google Scholar]
- 51. Bernhard EJ, Stanbridge EJ, Gupta S, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597-600 [PubMed] [Google Scholar]
- 52. McKenna WG, Bernhard EJ, Markiewicz DA, Rudoltz MS, Maity A, Muschel RJ. Regulation of radiation induced apoptosis in oncogene- transfected fibroblasts: influence of H-ras on the G2 delay. Oncogene. 1996;12:237-45 [PubMed] [Google Scholar]
- 53. Park JS, Qiao L, Su ZZ, et al. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20:3266-80 [DOI] [PubMed] [Google Scholar]
- 54. Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9:777-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374-8 [PubMed] [Google Scholar]
- 56. Gupta VK, Jaskowiak NT, Beckett MA, et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8:47-54 [DOI] [PubMed] [Google Scholar]
- 57. Kim IA, Bae SS, Fernandes A, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 2005;65:7902-10 [DOI] [PubMed] [Google Scholar]
- 58. Bernhard EJ, Kao G, Cox AD, et al. The farnesyl transferase inhibitor FTI-277 radiosensitizes H-ras transformed rat embryo fibroblasts. Cancer Res. 1996;56:1727-30 [PubMed] [Google Scholar]
- 59. Kasid U, Pfeifer A, Brennan T, et al. Effect of antisense c-raf-1 on tumorigenicity and radiation sensitivity of a human squamous carcinoma. Science. 1989;243:1354-6 [DOI] [PubMed] [Google Scholar]
- 60. Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200-11 [DOI] [PubMed] [Google Scholar]
- 61. Roskoski R., Jr. MEK1/2 dual-specificity protein kinases: structure and regulation. Biochem Biophys Res Commun. 2012;417:5-10 [DOI] [PubMed] [Google Scholar]
- 62. Marampon F, Gravina GL, Di Rocco A, et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sambade MJ, Camp JT, Kimple RJ, Sartor CI, Shields JM. Mechanism of lapatinib-mediated radiosensitization of breast cancer cells is primarily by inhibition of the Raf/MEK/ERK mitogen-activated protein kinase cascade and radiosensitization of lapatinib-resistant cells restored by direct inhibition of MEK. Radiother Oncol. 2009;93:639-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kurland JF, Voehringer DW, Meyn RE. The MEK/ERK pathway acts upstream of NF kappa B1 (p50) homodimer activity and Bcl-2 expression in a murine B-cell lymphoma cell line: MEK inhibition restores radiation-induced apoptosis. J Biol Chem. 2003;278:32465-70 [DOI] [PubMed] [Google Scholar]
- 65. Carter S, Auer KL, Reardon DB, et al. Inhibition of the mitogen activated protein (MAP) kinase cascade potentiates cell killing by low dose ionizing radiation in A431 human squamous carcinoma cells. Oncogene. 1998;16:2787-96 [DOI] [PubMed] [Google Scholar]
- 66. Qiao L, Yacoub A, McKinstry R, et al. Pharmocologic inhibitors of the mitogen activated protein kinase cascade have the potential to interact with ionizing radiation exposure to induce cell death in carcinoma cells by multiple mechanisms. Cancer Biol Ther. 2002;1:168-76 [DOI] [PubMed] [Google Scholar]
- 67. Chung EJ, Brown AP, Asano H, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15:3050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li J, Wang Y, Sun Y, Lawrence TS. Wild-type TP53 inhibits G(2)-phase checkpoint abrogation and radiosensitization induced by PD0166285, a WEE1 kinase inhibitor. Radiat Res. 2002;157:322-30 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Li J, Booher RN, et al. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001;61:8211-7 [PubMed] [Google Scholar]
- 70. Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732-42 [DOI] [PubMed] [Google Scholar]
- 71. Iliakis G, Nusse M. Arrest of irradiated G1, S, or G2 cells at mitosis using nocodazole promotes repair of potentially lethal damage. Radiat Res. 1984;99:346-51 [PubMed] [Google Scholar]
- 72. Tamamoto T, Ohnishi K, Takahashi A, et al. Correlation between γ-ray-induced G2 arrest and radioresistance in two human cancer cells. Int J Radiat Oncol Biol Phys. 1999;44:905-9 [DOI] [PubMed] [Google Scholar]
- 73. Huang H, Fletcher L, Beeharry N, et al. Abnormal cytokinesis after X-irradiation in tumor cells that override the G2 DNA damage checkpoint. Cancer Res. 2008;68:3724-32 [DOI] [PubMed] [Google Scholar]
- 74. Eriksson D, Lofroth PO, Johansson L, Riklund KA, Stigbrand T. Cell cycle disturbances and mitotic catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing radiation. Clin Cancer Res. 2007;13:5501-8 [DOI] [PubMed] [Google Scholar]
- 75. Syljuasen RG, Sorensen CS, Nylandsted J, Lukas C, Lukas J, Bartek J. Inhibition of Chk1 by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing radiation. Cancer Res. 2004;64:9035-40 [DOI] [PubMed] [Google Scholar]
- 76. Wei F, Yan J, Tang D, et al. Inhibition of ERK activation enhances the repair of double-stranded breaks via non-homologous end joining by increasing DNA-PKcs activation. Biochim Biophys Acta. 2013;1833:90-100 [DOI] [PubMed] [Google Scholar]
- 77. Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046-53 [DOI] [PubMed] [Google Scholar]
- 79. Hamed H, Hawkins W, Mitchell C, et al. Transient exposure of carcinoma cells to RAS/MEK inhibitors and UCN-01 causes cell death in vitro and in vivo. Mol Cancer Ther. 2008;7:616-29 [DOI] [PubMed] [Google Scholar]
- 80. Williams TM, Flecha AR, Keller P, et al. Cotargeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol Cancer Ther. 2012;11:1193-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marampon F, Gravina GL, Di Rocco A, et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shannon AM, Telfer BA, Smith PD, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res. 2009;15:6619-29 [DOI] [PubMed] [Google Scholar]
- 83. Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281-93 [DOI] [PubMed] [Google Scholar]
- 84. Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456-62 [DOI] [PubMed] [Google Scholar]
- 85. Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773-81 [DOI] [PubMed] [Google Scholar]
- 87. Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-14 [DOI] [PubMed] [Google Scholar]
- 89. Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265 :808-11 [DOI] [PubMed] [Google Scholar]
- 90. Lin A, Minden A, Martinetto H, et al. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286-90 [DOI] [PubMed] [Google Scholar]
- 91. Holbrook NJ, Liu Y, Fornace AJ., Jr. Signaling events controlling the molecular response to genotoxic stress. EXS. 1996;77:273-88 [DOI] [PubMed] [Google Scholar]
- 92. Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D. BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway: implication in the regulation of membrane ruffling. J Biol Chem. 2001;276:25066-72 [DOI] [PubMed] [Google Scholar]
- 93. Maizels ET, Mukherjee A, Sithanandam G, et al. Developmental regulation of mitogen- activated protein kinase-activated kinases-2 and -3 (MAPKAPK-2/-3) in vivo during corpus luteum formation in the rat. Mol Endocrinol. 2001;15: 716-33 [DOI] [PubMed] [Google Scholar]
- 94. Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Juretic N, Santibáñez JF, Hurtado C, Martínez J. ERK 1, 2 and p38 pathways are involved in the proliferative stimuli mediated by urokinase in osteoblastic SaOS-2 cell line. J Cell Biochem. 2001;83:92-8 [DOI] [PubMed] [Google Scholar]
- 97. Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem. 2001;268:6058-65 [DOI] [PubMed] [Google Scholar]
- 98. Kim SJ, Ju JW, Oh CD, et al. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002;277:1332-9 [DOI] [PubMed] [Google Scholar]
- 99. Taher MM, Hershey CM, Oakley JD, Valerie K. Role of the p38 and MEK-1/2/p42/44 MAP kinase pathways in the differential activation of human immunodeficiency virus gene expression by ultraviolet and ionizing radiation. Photochem Photobiol. 2000;71:455-9 [DOI] [PubMed] [Google Scholar]
- 100. Lee YJ, Soh JW, Dean NM, et al. Protein kinase C delta over expression enhances radiation sensitivity via extracellular regulated protein kinase 1/2 activation, abolishing the radiation-induced G(2)-M arrest. Cell Growth Differ. 2002;13: 237-46 [PubMed] [Google Scholar]
- 101. Narang H, Bhat N, Gupta SK, et al. Differential activation of mitogen-activated protein kinases following high and low LET radiation in murine macrophage cell line. Mol Cell Biochem. 2009;324:85-91 [DOI] [PubMed] [Google Scholar]
- 102. Wang X, McGowan CH, Zhao M, et al. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20:4543-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bulavin DV, Amundson SA, Fornace AJ. p38 and Chk1 kinases: different conductors for the G(2)/M checkpoint symphony. Curr Opin Genet Dev. 2002;2:92-7 [DOI] [PubMed] [Google Scholar]
- 104. Choi SY, Kim MJ, Kang CM, et al. Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J Biol Chem. 2006;281:7049-59 [DOI] [PubMed] [Google Scholar]
- 105. Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel. 2005;8:421-30 [PubMed] [Google Scholar]
- 106. Hynes J, Leftheri K. Small molecule p38 inhibitors: novel structural features and advances from 2002-2005. Curr Top Med Chem. 2005;5:967-85 [DOI] [PubMed] [Google Scholar]
- 107. O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549-63 [DOI] [PubMed] [Google Scholar]