Abstract
The activity of c-Jun N-terminal kinase (JNK) was initially described as ultraviolet- and oncogene-induced kinase activity on c-Jun. Shortly after this initial discovery, JNK activation was reported for a wider variety of DNA-damaging agents, including γ-irradiation and chemotherapeutic compounds. As the DNA damage response mechanisms were progressively uncovered, the mechanisms governing the activation of JNK upon genotoxic stresses became better understood. In particular, a recent set of papers links the physical breakage in DNA, the activation of the transcription factor NF-κB, the secretion of TNF-α, and an autocrine activation of the JNK pathway. In this review, we will focus on the pathway that is initiated by a physical break in the DNA helix, leading to JNK activation and the resultant cellular consequences. The implications of these findings will be discussed in the context of cancer therapy with DNA-damaging agents.
Keywords: DNA damage, UV irradiation, JNK
Introduction
In physiological conditions, eukaryote genomes are subjected to a variety of DNA-damaging events that may alter their coding information. Double-strand breaks (DSBs) of the DNA double helix are the most deleterious events that occur in the genome and can either be due to endogenous causes such as the metabolic production of reactive oxygen species (ROS) and replication fork collapse or exogenous causes including exposure to chemical genotoxic agents or ultraviolet (UV) irradiation. In order to maintain genome homeostasis, eukaryotes have evolved protective mechanisms that can detect damaged DNA, repair it and induce an appropriate cellular response including JNK signaling activation.1-7 of DSBs seems to rely on the initial detection of chromatin remodeling induced by the breakage that initiates a signaling cascade to activate repair systems. The 2 major mechanisms responsible for eliminating DSBs are the well-described nonhomologous end joining and homologous recombination repair systems, considered, respectively, as a fast and a slow repair system. The means of activation of these repair pathways are well known, and the first event described is the formation of nuclear foci, called irradiation-induced foci (IRIF), which contain a variety of proteins including the phosphorylated histone H2AX (γH2AX) that is common to every DNA damage response (DDR) described so far.8,9 When these mechanisms fail to repair damaged DNA, the persistence of the signal emerging from broken DNA leads to cell cycle arrest, apoptosis, or cell senescence. In cells compromised for cell cycle checkpoints, the persistence of damage in the genome during mitosis can lead to mutations or the missense recombination of chromatids and cell transformation. An initial observation has linked DNA damage to the activation of NF-κB, but the mechanism responsible for driving the nuclear DNA damage signal to the NF-κB cytoplasmic target has only recently been extensively described. The NF-κB family is a group of 5 transcription factors that bind DNA as dimers (namely RelA, RelB, c-Rel, p50, and p52). These transcription factors were initially discovered as inflammation-activated factors, but they can also be activated by a wide variety of signals including bacterial infection, presence of inflammatory cytokines, or DNA-damaging agents. The inactive form of NF-κB is sequestered in the cytoplasm through binding to its IκB inhibitory partner. NF-κB–activating signals induce the phosphorylation of IκB by a 3-subunit complex called IκB kinase (IKK) that is composed of at least 2 catalytically active kinases (IKKα and IKKβ) and a regulatory subunit, the NF-κB essential modifier (NEMO).10 In response to its phosphorylation, IκB undergoes ubiquitin-mediated proteasomal degradation, and the released NF-κB dimers can translocate to the nucleus and interfere with their target sequences. Although the observation that UV-induced DNA damage can activate NF-κB signaling was made a long time ago, the mechanisms responsible for driving the nuclear DNA damage signal to the NF-κB cytoplasmic target has only recently been extensively described.11,12 Mechanistic insight of JNK activation induced by DSB was shown by the discovery of DDR-driven activation of NF-κB and the consecutive production of tumor necrosis factor α (TNF-α) responsible for an autocrine feed-forward signaling loop through its type 1 receptor (TNFR-1) and the consequent activation of the JNK signaling pathway.13
DNA Damage Forward Signaling
Initiation of the cellular response to genotoxic stress relies on the efficient detection of any DNA lesion in the whole genome. While the events occurring shortly after DNA breakage are well described, the initial signal responsible for triggering these events has not yet been clearly identified. The proposed model for explaining the detection of DSBs stipulates that when a DSB occurs, the topological constraints due to DNA supercoiling are released, which induces the relaxation of the chromatin fiber.14-16 The associated conformational changes are proposed to recruit the wide variety of factors responsible for posttranslational modifications of the DNA- associated proteins. The earliest event in this process is histone poly (ADP- ribosylation), during which time enzymes called the poly (ADP-ribose) polymerases (PARP1, PARP2, and PARP3) catalyze the formation of poly (ADP-ribose) (PAR) and their covalent linkage to lysines in core histone proteins.9,17,18 Notably, the PAR chains formed by PARP1 serve as a binding site for chromatin and histone modifiers that inhibit replication and transcription around the site of the lesions.19 Very early after formation of the DNA damage, γH2AX is also detectable in close vicinity to the DSBs as well as other components of the DNA repair pathways that accumulate in so-called IRIF.20 Phosphorylation of histone H2AX is performed by kinases of the phosphoinositide-3-kinase–related protein (PIKK) family called DNA-dependent protein kinase (DNA-PK) and ataxia telangiectasia mutated (ATM).21 DNA-PK constitutes a DNA-binding subunit (Ku70 and Ku80) responsible for localization of the kinase subunit (DNA-PKcs) at DNA free ends.22 Although this composition would be a good candidate for signaling downstream events from DSBs, the current general consensus is that it does not play a central role in this phenomenon. Instead, while the intermediates responsible for ATM localization at DSBs are not yet well described, ATM has been shown to be activated through transautophosphorylation-induced dissociation of ATM-inactive dimers.17,23 Active ATM is shown to be prominently involved in the phosphorylation of H2AX and spreading of DSB signaling at the distance of IRIF.9,24,25 In turn, γH2AX, together with a factor called mediator of DNA checkpoint 1 (MDC1), was shown to be necessary for the recruitment of key elements involved in DSB signaling such as 53BP1, BRCA1, and p53 tumor suppressor protein.26,27 In consequence of these initial nuclear events, DDR signaling is transduced to cytoplasmic targets, among which NF-κB and the JNK pathway are prominently involved in the proliferation and survival of the cells.
From DNA Damage to NF-κB and JNK Activation
The demonstration that NF-κB activation can be induced by UV exposure suggested that it could be the mechanism responsible for triggering the DDR. Apparent contradictory results initiated a debate over whether UV-induced NF-κB activation is directly dependent on DNA damage signaling or not.28-30 The explanation seems to lie in the middle, with signals directly emanating from the nucleus due to the activation of DNA damage pathways combined with other events such as UV-induced formation of reactive species converging to activate NF-κB.31,32 Major breakthroughs confirming the existence of a nuclear-to-cytoplasmic signal transduction came from Miyamoto’s team, who broadly deciphered the mechanisms governing ATM- and NEMO-dependent nucleus-to-cytoplasm signaling responsible for NF-κB activation.33-36 A fundamental development confirming the existence of a nucleus-to-cytoplasm signaling pathway came from extending the results obtained with UV radiation activation of NF-κB to a variety of other DNA-damaging agents such as etoposide or camptothecin.36,37 Another convincing line of evidence comes from an experiment using Atm–/– mice: while control mice show the activation of IKK in response to whole body irradiation, Atm–/– mice fail to activate NF-κB signaling, demonstrating the requirement of ATM for transduction of the nuclear DNA damage–driven activation of NF-κB.38 Initial studies described the sumoylation of NEMO by the small ubiquitin-related modulator 1 (SUMO-1) as the driver for nuclear relocalization of IKK-unbound cytoplasmic NEMO proteins.33-39 While this sumoylation event was described to be ATM independent, the complete activation of NF-κB upon genotoxic damage also relies on nuclear NEMO posttranslational modifications, resulting in the export of an active form of NEMO. One of the earliest and critical events in DNA damage recognition and signaling is PAR formation by PARP-1, which has been demonstrated to play a fundamental role in the mechanism leading to NEMO nuclear posttranslational modifications.40 The automodification of PARP-1 was shown to drive the assembly of a complex consisting of ATM, NEMO, and the sumoylation factor inhibitor of activated STAT γ (PIASγ). Formation of this complex is concomitant with posttranslational modifications of NEMO that appear to be necessary for DNA damage–induced NF-κB activation: PIASγ transiently sumoylates NEMO on lysines 277 and 309, and ATM phosphorylates NEMO on serine S85.34,35 These initial modifications are required for the subsequent ubiquitination of NEMO by the protein cIAP1 (an inhibitor of apoptosis), and this modification is proposed to drive the nuclear export of a complex comprising at least NEMO and ATM.33 The multimodal interactions occurring in the nucleus and the cytoplasm between NEMO and its posttranslational modifiers appear to be partly dependent on the cell type and extent of the DNA damage. However, a common cytoplasmic effector of IKK activation by DNA damage is transforming growth factor β–activated kinase 1 (TAK1), a kinase that has already been shown to be an activator of NF-κB in other cellular contexts.41 Although the proposed models tend to differ depending on the cell type used, TAK1 was shown to be required for genotoxic-induced activation of NF-κB.35,42 Upon DNA damage– mediated activation of ATM and NEMO, TAK1 is activated through the ubiquitination of its regulatory partners and in turn phosphorylates IKKβ, which leads to the activation of NF-κB.13-35 The demonstration that UV-challenged IKKβ knockout cells lack the degradation of IκB and activation of NF-κB strongly implicates IKK involvement in DNA damage–induced activation of NF-κB.35,42,43 Recent studies also suggest that NF-κB activation can occur independently of IKK catalytic activity upon DNA damage. Using nonphosphorylatable forms of IκB, Tsuchiya and colleagues44 demonstrated that the IKK complex can play the role of an adaptor protein responsible for targeting IκB to ubiquitination independently of phosphorylation. Another mode of NF-κB activation by UV radiation was also suggested to occur via the DNA damage–induced phosphorylation of eukaryotic initiation factor 2α (eIF2α) that leads to the inhibition of IκB translation and the release of NF-κB transcription factors.45
The fate of cells subjected to DNA damage varies depending on the extent of the damage and the genetic background of the cells. The general paradigm stipulates that while the immediate response to DNA damage, and in particular DSBs, is rapid cell cycle arrest mainly based on the activation of p53, the capacity of the cell to repair the damage determines the rate of recovery and return to the normal cell cycle.8 If the damage is irreparable or incorrectly resolved, cells stop proliferating definitely and are directed towards apoptosis or senescence.16,46 NF-κB activity is usually described as a prosurvival factor driving resistance to anticancer drug therapies and thus fits well as a factor to promote cell survival during the time needed for DNA reparation.47 However, sustained NF-κB activation in response to DNA damage then appears contradictory to DDR-induced cell death. In a recent paper, Biton and Ashkenazi13 showed that the activation of NF-κB by genotoxic agents actually fits this 2-stage cellular response, and they proposed that this pathway may control a switch between a prosurvival and an apoptotic cellular response to DNA damage. In this model, mild DNA damage could promote an early-phase NF-κB response that would be responsible for cell survival during the time required to repair the injuries. In the case of extensive DNA damage, the initial NF-κB response would be maintained and reinforced by a TNF-α/TNFR-1 autocrine loop that is proposed to be responsible for the sustained activation of JNK3. In this model, NF-κB signaling therefore appears to be a fundamental promoter of DNA damage–induced apoptosis. In the next part, we will explore published data that may explain the role of the JNK signaling pathway in apoptosis.
Other Means of JNK Activation by DNA Damage
Since the initial description of JNK activation by DNA-damaging agents, few studies have deciphered the underlying mechanisms involved. Besides the recent breakthroughs described above implicating DNA damage–induced NF-κB activation as an upstream signal of JNK activation, other models explaining the initial observations made on UV-stimulated cells are scarce. The earliest explanation came from Westwick and colleagues,48 who suggested that ceramides could be activators of the stress kinases. Ceramides are lipid molecules present in the cell plasma membrane that have diverse functions in cellular signaling.49 Various cellular stresses have been proposed to modulate ceramide metabolism. Among them, DNA damage was shown to induce an augmentation of cellular ceramide levels that are responsible for the activation of JNK and NF-κB signaling pathways.50,51 More recently, Shim and colleagues52 have shown that TGF-β–mediated JNK and NF-κB activation are impaired in TAK1 knockout cells.53 This may imply that TAK1’s direct activation by NEMO after DNA damage directly drives JNK activation independently of TNFR. This complements Biton and Ashkenazi’s13 model stipulating that an autocrine feed-forward loop of TNF-α is responsible for the activation of JNK3 after genotoxic stress.54
JNK’s activation, as well as other MAPK activations, occurs through a double phosphorylation of a tyrosine and threonine residue in the consensus Thr-X-Tyr (where X is Glu, Gly, or Pro). This activation is negatively controlled by dual-specificity phosphatases that directly dephosphorylate the consensus motif to inactivate the kinase.55,56 Among these phosphatases, MAPK phosphatase 1 (MKP-1) is a cytoplasmic protein that was shown to be down-regulated in cells subjected to translation inhibition induced by severe DNA damage.57,58 The inhibition of MKP-1 translation therefore releases the negative control of JNK and is proposed to drive JNK activation in these conditions.
Cellular Outcomes of JNK Activation by DNA Damage
A wide variety of events occurring downstream of JNK activation, ranging from cytoskeleton remodeling to the regulation of cell proliferation or apoptosis, have been described. In this section, we will focus on JNK activities that may be relevant in the field of DDR, particularly in the control of apoptosis, DNA damage repair, and senescence.
As stated previously, H2AX phosphorylation is one of the most upstream events occurring after a DSB. ATM is the kinase usually described to be responsible for this phosphorylation; however, Lu et al.59 described that the phosphorylation of H2AX on serine S139 can also be achieved in vivo by JNK. Using either chemical inhibitors, a dominant-negative form of JNKs, and a Jnk2 knockout cell line silenced for JNK1 by siRNAs, they showed that H2AX phosphorylation in response to UV exposure is disrupted only in cells in which both JNK1 and JNK2 activities are hampered. The authors concluded that γH2AX formation is therefore redundantly dependent on JNK1 and JNK2 activities.59 Moreover, they confirmed that JNK is necessary for caspase-3–mediated apoptosis in response to UV exposure.59-61 The authors proposed a model whereby JNK translocation into the nucleus, where it meets its H2AX substrate, is a core event in the process, leading to UV-induced cell death. Moreover, Lawan and colleagues62 have shown that UV-induced H2AX phosphorylation and apoptosis are increased in mouse embryonic fibroblasts (MEFs) deficient for the dual-specificity phosphatase MKP-2. Interestingly, MKP-2 is localized specifically in the nucleus, pinpointing the suggestion that specific nuclear activity of JNK is central in JNK-induced apoptosis.57 Recently, another study showed that the phosphorylation of H2AX by JNK is a very early event in DNA damage–induced apoptosis.63 Consistent with the idea that H2AX is a substrate for JNK and that γH2AX is required for apoptosis, the authors also described the JNK-dependent pannuclear phosphorylation of H2AX that is proposed to promote the apoptosis of cells subjected to extensive DNA damage.63 The Fas receptor (Fas)/Fas ligand (Fas-L) is a potent system that induces apoptosis. When Fas-L binds to its receptor, it recruits pro–caspase-8 units that are cleaved into active caspase-8 forms, which in turn activate the caspase-3–dependent cell death pathway.64 Various genotoxic agents activate Fas-L expression through the activation of JNK and its prototypical targets of the AP-1 transcription factor family.65-68 Apoptosis of cells subjected to genomic insults can therefore also be mediated by a JNK/AP-1/Fas-L axis. Notably, the events leading to DNA damage–induced Fas-L expression are proposed to occur after a prolonged activation of JNK69. This raised the question of the role of a potential short-term JNK activity, which is perhaps involved in promoting DNA repair early on after DNA damage is detected.69,70 An interesting observation was made in the late 1990s in which cells deficient for the JNK prototypical target c-Fos transcription factor71 were shown to be more sensitive to UV-C radiation–induced apoptosis, and this sensitivity was due to an inefficient DNA repair activity.72 These results are supported by other reports involving members of the Fos-Jun family in DNA repair.73,74 A “ChIP-on-chip” study revealed the promoters binding to c-Jun and ATF2 shortly after the exposure of cells to a genotoxic cisplatin treatment.75 This study showed that genes encoding DNA repair factors are seemingly predominant among the genes up-regulated by c-Jun and ATF2 immediately after genomic insults.74 It is noteworthy that γH2AX is proposed to serve as a docking site for DNA repair machinery and is necessary for DNA repair and cell survival after DNA damage.75,76 JNK activity could therefore contribute to the activation of DNA damage repair factors through the phosphorylation of H2AX on S139 in an early phase after genomic insults. Conversely, sustained JNK activation in cells lacking DNA repair is thought to result in apoptosis.
Besides apoptosis, the lack of DNA damage repair can also lead to cellular senescence. Such permanent arrest of the cell cycle is accompanied by various phenotypes including the expression of β-galactosidase, modifications of the secretome of the cells to a so-called senescence-associated secretory phenotype (SASP), and the maintenance of DNA damage foci in the nucleus termed DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS).77-81 While JNK activity has never been implicated in the formation of DNA-SCARS or the expression of β-galactosidase, it is interesting to note that SASP is considered as an inflammatory-like phenotype and that some cytokines belonging to SASP such as IL-1α, IL-6, or IL-8 overlap with the JNK/AP-1–driven inflammatory secretory phenotype.82-86 Moreover, JNK’s homolog p38 MAPK was shown to be a potent inducer of SASP in normal human fibroblasts, mainly through its capacity to enhance NF-κB transcriptional activity.87 Surprisingly, while JNK’s role in inflammatory phenotypes was described in a variety of cellular contexts,88 there is still no report of JNK activity being involved in SASP induction. In support of this hypothesis, a recent study demonstrated that induction of the JNK inhibitory phosphatase MKP-1 by gluco-corticoids can counterbalance deleterious effects of JNK signaling in mice.89 Glucocorticoids are steroid hormones that signal through the glucocorticoid receptor (GR) and display potent anti-inflammatory effects. Using both GR signaling–deficient mice (GR dim/dim mice) and Mkp1–/– mice, Vandevyver and colleagues89 showed that endogenous glucocorticoids protect the mice from TNF lethal inflammation by up-regulating MKP-1 expression. Moreover, while Jnk1–/– mice displayed no differences in TNF-induced lethality compared to control mice, the Jnk2–/– mice showed better resistance to acute inflammatory stress. The authors concluded that GR signaling counteracts TNF/JNK2-induced inflammation through the up-regulation of MKP-1.89 Another engaging study has nicely echoed this report, linking glucocorticoids to SASP induction in human cells.86 In a screen designed to check for compounds able to repress SASP induction in normal human fibroblasts subjected to X-ray irradiation, Laberge and colleagues86 have shown that the glucocorticoids, corticosterone and cortisol, were able to decrease the secretion of some components of SASP. Notably, both of these glucocorticoids were able to decrease the production of proinflammatory cytokines such as IL-1α, IL-6, and IL-8 through their interaction with the GR.86 Although the authors proposed that GR-mediated NF-κB inhibition is the key mechanism responsible for the decreased secretion of inflammatory cytokines in glucocorticoid-treated DNA- damaged cells, the potential involvement of JNK in these events is an attractive hypothesis that may deserve future exploration.
The activity of the transcription factor p53, a master regulator of cell fate after DNA damage, has also been linked to JNK signaling. p53 is mainly described to play a pleiotropic tumor suppressor role, as its activation by posttranslational modifications can promote DNA repair, cell cycle arrest, apoptosis, and senescence.70, 90-93 Although over a dozen phosphorylation sites have been mapped in the p53 protein sequence, the phosphorylation of serine 15 by ATM is the best described means of activating p53 after DNA damage.92 S15 phosphorylation promotes p53 activation by disrupting the interaction of p53 with its inhibitor MDM2.94,95 Threonine 81 of the p53 sequence is a substrate for JNK and appears to be phosphorylated in a DNA damage–dependent manner.96-98 This modification was shown to stabilize p53 and enhance its transcriptional activity, and it appeared to be crucial for p53 activity after genomic insult.97-100 A recent exhaustive study utilizing Drosophila and mammalian cell line models showed that p53 expression is able to potentiate JNK activity.101 In p53-deficient Drosophila wing imaginal discs irradiated with high doses of γ-rays, JNK activation and apoptosis rates were lower than in the controls. Consistent results were found in p53 knockout MEFs in which the mechanism revealed a binding domain for JNK to p53, where the interaction of JNK and p53 avoids JNK dephosphorylation by MKP-5.101 As JNK’s capacity to phosphorylate c-Jun is preserved even when bound to p53, the authors proposed a model describing that simultaneous activation of JNK and p53 can cooperate to reinforce JNK-induced apoptosis.101 Conversely, p53 binding to JNK was also described to hamper JNK activation, thus protecting the cells from UV-induced apoptosis in a lung carcinoma cell line.102 An explanation for the discrepancies between these 2 studies could stem from the fact that the activity of all 3 jnk gene products was considered as a whole unit in these reports. However, JNK1 and JNK2 are known to mediate opposite effects on p53, with JNK1 proposed to negatively regulate p53, while JNK2 acts as a positive regulator of p53.103
Overall, the results presented here illustrate the complex interactions involving JNKs in the regulation of the cellular response to DNA damage (Figure 1). In particular, no future studies should exclude assessment of the individual proteins arising from the expression of the 3 jnk genes. Moreover, cell type specificities and the existence of a multiphase cellular response with the immediate activation of pathways promoting cell cycle arrest and survival followed by factors responsible for cell cycle arrest, apoptosis, or senescence can lead to a further variety of responses. Transferring these findings to the clinic proves even more difficult, as additional parameters such as the interactions occurring between different cell types intervene in complex systems such as in vivo tumor growth. In the next part, we will examine some results and hypotheses that may prove relevant in linking genomic stress, JNK activity, and cancer.
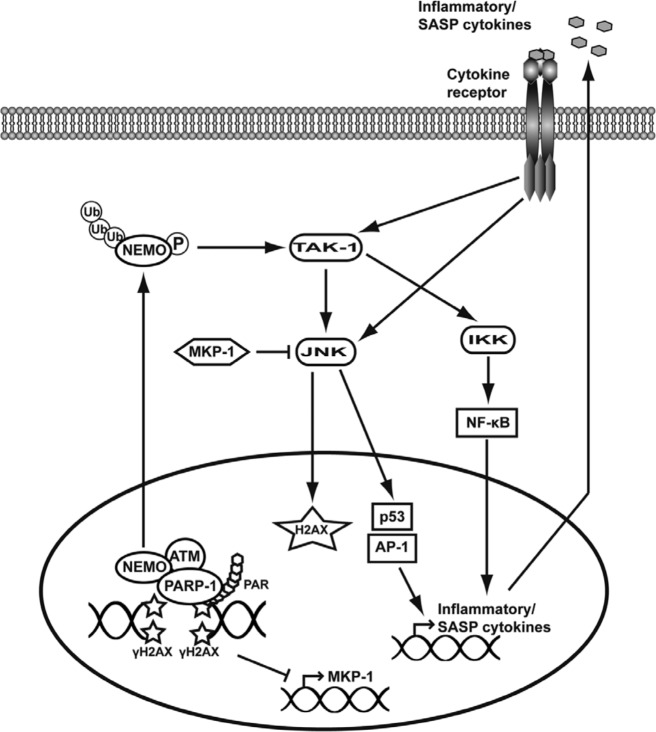
Figure 1.
Schematic representation of the pathways proposed to lead to JNK activation following DNA damage. In response to DNA damage, H2AX phosphorylation, recruitment of PARP-1, and subsequent PAR formation at the sites of DNA breakage drive the assembly of a complex comprising at least ATM, NEMO, and PARP-1. This complex is necessary for NEMO posttranslational-activating modifications, such as sumoylation by PIASγ (not represented here), subsequent phosphorylation by ATM, and ubiquitination by cIAP1 (not represented here). Active NEMO is then exported out of the nucleus, where it activates the kinase TAK1, which in turn phosphorylates IKK and JNK directly. Subsequent activation of NF-κB and JNK targets p53, and the transcription factors of the AP-1 family drive the expression of inflammatory cytokines that are proposed to participate to a feed-forward loop responsible for sustained JNK activation. JNK’s kinase activity on H2AX is also proposed to reinforce a DDR. The general repression of transcription after extensive DNA damage is also proposed to account for the activation of JNK via inhibition of the expression of MKP-1, a phosphatase that negatively regulates JNK.
Potential Implications of DNA Damage–Driven JNK Activation in Cancer
In normal conditions, mice and cells depleted of either one jnk or another do not display massive functional difference, pinpointing redundancies in their functions during development and normal cell growth. However, challenging the same cells or animals with nonphysiological constraints such as tumor-inducing or DNA-damaging agents revealed nonredundant and specific roles for each JNK protein.13,104-106 Most striking are the adverse effects of jnk1 and jnk2 knockouts on skin tumor formation induced by treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA). While jnk2–/– mice are less prone to tumor formation in a TPA skin carcinogenesis protocol, jnk1–/– mice exposed to the same protocol form more tumors than the controls.104,105 However, studies concerning the structure of the skin in these mice have shown that jnk1–/– mice display a thinner epithelial layer than jnk2–/– mice, suggesting that differences in the susceptibility toward TPA treatment may result from structural rather than functional differences in the skin epithelium.107-108 The study of JNK and DNA damage interactions may also lead to interesting discoveries in the field of oncogene-induced tumorigenesis. Indeed, oncogene-induced replicative stress leads to genetic instability and activation of the DDR. Senescence or apoptosis of genetically unstable cells therefore represents a major barrier to the transformation of cells.109 When this barrier is lost, because of a p53 mutation for example, the resulting genomic instability is proposed to drive the transformation of cells.110,111 While JNK activation has been linked to exposure to a variety of DNA-damaging agents, little is known about the capacity of oncogene-induced replicative stress to activate JNK signaling pathways. A recent paper by Chen and colleagues106 described the oncosuppressive role of JNK2 in a breast cancer model of mice expressing the polyoma middle T antigen (PyV MT) that spontaneously developed mammary tumors.112 The PyV MT/jnk2–/– mice developed earlier and more frequent tumors as compared to control mice. Moreover, the combined depletion of jnk2 and PyV MT expression induced higher genomic instability with increased aneuploidy and chromosomal rearrangements. Interestingly, nuclei of the tumor cells also displayed less γH2AX and 53BP1 signaling, suggesting that JNK2 may play a role in counterbalancing PyV MT–induced replicative stress by establishing an appropriate DDR.106 Consistently, the inhibition of JNK2 was shown to induce chromosome segregation defects, resulting in polyploidy in human cervical carcinoma (HeLa) cells and human small-cell lung carcinoma (Calu-1).113 The authors of this study proposed that JNK1 functions in promoting apoptosis while JNK2 rather regulates progression in the cell cycle.113-115 One could argue, however, that the possible involvement of JKN2 in DNA repair has not been addressed in these studies and hypothesize that JNK2 may be involved more broadly in the maintenance of genome homeostasis. JNK2 disruption would therefore lead to unresolved DNA damage without cell cycle arrest and finally result in the propagation of genomic instability and cancer promotion. Consistent with this idea, the inhibition of basal JNK activity in established breast cancer cell lines was shown to lead to cell cycle aberrations and endoreduplication.116 More recently, persistent DNA damage induced either by chronic treatment with a DNA-damaging drug or by destabilization of telomeres was shown to lead to similar phenotypes in p53-deficient cells, with the occurrence of endomitosis and tetraploidization events.117 Taken together, these results allow us to postulate that the disruption of JNK2 activity could participate in DNA damage–induced genetic instability and oncogenesis.
Another potential link between JNK signaling, DNA damage, and cancer was discovered in a study showing that αV integrin is overexpressed in tissue samples from patients with radioresistant nasopharyngeal carcinoma (NPC).118 Using a NPC cell line cultivated as spheroids, the authors showed that the cell radioresistance is mediated by αV integrin–induced activation of JNK and that JNK inhibition restores the radiosensitivity of the cells.118 Therefore, studying the role of JNK activity in response to DNA damage could lead to a better understanding of the processes governing the response of cancer cells to DNA-damaging agents.
Further extensive research is required in order to clearly decipher the links existing between DNA damage, JNK signaling, and cancer. In particular, the role of individual JNK isoforms has to be addressed in cells challenged with DNA damage caused by the exposure to DNA-damaging agents such as γ-irradiation or oncogene-induced genomic instability.
Acknowledgments
The authors thank Dr. Scott Parks for editorial assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Institut National pour la Santé et la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS) and the Centre Scientifique de Monaco, the National Institute of Cancer (INCA), the Association for Cancer Research (ARC), the Fondation de France, the Fondation pour la Recherche Médicale (FRM), the “Association pour la Recherche sur les Tumeurs du Rein (ARTuR)”, and ROCHE France.
References
- 1. Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135-48 [DOI] [PubMed] [Google Scholar]
- 2. Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025-37 [DOI] [PubMed] [Google Scholar]
- 3. Chen YR, Meyer CF, Tan TH. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J Biol Chem. 1996;271:631-4 [DOI] [PubMed] [Google Scholar]
- 4. Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation: duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929-36 [DOI] [PubMed] [Google Scholar]
- 5. Liu ZG, Baskaran R, Lea-Chou ET, et al. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273-6 [DOI] [PubMed] [Google Scholar]
- 6. Kharbanda S, Bharti A, Pei D, et al. The stress response to ionizing radiation involoves c-Abl-dependent phosphorylation of SHPTP1. Proc Natl Acad Sci U S A. 1996;93:6898-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asur R, Balasubramaniam M, Marples B, Thomas RA, Tucker JD. Involvement of MAPK proteins in bystander effects induced by chemicals and ionizing radiation. Mutat Res. 2010; 686:15-29 [DOI] [PubMed] [Google Scholar]
- 8. Belyaev IY. Radiation-induced DNA repair foci: spatio-temporal aspects of formation, application for assessment of radiosensitivity and biological dosimetry. Mutat Res. 2010;704:132-41 [DOI] [PubMed] [Google Scholar]
- 9. Thompson LH. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: the molecular choreography. Mutat Res. 2012;751:158-246 [DOI] [PubMed] [Google Scholar]
- 10. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695-708 [DOI] [PubMed] [Google Scholar]
- 11. Herrlich P, Karin M, Weiss C. Supreme EnLIGHTenment: damage recognition and signaling in the mammalian UV response. Mol Cell. 2008;29:279-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCool KW, Miyamoto S. DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol Rev. 2012;246:311-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92-103 [DOI] [PubMed] [Google Scholar]
- 14. van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19:207-17 [DOI] [PubMed] [Google Scholar]
- 15. Krishnakumar R, Kraus WL. PARP-1 regulates chromatin structure and transcription through a KDM5B-dependent pathway. Mol Cell. 2010;39:736-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lukas C, Savic V, Bekker-Jensen S, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243-53 [DOI] [PubMed] [Google Scholar]
- 17. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499-506 [DOI] [PubMed] [Google Scholar]
- 18. Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou DM, Adamson B, Dephoure NE, et al. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci U S A. 2010;107:18475-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lobrich M, Shibata A, Beucher A, et al. gammaH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2010;9:662-9 [DOI] [PubMed] [Google Scholar]
- 21. Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol. 2001;13:225-31 [DOI] [PubMed] [Google Scholar]
- 22. Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131-42 [DOI] [PubMed] [Google Scholar]
- 23. Wilson PF, Nham PB, Urbin SS, et al. Inter-individual variation in DNA double-strand break repair in human fibroblasts before and after exposure to low doses of ionizing radiation. Mutat Res. 2010;683:91-7 [DOI] [PubMed] [Google Scholar]
- 24. Kozlov SV, Graham ME, Peng C, et al. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 2006;25:3504-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. So S, Davis AJ, Chen DJ. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol. 2009;187:977-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. FitzGerald JE, Grenon M, Lowndes NF. 53BP1: function and mechanisms of focal recruitment. Biochem Soc Trans. 2009;37:897-904 [DOI] [PubMed] [Google Scholar]
- 27. O’Donovan PJ, Livingston DM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 2010;31:961-7 [DOI] [PubMed] [Google Scholar]
- 28. Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442-5 [DOI] [PubMed] [Google Scholar]
- 29. Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994;102:422-7 [DOI] [PubMed] [Google Scholar]
- 30. Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006;13:773-84 [DOI] [PubMed] [Google Scholar]
- 31. Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95:13012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bender K, Gottlicher M, Whiteside S, Rahmsdorf HJ, Herrlich P. Sequential DNA damage- independent and -dependent activation of NF-kappaB by UV. EMBO J. 1998;17:5170-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141-6 [DOI] [PubMed] [Google Scholar]
- 34. Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol. 2006;8:986-93 [DOI] [PubMed] [Google Scholar]
- 35. Wu ZH, Wong ET, Shi Y, et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell. 2010;40:75-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyamoto S. Nuclear initiated NF-kappaB signaling: NEMO and ATM take center stage. Cell Res. 2011;21:116-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piret B, Schoonbroodt S, Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999;18:2261-71 [DOI] [PubMed] [Google Scholar]
- 38. Li N, Banin S, Ouyang H, et al. ATM is required for IkappaB kinase (IKKk) activation in response to DNA double strand breaks. J Biol Chem. 2001;276:8898-903 [DOI] [PubMed] [Google Scholar]
- 39. Huang X, Traganos F, Darzynkiewicz Z. DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle. 2003;2:614-9 [PubMed] [Google Scholar]
- 40. Stilmann M, Hinz M, Arslan SC, et al. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IkappaB kinase activation. Mol Cell. 2009;36:365-78 [DOI] [PubMed] [Google Scholar]
- 41. Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087-95 [DOI] [PubMed] [Google Scholar]
- 42. Yang Y, Xia F, Hermance N, et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol. 2011;31:2774-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hinz M, Stilmann M, Arslan SC, et al. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell. 2010;40:63-74 [DOI] [PubMed] [Google Scholar]
- 44. Tsuchiya Y, Asano T, Nakayama K, et al. Nuclear IKKbeta is an adaptor protein for IkappaBalpha ubiquitination and degradation in UV-induced NF-kappaB activation. Mol Cell. 2010;39:570-82 [DOI] [PubMed] [Google Scholar]
- 45. O’Dea EL, Kearns JD, Hoffmann A. UV as an amplifier rather than inducer of NF-kappaB activity. Mol Cell. 2008;30:632-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297-309 [DOI] [PubMed] [Google Scholar]
- 48. Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689-92 [DOI] [PubMed] [Google Scholar]
- 49. Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383-92 [DOI] [PubMed] [Google Scholar]
- 51. Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012;24:835-45 [DOI] [PubMed] [Google Scholar]
- 52. Shim JH, Xiao C, Paschal AE, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu Y, Ge N, Xie M, et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem. 2008;283:24497-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci U S A. 1997;94:9792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917-23 [DOI] [PubMed] [Google Scholar]
- 56. Bermudez O, Pages G, Gimond C. The dual-specificity MAP kinase phosphatases: critical roles in development and cancer. Am J Physiol Cell Physiol. 2010;299:C189-202 [DOI] [PubMed] [Google Scholar]
- 57. Rosini P, De Chiara G, Bonini P, et al. Nerve growth factor-dependent survival of CESS B cell line is mediated by increased expression and decreased degradation of MAPK phosphatase 1. J Biol Chem. 2004;279:14016-23 [DOI] [PubMed] [Google Scholar]
- 58. Hamdi M, Kool J, Cornelissen-Steijger P, et al. DNA damage in transcribed genes induces apoptosis via the JNK pathway and the JNK-phosphatase MKP-1. Oncogene. 2005;24:7135-44 [DOI] [PubMed] [Google Scholar]
- 59. Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870-4 [DOI] [PubMed] [Google Scholar]
- 61. Papadakis ES, Finegan KG, Wang X, et al. The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 2006;580:1320-6 [DOI] [PubMed] [Google Scholar]
- 62. Lawan A, Al-Harthi S, Cadalbert L, et al. Deletion of the dual specific phosphatase-4 (DUSP-4) gene reveals an essential non-redundant role for MAP kinase phosphatase-2 (MKP-2) in proliferation and cell survival. J Biol Chem. 2011;286:12933-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Feraudy S, Revet I, Bezrookove V, Feeney L, Cleaver JE. A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci U S A. 2010;107:6870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nagata S. Fas-mediated apoptosis. Adv Exp Med Biol. 1996;406:119-24 [DOI] [PubMed] [Google Scholar]
- 65. Kasibhatla S, Brunner T, Genestier L, et al. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543-51 [DOI] [PubMed] [Google Scholar]
- 66. Brozovic A, Fritz G, Christmann M, et al. Long-term activation of SAPK/JNK, p38 kinase and fas-L expression by cisplatin is attenuated in human carcinoma cells that acquired drug resistance. Int J Cancer. 2004;112:974-85 [DOI] [PubMed] [Google Scholar]
- 67. Lauricella M, Emanuele S, D’Anneo A, et al. JNK and AP-1 mediate apoptosis induced by bortezomib in HepG2 cells via FasL/caspase-8 and mitochondria-dependent pathways. Apoptosis. 2006;11:607-25 [DOI] [PubMed] [Google Scholar]
- 68. Gupta P, Dixit D, Sen E. Oncrasin targets the JNK-NF-kappaB axis to sensitize glioma cells to TNFalpha-induced apoptosis. Carcinogenesis. 2013;34:388-96 [DOI] [PubMed] [Google Scholar]
- 69. Mansouri A, Ridgway LD, Korapati AL, et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245-56 [DOI] [PubMed] [Google Scholar]
- 70. Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440-50 [DOI] [PubMed] [Google Scholar]
- 71. Kaina B, Haas S, Kappes H. A general role for c-Fos in cellular protection against DNA-damaging carcinogens and cytostatic drugs. Cancer Res. 1997;57:2721-31 [PubMed] [Google Scholar]
- 72. Christmann M, Tomicic MT, Aasland D, Kaina B. A role for UV-light-induced c-Fos: stimulation of nucleotide excision repair and protection against sustained JNK activation and apoptosis. Carcinogenesis. 2007;28:183-90 [DOI] [PubMed] [Google Scholar]
- 73. MacLaren A, Black EJ, Clark W, Gillespie DA. c-Jun-deficient cells undergo premature senescence as a result of spontaneous DNA damage accumulation. Mol Cell Biol. 2004;24:9006-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayakawa J, Mittal S, Wang Y, et al. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol Cell. 2004;16:521-35 [DOI] [PubMed] [Google Scholar]
- 75. Bassing CH, Chua KF, Sekiguchi J, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci U S A. 2002;99:8173-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Celeste A, Difilippantonio S, Difilippantonio MJ, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187-95 [DOI] [PubMed] [Google Scholar]
- 79. Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Coppe JP, Patil CK, Rodier F, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rodier F, Munoz DP, Teachenor R, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44 [DOI] [PubMed] [Google Scholar]
- 83. Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537-49 [DOI] [PubMed] [Google Scholar]
- 84. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Laberge RM, Zhou L, Sarantos MR, et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11:569-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vandevyver S, Dejager L, Van Bogaert T, et al. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J Clin Invest. 2012;122:2130-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Smith ML, Chen IT, Zhan Q, O’Connor PM, Fornace AJ., Jr Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene. 1995;10:1053-9 [PubMed] [Google Scholar]
- 91. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323-31 [DOI] [PubMed] [Google Scholar]
- 92. Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027-36 [DOI] [PubMed] [Google Scholar]
- 94. Tao W, Levine AJ. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci U S A. 1999;96:6937-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tao W, Levine AJ. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci U S A. 1999;96:3077-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Milne DM, Campbell LE, Campbell DG, Meek DW. p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J Biol Chem. 1995;270:5511-8 [DOI] [PubMed] [Google Scholar]
- 97. Buschmann T, Potapova O, Bar-Shira A, et al. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol Cell Biol. 2001;21:2743-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci U S A. 1998;95:10541-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fujita D, Tanabe A, Sekijima T, et al. Role of extracellular signal-regulated kinase and AKT cascades in regulating hypoxia-induced angiogenic factors produced by a trophoblast-derived cell line. J Endocrinol. 2010;206:131-40 [DOI] [PubMed] [Google Scholar]
- 101. Gowda PS, Zhou F, Chadwell LV, McEwen DG. p53 binding prevents phosphatase-mediated inactivation of diphosphorylated c-Jun N- terminal kinase. J Biol Chem. 2012;287:17554-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lo PK, Huang SZ, Chen HC, Wang FF. The prosurvival activity of p53 protects cells from UV-induced apoptosis by inhibiting c-Jun NH2-terminal kinase activity and mitochondrial death signaling. Cancer Res. 2004;64:8736-45 [DOI] [PubMed] [Google Scholar]
- 103. Tafolla E, Wang S, Wong B, Leong J, Kapila YL. JNK1 and JNK2 oppositely regulate p53 in signaling linked to apoptosis triggered by an altered fibronectin matrix: JNK links FAK and p53. J Biol Chem. 2005;280:19992-9 [DOI] [PubMed] [Google Scholar]
- 104. Chen N, Nomura M, She QB, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908-12 [PubMed] [Google Scholar]
- 105. She QB, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343-8 [PubMed] [Google Scholar]
- 106. Chen P, O’Neal JF, Ebelt ND, et al. Jnk2 effects on tumor development, genetic instability and replicative stress in an oncogene-driven mouse mammary tumor model. PLoS One. 2010;5:e10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Weston CR, Wong A, Hall JP, et al. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proc Natl Acad Sci U S A. 2004;101:14114-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801-9 [DOI] [PubMed] [Google Scholar]
- 110. Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043-7 [DOI] [PubMed] [Google Scholar]
- 111. Duelli DM, Padilla-Nash HM, Berman D, et al. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr Biol. 2007;17:431-7 [DOI] [PubMed] [Google Scholar]
- 112. Siegel PM, Hardy WR, Muller WJ. Mammary gland neoplasia: insights from transgenic mouse models. Bioessays. 2000;22:554-63 [DOI] [PubMed] [Google Scholar]
- 113. MacCorkle RA, Tan TH. Inhibition of JNK2 disrupts anaphase and produces aneuploidy in mammalian cells. J Biol Chem. 2004;279:40112-21 [DOI] [PubMed] [Google Scholar]
- 114. Butterfield L, Storey B, Maas L, Heasley LE. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J Biol Chem. 1997;272:10110-6 [DOI] [PubMed] [Google Scholar]
- 115. Tseng SH, Wang CH, Lin SM, et al. Activation of c-Jun N-terminal kinase 1 and caspase 3 in the tamoxifen-induced apoptosis of rat glioma cells. J Cancer Res Clin Oncol. 2004;130:285-93 [DOI] [PubMed] [Google Scholar]
- 116. Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596-604 [DOI] [PubMed] [Google Scholar]
- 117. Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ou J, Luan W, Deng J, Sa R, Liang H. alphaV integrin induces multicellular radioresistance in human nasopharyngeal carcinoma via activating SAPK/JNK pathway. PLoS One. 2012;7: e38737. [DOI] [PMC free article] [PubMed] [Google Scholar]