Abstract
Mitogen-activated protein kinases (MAPKs) mediate a wide variety of cellular behaviors in response to extracellular stimuli. One of the main subgroups, the p38 MAP kinases, has been implicated in a wide range of complex biologic processes, such as cell proliferation, cell differentiation, cell death, cell migration, and invasion. Dysregulation of p38 MAPK levels in patients are associated with advanced stages and short survival in cancer patients (e.g., prostate, breast, bladder, liver, and lung cancer). p38 MAPK plays a dual role as a regulator of cell death, and it can either mediate cell survival or cell death depending not only on the type of stimulus but also in a cell type specific manner. In addition to modulating cell survival, an essential role of p38 MAPK in modulation of cell migration and invasion offers a distinct opportunity to target this pathway with respect to tumor metastasis. The specific function of p38 MAPK appears to depend not only on the cell type but also on the stimuli and/or the isoform that is activated. p38 MAPK signaling pathway is activated in response to diverse stimuli and mediates its function by components downstream of p38. Extrapolation of the knowledge gained from laboratory findings is essential to address the clinical significance of p38 MAPK signaling pathways. The goal of this review is to provide an overview on recent progress made in defining the functions of p38 MAPK pathways with respect to solid tumor biology and generate testable hypothesis with respect to the role of p38 MAPK as an attractive target for intervention of solid tumors.
Keywords: mitogen-activated protein kinase (MAPK), p38 signaling pathway, solid tumors
Background
Mitogen-activated protein kinase (MAPK) signal transduction pathways are evolutionarily conserved among eukaryotes and have been implicated to play key roles in a number of biological processes, including cell growth, differentiation, apoptosis, inflammation, and responses to environmental stresses.1,2 They are typically organized in 3-tiered architecture consisting of a MAPK, a MAPK activator (MAPK kinase), and a MAPKK activator (MAPKK kinase). The MAPK pathways can be regulated at multiple levels as well as via multiple mechanisms, of which the regulation of mitogen-activated protein kinase kinase kinase (MAPKKK/MAP3K) has been proved to be the most challenging due to the great diversity and versatility between different modules at this level. The complex array of growth factors and other ligands that can initiate intracellular cell signaling requires a very high level of coordination among the different proteins involved. The cascade events can thus amplify the incoming signal and generate a threshold subject to multiple activation cascades. The transduction of exogenous signals is achieved through sequential phosphorylation and activation of the components in the individual cascade leading to the activation of certain transcription factors and other kinases.3,4 The specificity is less clearly defined for elements upstream of the MAPKK modular level because the MAPKKK are highly promiscuous and can interact with and activate a number of downstream components. When stimulated, MAPKs phosphorylate their specific substrates at serine and/or threonine residues; however, substrate selectivity is often conferred by specific interaction motifs located on physiological substrates, including phospholipases, transcription factors, and cytoskeletal proteins, which can either positively or negatively regulate the substrate or ultimately regulate the entire signaling pathway.5-7 Whereas many kinases gain their activation via autophosphorylation, yeast MAPK Hog1 is usually phosphorylated by specific kinases MAPKK Pbs2, via an autophosphorylation activity that is induced by osmotic pressure,8 suggesting a key aspect to understand how adaptation to external stimuli is accomplished.
Signaling by MAPKs
There are 4 major MAP kinase (MAPK) pathways: the extracellular-signal regulated kinase (ERK, also known as p42/44 MAP kinase), c-jun N-terminal kinase (JNK, also known as stress activated protein kinase-1 [SAPK1]), Big MAP kinase (BMK, also known as ERK5), and p38 MAPK (also known as SAPK2/RK). In general, ERK1 and ERK2 are key transducers of proliferation signals and are often activated by mitogens. BMK is activated by various stimuli such as oxidative stress and growth factors. In contrast, SAPKs/JNKs and p38 are poorly activated by mitogens but strongly activated in cells in response to stress signals, growth factors, and inflammatory cytokines.9,10 The transfer of signal between a MAPK and its immediate upstream kinase (MAPKK) is highly specific; with p42/p44 (ERK) MAP kinases being phosphorylated exclusively by MAP/ERK kinase (MEK) 1 and 2; p38 MAP kinase is selectively activated by MAP kinase kinases (MKK) 3 and 6, while JNK is activated by MKK7 and MKK4. In most conditions though, MKK4 can sometimes activate p38 MAP kinase when overexpressed.11 MKK6 can phosphorylate the 4 p38 MAPK family members, while MKK3 phosphorylates p38α, p38γ, and p38δ but not p38β. Both will also phosphorylate JNK isoforms.12
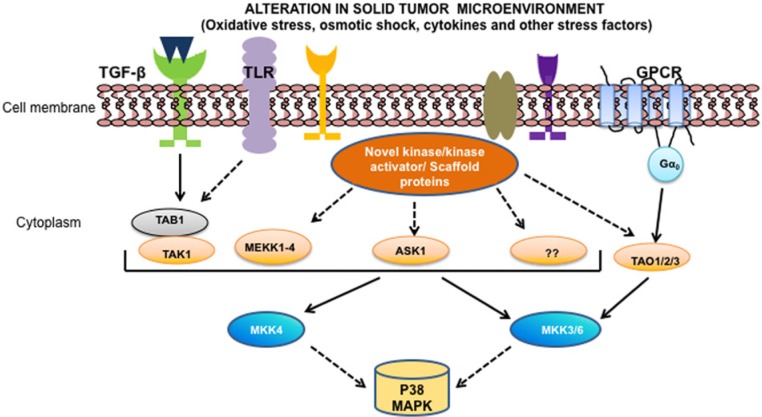
Several MAP3Ks have been shown to trigger p38 MAPK activation (Figure 1); they include ASK1 (apoptosis signal-regulating kinase 1), DLK1 (dual-leucinezipper-bearing kinase 1), TAO (thousand-and-one amino acid) 1 and 2, TPL2 (tumor progression loci 2), MLK3 (mixed lineage kinase 3), MEKK3 (MEK kinase 3) and MEKK4, and ZAK1 (leucine zipper and sterile-α motif kinase 1).12 The diversity of MAP3Ks and their regulatory mechanisms may provide the ability to respond to a wide range of stimuli and to integrate p38 MAPK activation with other signaling pathways. It should be noted that some MAP3Ks that trigger p38 MAPK activation can also activate the JNK pathway.12 The mechanism of p38 MAPK activation is mediated by dual phosphorylation at the Thr-Gly-Tyr motif and is generally activated by environmental stresses, including heat, osmotic and oxidative stresses, as well as inflammatory cytokines and transforming growth factor-β (TGF-β) signaling.13 Recent reports suggest that GPCR (G protein-coupled receptor) can also regulate cellular growth and differentiation by controlling p38 MAPK activity. GPCR stimulation results in the activation of heterotrimeric G protein that transmits the signal to TAOs, which then activate p38 MAPK cascades.14,15 The upstream kinases acting on p38 include MKKs 4, and 3/6 has preferential effects on different p38 isoforms,16 which are in turn activated by MEKKs, MLKs, and ASK1. GTPases are responsible for the transmission of stress stimuli to the MAPKKKs of this pathway, including the Racs, Rhos, and Cdc42s. Recently, a major concept change for MAPK regulation was developed for p38α. The p38α enzyme is activated by TAK1-TAB1 kinase enzyme system,17 but TAB1 is not a MKK and TAB1 appears to be an adaptor or scaffolding protein and has no known catalytic activity. This is the first demonstration that another mechanism exists for the regulation of MAPKs in addition to the MKKK-MKK-MAPK regulatory module. This important observation indicates that other adaptor proteins should be scrutinized for potential roles in regulating MAPK activity. Transforming growth factor-β (TGF-β) activated kinase 1 (TAK1) belongs to the MAPKKK family. This serine/threonine kinase is a key intermediate in inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1)18,19 as well as TGF-β20 mediated signaling pathways. In the absence of TGF-β stimulation, TAK1 associates with TGF-β receptor through complex formation with TAB2 and TRAF6. On TGF-β stimulation, autopolyubiquitination of TRAF6 leads to polyubiquitination (Ub) of TAK1, by which TAK1 is released from the receptor complexes. The released TAK1 interacts with TAB1, which in turn induces autophosphorylation of TAK1 for activation.21-23 Activated TAK1 has the capacity to stimulate its downstream MAPK and NF-κB-inducing kinase-IκB kinase cascades.24 The former activates c-jun N-terminal kinase (JNK) and p38 MAPK while the latter activates NF-κB.18,25,26
Figure 1.
Schematic representation of the activation of p38 MAPK. Numerous physical and chemical stresses, including oxidative stress and osmotic shock, cytokines, and growth factors such as TGF-β (transforming growth factor-β) activate p38 MAPKs through complex kinase cascades signaling pathways. The mechanism of p38 MAPK activation is mediated by dual phosphorylation (at Thr-Pro-Tyr motif) by MAPK kinase 4 (MKK4) and MKK3/6. Upstream of the MKKs are their MAPKKKs, which include MEKKs 1-4, a member of the mixed-lineage kinases (MLK3) and ASK-1. In turn, these are activated by cellular intermediates, like GTPases, kinase activator, or receptor adapter proteins, among others, which transmit the stimulus to the kinase cascades. TGF-β also activates Smad-independent signaling pathways, including TAK1, which in turn triggers the activation of downstream p38 MAPK signaling cascades. GPCR (G protein-coupled receptor) stimulation results in the activation of heterotrimeric G protein that transmits the signal to TAOs (thousand-and-one amino acid), which then activate p38 MAPK cascades. Once activated, the different p38MAPKs either phosphorylate cytoplasmic targets or translocate into the nucleus leading to the regulation of transcription factors involved in cellular responses.
p38 MAPK modulates cellular functions by activation of a number of downstream targets (Figure 2). The different p38 isoforms defined to date (p38α, p38β, p38γ, p38δ) vary, based on their substrate specificity. The α and β isoforms of p38 are responsible for the activation of heat shock proteins (hsps) 25, 27, and the MAPK-activated protein (MAPKAP)-2 or MK2. The γ and δ isoforms of p38 activate ATF2.27 Other transcription factors affected by the p38 family include STAT1, Max/Myc complexes, MEF-2, Elk-1, and CREB through the activation of MSK1/2. Other substrates of the p38 signaling pathway include Pax6, ETS1, PRAK, MK3, RARα, HMGN1, Histone H3, ER8, Activator Protein 1 (AP-1), ATF1, NF-κB, and CHOP for regulation of gene expression, affecting cell motility, transcription, and chromatin remodeling.28-31
Figure 2.
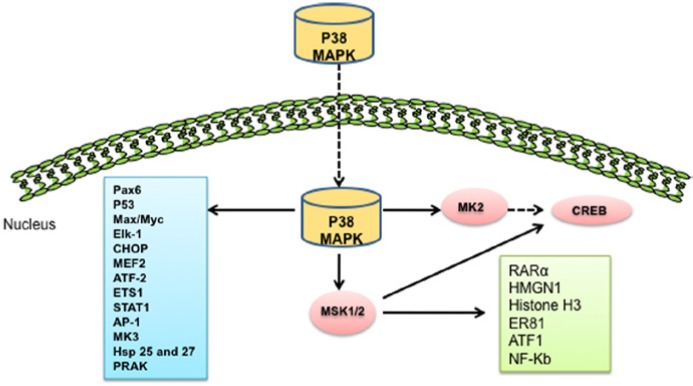
Schematic diagram of the downstream targets of p38 MAPK. Activated p38 MAPK regulates downstream targets including several kinases and transcription factors. These kinases include PRAK (p38-related/activated protein kinase), MSK1/2 (mitogen- and stress-activated protein kinase-1/2), and MK2 (MAPK-activated protein kinase 2). Some important transcription factors phosphorylated by p38 MAPK include tumor suppressor protein p53, CHOP (C/EBP-homologous protein), STAT1 (signal transducers and activators of transcription-1), CREB (cAMP response element-binding protein), NF-κB, Max/Myc complexes, and so on. Other substrates of the p38 signaling pathway include Pax6, Elk-1, MEF2, ATF-2, Hsp 25 and 27, ETS1, MK3, RARα, HMGN1, Histone H3, ER81, Activator Protein 1 (AP-1), and ATF1 for regulation of gene expression, affecting cell motility, transcription, and chromatin remodeling.
p38 MAPK Signaling
The p38 MAPK family consists of 4 isoforms—p38-MAPKα, p38-MAPKβ, p38-MAPKγ, and p38-MAPKδ—each encoded by a separate gene with differences in substrate specificity and tissue distribution (Table 1).32 The most common form, p38α, was first isolated as a 38-kDa protein rapidly tyrosine phosphorylated in response to lipopolysaccharide (LPS) stimulation. Of these, p38α and p38β are ubiquitously expressed while p38γ and p38δ are differentially expressed depending on tissue type.33 The p38 MAPK pathways are activated in response to various environmental and cellular stresses, inflammation, and other signals.34 The 4 vertebrate isoforms of p38 are characterized by the presence of the conserved Thr-Gly-Tyr (TGY) phosphorylation motif in their activation loop.35 Sequence comparisons have shown that each p38 isoform shares ~60% identity within the p38 group but only 40% to 45% to the other 3 MAP kinase family members. This motif is phosphorylated by MEK3 and MEK6, which themselves are activated by various MAPKKKs that are induced by physical and chemical stresses, such as oxidative stress, hypoxia, X-ray and UV irradiation, and cytokines.36 In some instances, p38 can also be activated by MEK4, a kinase that is better known as an activator of JNK. Once activated, p38 proteins can translocate from the cytosol to the nucleus where they phosphorylate serine/threonine residues of their many substrates. In addition to its role in stress responses, recent studies have suggested a role for p38 MAPK in mediating pathways leading to cell apoptosis and growth inhibitory signals.37 However, other studies with malignant cells have shown that activation of p38-MAPK signaling pathways may produce just the opposite effect: anti-apoptotic and proliferative effects and various cell survival pathways.38 These studies suggest that tumor cell growth might be regulated by coordination between cell proliferation and apoptosis. The inability of malignant cells to respond to p38 MAPK signaling toward apoptosis could possibly be due to alterations in regulators of apoptosis that help in cell survival.39 The emerging views on how these pathways might affect human cancers, as well as evidence from human genetic studies, are discussed below and are summarized in Table 2.
Table 1.
Phenotypes of p38 Mitogen-Activated Protein Kinase Knockout Mice.
| MAPK | Distribution by Immune orInflammatory Cellsa | Phenotype | Disease and Cancer Model | References |
|---|---|---|---|---|
| p38α | All cells | Placental defect, death by E12.5 | Not applicable | 260, 261 |
| p38β | T cells | No phenotype | Not determined | 249 |
| p38γ | — | No phenotype | Not determined | 262 |
| p38δ | Tcells, macrophages/monocytes and neutrophils | Impaired insulin secretion and survival of pancreatic β-cells | Diabetes | 263 |
| Skin cancer (DMBA and TPA) and lung cancer (KrasG12V) induced | 264 |
Tissue distribution as described in Hale et al.236 p38γ is not expressed by immune or inflammatory cells.
Table 2.
Role of p38 MAPK in Human Cancers.
p38 MAPK Pathways in Human Cancer
Several studies have suggested that p38 MAPK plays an important role in prostate cancer,40-42 breast cancer,43,44 bladder cancer,45,46 liver cancer,47 lung cancer,48 transformed follicular lymphoma,49,50 and leukemia.51 Therefore, the relevance of the p38 MAPK pathway in the context of tumor formation has yet to be investigated. The activation of p38 MAPKs has been reported to contribute to the epithelial-mesenchymal transition (EMT) of cells in the primary tumor, to the acquisition of invasion and migrating capabilities, and to the extravasation of migrating tumor cells.52,53 In contrast, p38 MAPK inhibition has been correlated with the resistance to anoikis, which allows circulating cancer cells to survive.54 Tumor cell dormancy has been associated with high p38 MAPK activity in combination with low activity of the ERK1/2 pathway. Analysis of the phenotype of mice disrupted in both the MEK3 and MEK6 genes and the p38α gene has led to the suggestion that p38 can function as a tumor suppressor. The transforming potential of oncogenes is increased in fibroblasts from these animals as well as their tumorigenic potential in nude mice.55-58 Suppression of p38 function also plays a critical role in Ras-induced transformation.59,60 The tumor suppressive effects of p38 are involved in both the activation of p53 and in p53-induced apoptosis and acts as negative regulator of cell cycle progression.57,61-63 p38 is also activated by oncogenic stresses and plays an important role in Ras-induced senescence in mouse embryo fibroblasts.64 Overall, it suggests that decreasing p38 activity plays an important role in cancer.
The significance of p38 MAPKs in controlling cellular responses to the environment and in regulating gene expression, cell growth, and apoptosis has made them a priority for research related to many human diseases.65 Therefore, p38 MAPK signaling pathways appear to be a distinguished molecular feature as molecular targets for drug development, and inhibitors of MAPKs will undoubtedly be one of the next groups of drugs developed for the treatment of different solid tumors of the prostate, breast, bladder, liver, and lungs.
p38 MAP Kinase and the Prostate
Prostate cancer is the second-leading cause of cancer deaths in men, after lung and bronchial cancer, according to the National Cancer Institute. Diseases of the prostate can vary from benign enlargement (benign prostatic hyperplasia [BPH]) causing discomfort to prostatic intraepithelial neoplasia and prostate carcinoma, which are a tremendous source of morbidity and mortality in aging males.66 Most of the prostate cancer deaths are due to the emergence of an androgen-resistant phenotype of prostate cancer either by certain co-activators or through mitogen-activated cell signaling activities,67,68 which lead to the overexpression of anti-apoptotic genes and survival of the cancer cells. Unfortunately, treatment options for these androgen-resistant prostate cancer patients are few and generally ineffective. These facts underline the need to develop new therapies that will improve outlook for hormone-independent prostate cancer.
The effects of various manipulations, both genetic and chemical, including growth factors, chemical modifiers, and androgens on prostatic cells, have been described so far. Despite these studies, the structure and function of the MAP kinase pathways in prostate are far from being clearly understood. In normal human prostate tissue, p38 MAP kinase protein is present in the basal cells and epithelial cells of the prostate gland, but Magi-Galluzzi et al. show it to be absent in the epithelial layer.69 While likely present, it is not normally activated in epithelial tissue samples that have been studied, but has stronger activity in the prostatic stroma similar to ERK. Epithelial p38 MAP kinase can become active in situations of neoplasia and benign hypertrophy of the prostate gland.70 In a study using TRAMP mice, strong epithelial p38 MAP kinase activation was shown to be present in intraepithelial neoplasia and well-differentiated tumors but was probably lost in poorly differentiated tumors.71 Similar to JNK, p38 MAP kinase is a kinase primarily activated by external stresses, some of which are listed in Table 3. Many growth and endocrine factors are able to activate p38 MAP kinase, including FGF-1 and -2, keratinocyte-derived growth factor (KGF), IL-6, heparin binding epidermal growth factor (HB-EGF), ATP, vitamin D, and Neu differentiation factor (NDF).72-76 Other factors that provoke stress response (phorbol-12-myristate-13-acetate [PMA], carbachol, osmotic shock, UV light, and anisomycin)77 are also known to cause p38 activation. Most of the data regarding the effect of these inducers were obtained in studies using cell lines (mostly DU145 and LNCaP) but the PC3 cells are conspicuously absent from these studies. AT3 cells have been used to show drug resistance in prostate cancer due to the inhibition of p38 together with the activation of ERK and JNK by Id-1.78 Response of p38 MAP kinase to various hormones can range in time from several minutes to several hours, which suggests indirect inducible activation of p38 MAPK.
Table 3.
p38 MAP Kinase Activating Agents in Prostatic Cells.
| Agent | Cell Line | Apoptosis | References |
|---|---|---|---|
| FGF-1 | DU145 | ? | 74 |
| FGF-2 | DU145 | ? | 74 |
| Keratinocyte growth factor (FGF-7) | DU145 | ? | 74 |
| IL-6 | LNCaP | ? | 72 |
| TGF-β | PC3 | + | 94 |
| Adenosine triphosphate | 1E8, 2B4 | ? | 89 |
| Neu differentiation factor (NDF)/Heregulin | LNCaP | ? | 76 |
| Vitamin D | LNCaP | ? | 268 |
| Selenite | DU145 | ? | 269 |
| Arsenic trioxide | DU145, PC3 | − | 270 |
| PEITC | PC3 | − | 271 |
| Tyrphostin AGE82 | LNCaP | + | 272 |
| FTY720 | DU145 | − | 91 |
| Phorbol esters | LNCaP | + | 83 |
| Sodium butyrate | DU145 | + | 273 |
| IL-1 | Patient samples | − | 80 |
| TNF-α | LNCaP | − | 274 |
Protein Signaling
Intracellular signaling prior to the p38 MAP kinase module appears to be complex and incompletely described. Many of the factors that have not been shown to act in other MAPK pathways like IL-1 and TNF-α have been shown to activate the p38 pathway.79,80 Activation of p38 via IL-1 is through MEK-6 and has been attributed to cell proliferation. Activation of JNK via TNF-α is mediated by MEK-4 and can cause apoptosis in prostate carcinoma,79 but the pathway can be diverted to cell proliferation via the activation of p38 by MEK-4. Protein kinase C is able to activate p38 MAP kinase, although perhaps indirectly.10 The JAK/STAT family of proteins also appears to be able to activate p38 MAP kinase while PAR4 loss causes impairment of p38 MAP kinase phosphorylation.81
Results of p38 Activation
Metastatic cancer of the prostate occurs through the concerted proliferation, migration, adhesion, and differentiation of normal prostate cells. These activities are brought about by many intrinsic growth factors like epidermal-derived growth factor (EGF), transforming growth factor (TGF)-α, and insulin-like growth factor (IGF).82 The cell surface receptors for these factors, on binding their ligands, induce intrinsic kinase activities. The kinases subsequently initiate signal transduction cascades including Ras/Raf/ MAPK, phosphoinositol 3-kinase (PI3K)-Akt, signal transducers and activators of transcription (STAT), and protein kinase C pathways. After activation, the cytosolic proteins translocate to the nucleus and initiate gene expression through the activation of transcription factors. Continuous cell proliferation requires the activity of p38 in most of the cancers studied. This role is carried out by the activation of downstream targets like ATF-2 and Elk-1. Activating transcription factor (ATF-2) has been reported in the control of cell cycle progression.83 Enhancement of cell proliferation by ATF-2 has been reported in some neoplastic cells such as skin adenocarcinoma84 and melanoma.85 Experimental studies in rat ventral prostate has shown that reduction in p-Elk-1 is associated with an enhancement in apoptosis,86 while in the human prostate cell lines DU145 and PC-3, its presence has been correlated with cell proliferation.87 Recent evidence provided by Ricote et al.80 shows that overexpression of α-PAK, MEK-6, p38, p-Elk-1, and p-ATF-2 occurs in benign prostate hyperplasia and more intensely in prostate cancer patients, enhancing cell proliferation and survival. In BPH proliferation can be counteracted in part by TNF-α/JNK/AP-1 mediated apoptosis, but in tumors from patients and LNCaP cells, this pathway is diverted to p38 and proliferation.88
Chen et al.89 demonstrated that ATP mediated P2Y (metabotropic GPCR family) receptor induced prostate cancer cell invasion is regulated through the involvement of p38. The p38 MAP kinase is well known to activate the transcription factor NF-Kappa B90 and may also have some role in activating caspases.91 p38 is also able to increase the expression of certain proteins including chromium induced HIF-1α expression, upregulation of the U-PA promoter in PC3 cells, and PSA induced by IL-6 in LNCaP cells.78,92,93 A decrease in the activation of actin stress fibers in DU145 cells was observed when p38 was inhibited,74,94 probably by growth factors. Kim et al.75 demonstrated neuroendocrine differentiation in LNCaP cells via a p38 MAP kinase dependent mechanism. Some authors have postulated that the determination of the ratio of p-ERK to p-p38 MAP kinase might be able to predict the in vivo behavior of cancer, including the prostate.95 Skjoth and Issinger96 suggest that the presence of an active p38 may be responsible for the high sensitivity to apoptosis induction leading to cell death, p38 MAP kinase is not a requisite for cellular apoptosis, and clearly the balance of p38 MAP kinase activation versus other signals probably determines cellular outcome in which the PI3K/Akt pathway has an apparent role. Previous studies demonstrated that inhibition of p38 MAP kinase pathway resulted in inhibition of the DNA synthesis, growth, and proliferation of PC3 cells.97 Additional studies further evaluate the function of p38 MAP kinase signaling pathway in other prostate cancer cell lines. In addition, it has also been demonstrated that prostate cancer cell invasion is mediated through the p38 MAPK pathway; this leads to the phosphorylation of heat shock protein 27, which in turn regulates MMP-2 activation and cell invasion.98 The activation of MMP-2 and the invasiveness have been shown to be mediated through p38 MAPK, but not ERK, signaling in human melanoma cells.99 Studies from our laboratory have demonstrated a critical role for p38 MAP kinase activation in response to hypoxia/reoxygenation and emergence of castrate resistant phenotype in prostate cancer.41 We have observed that p38 MAPK associates AR in immunoprecipitation assays and that inhibition of p38 MAPK results in decrease in AR levels and activity in prostate cancer cells in culture (S. Koul et al., unpublished observations). Given the critical role of AR signaling in prostate cancer, p38 MAPK may thus offer an attractive alternate target in management of prostate cancer.
p38 MAP Kinase and the Breast
Breast cancer is the second most common cancer in women in the United States, after skin cancer, according to the Mayo Clinic. Breast cancer is a heterogeneous disease with several subtypes that have been identified through analysis of gene expression patterns.100 Metastatic breast tumors represent the final lethal stage of the disease, with cancer cells from the primary mammary gland site having spread to a secondary site (e.g., bone, lung, or liver). The cells from the initial tumor that is able to invade the surrounding tissue by a degrading basement membrane disperse through the lymph and vascular systems to produce metastasis. They usually undergo EMT, which provides epithelial cell plasticity and gain a proliferative and migratory/invasive phenotype with an ability to degrade and migrate through ECM.101 A range of growth factors and cytokines (e.g., human growth factor, FGF, and TGF-β) have been found to trigger EMT.102-106 But in particular, TGF-β signals through transmembrane receptor kinases activate p38 through both Smad-dependent and Smad-independent mechanisms and the MAPK4 pathway.107,108 TGF-β inhibits growth of normal epithelial cells but induces proliferation and EMT in immortalized, phenotypically normal MCF10A cells and in cells from advanced carcinomas.109 Previous studies have shown that p38 MAPK is strongly activated by a mutant form of H-Ras in human breast epithelial cells.110,111 Not only the small G-proteins but the large G-proteins such as GPCR and regulators of G-protein signaling (RGS) proteins are also involved in the p38 MAPK signaling pathway.112,113
The role of MAPKs and their endogenous negative regulators, mitogen-activated protein kinase phosphatases (MKPs) in chemotherapy resistance is well established. MKPs dephosphorylate MAPK are part of the dual-specificity family of phosphatases. MAPKs have been shown to be involved in resistance to tamoxifen, and MKPs have been linked to resistance to treatment with doxorubicin, mechlorethamine, paclitaxel, proteasome inhibitors, and oxidative-stress-induced cell death in breast cancer.114 Growing evidence suggests that modulating MKP-1 activity could be a viable option to make breast cancer chemotherapy more effective.114 Inhibition of p38 signaling by overexpression of MKP-1 also increased resistance to H2O2-induced death in MCF7 breast cancer cells, with a correlation between MKP-1 induction and the disappearance of phosphorylated MAPKs, suggesting that MKP-1 might play a physiologic role in the inactivation of oxidative-damage-induced MAPK activities.115
Protein Signaling
p38 MAPK exerts its biological effects by activating several transcription factors that are involved in various cellular functions such as apoptosis, cell growth, and differentiation. On activation of p38 MAPK, transcription factors present in the cytoplasm or nucleus become phosphorylated and activated, leading to expression of target genes resulting in a biological response. These transcription factors include an activating transcription factor (ATF)-1, ATF-2, GADD153, myocyte enhancer factor (MEF) 2C, CREB, C/EBP homologous protein (CHOP), p53, and NF-κB.31,116-119 ATF-2, a substrate of p38 MAPK, is a possible partner of c-jun in the activator protein (AP)-1 complex. A recent study has demonstrated that ATF-2 mediates MMP-2 transcriptional activation induced by p38 MAPK in MCF10A, human breast epithelial cells.120 p38 MAPK can modulate transcriptional upregulation of TNF-α expression and regulate NF-κB-dependent gene expression in LPS-stimulated macrophages.121,122 Genes that are regulated by p38 MAPK have been revealed by using dominant negative mutants of the upstream activators of p38 MAPK, MKK3, and MKK6, as well as pharmacological inhibitors of p38 MAPK. Expression of many cytokines, transcription factors, cell surface receptors, and enzymes including IL-1, IL-6, IL-8, TNF-α, iNOS, COX-2, MMP-1, MMP-2, MMP-9, MMP-13, c-jun, c-fos, junB, and LDL receptor have been demonstrated to be regulated by the p38 MAPK pathway.120 p38α regulates the induction of the pro-inflammatory mediator cyclooxygenase 2 (COX2), which could potentially contribute to breast cancer progression.123 Further studies identifying p38 MAPK-regulated genes would be of great importance to understand the p38 MAPK signaling pathway and application of its inhibitors for therapeutic use.
Results of p38 Activation
Activated p38 phosphorylates and activates many transcription factors (including activating transcription factor-2, Max, myocyte enhancer factor-2, Mac, p5,3 and Stat1),124,125 which are involved in regulating existing invasive behavior in cultured cells: phospho-p38, which is elevated in cultured invasive breast cancer cells. Constitutive p38 activity induces the overproduction of the pro-invasive uPA,126 which might have an important role in invasion and metastasis of breast cancer.127 Also, treatment of the invasive BT549 breast cancer cells with a p38 MAPK inhibitor diminished both uPA and uPAR expression and inhibited the ability of these cells to invade matrigel.126,128 Furthermore, activated Src overexpression was shown to activate p38 during TGF-β-induced breast cancer cell proliferation and invasion.129 Studies on mammary cells indicate that WAVE-3 (a regulator of cytoskeletal dynamics and cell motility) regulates breast cancer progression, invasion, and metastasis through the p38 pathway and MMP production. Knockdown of WAVE-3 using small interfering RNA in triple negative MDA-MB-231 breast cancer cells decreases p38 activity but not AKT, ERK1/2, or JNK.130 Elevated phospho-p38 levels have been associated with high expression of EGFR and ErbB2 as well in tamoxifen-resistant xenografts.131 p38 would not be seen as driving apoptosis in this context; it acts to support nuclear functions of the estrogen receptor.132 Interestingly, a relationship between phospho-p38 level and lymph node metastasis was identified in human breast cancer samples.131
Recent studies indicated that the p38 MAPK pathway regulates the expression of the MMP family,120,133,134 which is significantly involved in tumor invasion and metastasis formation in various cell systems.135 Activation of the p38 MAPK pathway plays a crucial role in the invasive phenotype of transformed squamous epithelial cells.127,136 The p38 MAPK pathway stimulated MMP-9 expression/secretion and in vitro invasion of human squamous carcinoma cell lines.137 The activation of p38 MAPK is critical for induction of the expression of invasion-associated MMPs such as MMP-13, MMP-1, and MMP-9 in transformed keratinocytes.138,139 p38 MAPK is activated in invasive H-Ras MCF10A human breast epithelial cells but not in noninvasive N-Ras MCF10A cells.110 It has been shown that H-Ras-specific activation of the Rac/MKK-6/p38 MAPK signaling pathway upregulated MMP-2, leading to invasion and migration of MCF10A cells.134 The p38 MAPK pathway activates ATF-2, which then results in transcriptional activation of MMP-2 through binding to the functional AP-1 site.120 Phosphorylation of heat shock protein 27 by p38 MAPK has been shown to induce the migration of MDA-MB-231 breast cancer cells on a laminin-5 coated dish.140 It was also suggested that p38 MAPK might be critical in heregulin-b1-mediated MMP-9 induction in breast cancer cells.141 p38 signaling was found to be required for resveratrol-induced apoptosis in triple negative MDA-MB-231 breast cancer cells,62,142 whereas p38 was not required for vitamin E succinate–induced apoptosis of MDA-MB-435 breast cancer cells.143 Recently, it has been demonstrated that breast carcinoma cells in effusions have markedly different expressions of key molecules that are involved in the biology of breast tumors, as well as in cancer invasion and metastasis in general, including tyrosine kinase receptors, hormone receptors, matrix metalloproteinases, Ets transcription factors, and angiogenic molecules.144,145 Expression of several of these molecules was found to correlate with more aggressive clinical behaviour.145,146 Carcinoma cells in effusions showed significant upregulation of p-p38, which would make sense biologically in a microenvironment lacking access to vessels and therefore considerably deprived of oxygen and nutrients, as well as to the often beneficial cross-talk with peritumoral myofibroblasts in solid tumors. The fact that the upregulation of phopho-p38 does not trigger apoptosis provides further proof to the adaptive capabilities of these cells in suboptimal growth conditions. One target that is known is mitogen-activated protein kinase activated protein kinase 2 (MAPKAP-K2), which phosphorylates RNA binding effectors, such as tristetraprolin, heterogeneous ribonuclear protein A0, and poly (A) binding protein. These molecules are involved in reducing the stability of MKP-1, vascular endothelial growth factor, and MMP mRNA.147 p38 phosphorylation prevents the effectors from carrying out their function, thereby increasing the stability of their target mRNAs.147
p38 MAP Kinase and the Bladder
Bladder cancer, the fourth most common malignancy in men, the ninth in women in the United States, and the tenth frequent cancers worldwide, is characterized by frequent recurrence and poor clinical outcome when tumors from noninvasive flat and papillary urothelial neoplasia progress to muscle invasive or metastatic disease.148 More than 90% of bladder cancers are transitional cell carcinoma (TCC), which is characterized by a multifocal origin and an immunogenic cancer. Although transurethral resection (TUR) of tumor combined with intravesical BCG immunotherapy has become the standard treatment for this type of bladder cancer, the tumor recurrence rate is still high, and most of which will progress to a higher stage or grade.149,150
In our previous studies we found that p38 MAPK is activated during the log phase growth of bladder cancer cells and activation of MAPK is a frequent event in tumor progression and metastasis.45 As metastatic disease is the principal cause of death in cancer patients, a greater understanding of tumor invasion and metastasis is essential and should lead to the identification of new therapeutic targets. There is increasing evidence to indicate that several MMPs in tumor invasion not only have a direct role in tumor invasion by degrading extracellular matrix protein, but as a consequence they also have an important role in maintaining the tumor microenvironment and thus promoting tumor growth. Our previous findings also suggest that p38 MAPK modulates MMP-2 and MMP-9 expression, stability, and activity as well as invasion of bladder cancer cells.46 Recently, it was demonstrated that exposure to LPS caused a remarkable increase in the level of phosphorylated p38 in T24 cells and p38 played a key role in LPS induced IL-6 syntheses.151-153 However, these studies focused on the signaling pathway of regulating IL-6 syntheses in normal cells including airway smooth muscle cells, biliary epithelial cells, and some immune cells.154,155 Very little is known about the mechanisms by which IL-6 is regulated in bladder cancer cells. Interleukin-6 is a pleiotropic cytokine with both pro- and anti-inflammatory roles on immune and nonimmune cells.156 Recent studies suggested that elevated levels of IL-6 in the serum and urine of patients suffering from bladder cancer correlated with tumor size and grade.157 Therefore, tumors associated IL-6 were reported to function as an autocrine growth factor for bladder cancer.158 Previous studies also found that the activation of TLR4 by LPS increased the secretion of IL-6 in a dose- and time-dependent manner and was regulated positively by activation of p38. Horwood et al. demonstrated that LPS-induced expression of IL-6 in human monocytes/macrophages was significantly less sensitive to p38 MAPK inhibition than TNF; it might be explained by this kind of double-modulation mode.159 Moreover, recent findings demonstrate that activation of PI3K/Akt pathway neutralizes the IL-6 induction effect of p38 activation in bladder cancer cells and may contribute to an improved understanding of the overall regulatory process of IL-6 expression through TLR4 signaling. It has also been shown that p38 is essential for the downregulation of COX-2 expression by sulforaphane (SFN), a dietary isothiocyanate in human bladder T24 cells. SFN suppressed both COX-2 mRNA and protein levels by inhibiting NF-κB DNA-binding to the COX-2 promoter. It is known that COX-2 plays a role in cell proliferation, metastasis, and angiogenesis, and thus recent findings suggest that targeting COX-2 through p38 MAPK is a novel mechanism for cancer chemoprevention by SFN in human bladder cancer. Therefore, SFN activated p38 MAPK, and then inhibited NF-κB binding to the COX-2 promoter as well as the downregulation of COX-2 expression, suggesting that activators of p38 MAPK may be beneficial in the down-regulation of COX-2 and, in combination with SFN, may provide potential therapeutic strategies for bladder cancer.
Protein Signaling
Several studies have identified that p38 MAPK signal transduction pathways are involved in the regulation of MMP-9 expression in several bladder cancer cell lines.46,160-162 A recent study identified potential cisacting elements of the p38 MAP kinase signal pathway–mediated control of the MMP-9 gene in HT1376 bladder cancer cells in response to TNF-α. Although multiple signaling pathways were previously shown to be involved in MMP-9 expression in different cell types,141,163 a recent study reported that TNF-α upregulates MMP-9 via the simultaneous activation of different pathways in bladder cancer cells. This finding has broadened the understanding of the regulation of this important molecule, which is involved in urinary bladder tumor invasion and metastasis. The transcription factors, NF-κB, AP-1, and Sp-1, were the focus of this investigation into how p38 MAP kinase mediates MMP-9 expression, as they are necessary for MMP-9 secretion. The transcription factors that are involved in p38 MAP kinase-mediated control of the MMP-9 gene in HT1376 cells in the presence of TNF-α were identified. Therefore, it clearly shows that TNF-α regulates MMP-9 expression through NF-κB, AP-1, and Sp-1 cis-elements of the gene promoter, and p38 MAP kinase mediates TNF-α-induced MMP-9 expression by coordinating the regulation of the binding activity of the transcription factors, NF-κB, AP-1, and Sp-1, in HT1376 cells.107
Results of p38 Activation
It has been demonstrated that p38 MAPK plays an important role in the expression of both ARE-dependent enzymes and COX-2 manipulated by sulforaphane (SFN). p38 activation positively modulates the induction of GSTA1-1 and TR-1 by SFN and enhances the effect of SFN on downregulation of COX-2. In accordance with these findings, positive regulation of p38 MAPK was also reported in other ARE-dependent enzymes, such as HO-1, γ-GCS.164,165 However, only a few studies have reported the converse, that p38 MAPK negatively controls ARE-dependent enzymes. Keum et al.166 found that p38 MAPK overexpression suppressed SFN-induced heme oxigenase-1 (HO-1) expression in human hepatoma HepG2 cells. The underlying mechanism remains to be clarified and the specificities in stimuli and cell types should also be considered. In nonstimulated cells, Nrf2 is sequestered in the cytoplasm by its inhibitor Keap1. On activation mediated by p38 MAPK following exposure to SFN, Nrf2 translocates into the nucleus and binds to ARE sites in the promoter regions of many detoxification and antioxidant genes, leading to coordinated upregulation of downstream targets that enhance cellular detoxification and antioxidative potency.167 Previous studies have revealed that intracellularly, hypericin-PDT-induced photodynamic therapy (PDT) cell death is mediated by endoplasmic reticulum (ER) Ca2+ store emptying and executed through caspase-dependent or caspase-independent pathways.168 However, hypericin-PDT also activates rescuing responses, chiefly governed by the activation of p38 MAPK.169-171 The pleiotropic p38 MAPK appears to contribute to carcinogenesis by regulating growth factor expression,43,49,50 inflammatory,40 and invasion-associated genes, including members of the MMP family.44,46,136,139 Previous results confirm the crucial role of p38 MAPK in modulating immune and inflammatory responses by upregulation. For example, COX-2, IL8, and CSF2 additionally assign novel important molecular functions to this pathway in PDT signaling by inducing genes including AKR1C1, AKR1C3, GCLC, NQO1, as well as HMOX1. These cytoprotective genes are induced by oxidants and electrophilic carcinogens through activation of the redox-dependent Keap1/Nrf2 pathway.172,173 Accordingly, it was observed that the activation of p38 MAPK signaling pathways by PDT contributes to HO-1 protein induction and survival through a mechanism involving nuclear accumulation of Nrf2.174
Furthermore, a previous study reveals that MMP-13, a member of the collagenase subfamily of MMPs, is a p38 MAPK target. MMP-13 is expressed at the invading edges of urinary bladder transitional cell carcinoma (TCC) in vivo and is regulated in vitro in T24 cells stimulated by TNF-α through p38 MAPK.175 MMP-13 upregulation in bladder cancer cells after PDT is remarkably p38 MAPK dependent, suggesting that p38 MAPK inhibition could be required to halt the invasiveness of cancer cells escaping photokilling. Thus, the role of p38 MAPK-MMP-13 cascade in the modulation of tumor growth, as well as the immune response in vivo, deserves to be further addressed.176 Interestingly, the marked activation of angiogenesis in urinary bladder lamina propria with chronic radiation cystitis was associated with a dramatic increase of p38 and p65 cytoplasmic and nuclear expression in endothelial cells. These findings indicate a critical role for the p38 MAPK cascade and the NF-kB p65 subunit in endothelial cell activation as an important component in the pathogenesis of Chernobyl cystitis.177 T24 cells have an activating ras mutation,178 and previously it has been shown that T24 cells have very low NF1 tumor-suppressor gene expression levels.179 As the NF1 gene has been suggested to have inhibitory effects on ras, high ras activation in T24 cells could lead to activation of MAPK pathways, for example, p38 when compared to RT4 cells.175
p38 MAP Kinase and the Liver
Liver cancer is one of the most common forms of cancer around the world, but is uncommon in the United States, according to the Mayo Clinic. However, its rates in America are rising. MAPKs are essential components of intracellular signal transduction and are activated in response to various extracellular stimuli, including growth factors, cytokines, and environmental stress. Proinflammatory cytokines such as TNF-α and IL-1 are elevated in liver cell injury induced by hepatitis B and C virus infection.180,181 Accordingly, these cytokines may activate the p38 MAPK pathway in chronic liver diseases. Of 4 distinct MAPK groups, p38 MAPKs are phosphorylated on Tyr and Thr by a stress-induced signal transduction pathway and activated by proinflammatory cytokines and environmental stress.10,182 It has been demonstrated that activation of p38 and concurrent inhibition of ERK are critical for induction of apoptosis in rat PC-12 pheochromocytoma cells and other cell lines.183-188 Recent studies indicate the enzymatic activity of p38 MAPK requires dual phosphorylation of threonine and tyrosine residues by members of the MAPK kinases (e.g., MKK6 and MKK3). MKK6 is identified as a common activator of entire isoforms, whereas MKK3 activates some isoforms of p38 MAPK.189 Because resistance to apoptosis is a molecular event underlying unrestricted cell growth other than acceleration of cell cycle, a recent study focuses on determining how p38 MAPK and MKK6 activations participate in the apoptosis of human hepatocellular carcinoma (HCC).
Protein Signaling
Recent studies indicate apoptosis is induced by genotoxic stresses, such as UV, X-ray, H2O2, heat,190,191 and cell surface receptor Fas.192,193 Consistently, blockade of JNK activation promotes cell survival and stimulates p38 MAPK mediated by MKK3/6 and MKK4/7 in human hepatoma cell lines.47 In addition, it is established that p38 MAPK cascade is involved in the apoptotic pathway, mutant MKK6-induced p38 MAPK activation inhibited cell growth, and induced apoptosis in the 2 hepatoma cell lines, HepG2 and HuH7.47 Alterations in the apoptosis pathways might lead to unrestricted malignant cell growth. Therefore, there has been recent interest in the interactions between the MAPK pathways and apoptosis because caspases participate in one of the major death process pathways.194 These observations indicate that activation of p38 MAPK enhances caspase 3 activities, accompanied by apoptosis of hepatoma cell lines.
Results of p38 Activation
Recent findings suggest that p38 MAPK modulates the caspase cascade downstream of Bid cleavage and upstream of cytochrome c release in human hepatoma cell lines. Recently, activation of MAPK superfamily in human HCC tissue specimens was evaluated. p38 MAPK and MKK6 activities in human HCCs were significantly lower than in adjacent uninvolved liver tissue specimens,47 whereas ERK1/2 activity was significantly higher in malignant lesions from the same tissue specimens. There is a significant positive correlation between p38 MAPK and MKK6 activities in human HCC tissue specimens,47 which has been clarified by transfecting mutant MKK6 in the modulation of caspase cascade through p38 MAPK activation. The proapoptotic protein Bid, a member of the bcl-2 family, is a substrate for caspase-8.194 Cleavage of Bid is mediated by p38 MAPK during apoptosis in human leukemic cells.195 Since caspase-8 activation and Bid cleavage in MKK6-transfected hepatoma cells was not observed, caspase-8 and Bid may not be involved downstream of MKK6-p38 MAPK in hepatoma cells. On the other hand, mitochondria are influenced by proapoptotic signal transduction through the p38 MAPK pathway. ASK1, a MAPK kinase kinase that activates p38 MAPK and JNK/SAPK via MKK3/6 and MKK4/7, induces cell death-mediated cytochrome c release from the mitochondria and caspase activation.196
The attenuation in the p38 MAPK and MKK6 activities in human HCC tissue specimens may account for the resistance to apoptosis, leading to unrestricted cell growth of human HCCs.197 The p38 MAPK inhibitor completely blocked p38 MAPK activity and markedly suppressed cell death induced by TNF-α treatment.198 It was established that TNF-α receptor levels were reduced in HCCs.199 Therefore, reduced TNF-α apoptotic signals might account, in part, for the downregulation of p38 MAPK cascade in human HCCs. Conversely, there are reports of MAPK regulation through dephosphorylation by specific protein phosphatases200,201 and inhibition of p38 MAPK activation through protein phosphatase 2C alpha.201 The functional alternation of protein phosphatase would serve as another mechanism for the downregulation of the p38 MAPK cascade. Some anticancer drugs induce apoptosis in human cancer cells in association with the p38 MAPK activation.202,203 Recent studies suggest that p38 MAPK activation induces apoptosis in hepatoma cells. p38 MAPK activation by anticancer drugs or other chemical agents may be a potent approach to targeted therapy for human HCCs. Overall, in vitro findings in hepatoma cell lines and the in vivo observations of human HCCs suggest that diminished p38 MAPK and MKK6 activities may account, in part, for the resistance to apoptosis, leading to unrestricted cell growth of human HCCs. In support of this notion, p38 activity has been shown to be reduced in hepatocellular carcinomas in comparison to adjacent normal tissue, with tumor size inversely related to p38 activity.47
p38 MAP Kinase and the Lung
Lung and bronchial cancer is the top killer cancer in the United States. p38 MAPK is a ubiquitous and highly conserved, proline-directed serine/threonine protein kinase considered important in many processes that are critical to the inflammatory response and tissue remodeling204 that are hallmarks of pulmonary diseases such as chronic obstructive pulmonary disease (COPD).205 Although a role for p38 kinase inhibitors in the treatment of pulmonary disease has only recently been postulated,205,206 it is well known that inflammatory cytokines and chemokines are capable of regulating or supporting chronic airway inflammation.207 Several reports support the association of p38 kinase activation with a plethora of pulmonary events: LPS and TNF-α-induced intercellular adhesion molecule-1 expression on pulmonary microvascular endothelial cells,208 MMP-9 activation,138 hypoxia-induced stimulation of pulmonary arterial cells,209 hyperosmolarity-induced IL-8 expression in bronchial epithelial cells,210 and enhanced eosinophil trafficking and survival.211,212
Protein Signaling
Although there is a scantiness of reports specifically addressing the role of p38 kinase in pulmonary disease, it is known that inflammatory cytokines play an important role in airways inflammation. Thus, cytokines such as TNF-α, IFN-γ, IL-4, IL-5, IL-6, and chemokines such as IL-8, regulated on activation normal T-cell expressed and secreted (RANTES), and eotaxin have all been shown to be capable of regulating or supporting chronic airway inflammation,207 suggesting that p38 MAPK inhibition would have an impact in models that reflect chronic lung diseases.207 There is also evidence for an involvement of p38 kinase in allergic mechanisms. For example, human monocyte IL-4-induced release of soluble cellular differentiation antigen 23 was dramatically inhibited by the p38 kinase inhibitor SB 203580,213 suggesting IgE synthesis also might be regulated by p38 kinase activity. In addition, aggregated IgA- or IgG-stimulated eosinophil degranulation has been shown to involve the MAP kinases and phosphatidylinositol 3-kinase.214
Results of p38 Activation
TNF-α and IL-1β have been implicated as mediators of LPS-induced airway inflammation215 by stimulating the release of chemotactic factors from alveolar macrophages and airway epithelial cells and by upregulating the expression of leukocyte and endothelial adhesion molecules.216 Furthermore, instillation of recombinant TNF-α into the tracheobronchial tree induces chemokine release in bronchoalveolar lining fluid217 and upregulation of intercellular adhesion molecule-1 (ICAM-1) on pulmonary vascular endothelium.218 Several therapeutic strategies have been employed to control the release of pro-inflammatory cytokines such as IL-1 and TNF-α. Recently, attention has focused on the bicyclic pyridinyl imidazole class of compounds such as SB 203580 and SB 202190, which are potent inhibitors of TNF-α and IL-1β release.219-222 The intracellular target of these compounds is the p38 mitogen-activated protein kinase,221 a key component in cytokine and stress-induced signal transduction pathways.223 Analysis of the inhibitory mechanisms of these compounds indicated that the site of action was primarily at the translational level.222,224
Several lines of evidence suggest the involvement of TNF-α as a mediator of LPS-induced airway inflammation215,225-229 mediated through the 55-kDa type 1 TNF receptor (TNFR1). It has been demonstrated that TNFR1-deficient mice exposed to LPS aerosol showed a persistent reduction in neutrophil recruitment to the air spaces of the lungs, in comparison with wild-type animals, that was associated with depressed chemokine levels in BAL fluid.230 Studies in monocytes172 and alveolar macrophages231 have shown that the p38 MAPK pathway is critical for LPS-induced cytokine release. Specific inhibition of the p38 kinase pathway by SB 203580 resulted in reduced cytokine release secondary to a defect in translation. In alveolar macrophages, the regulatory effect of p38 kinase on LPS-induced TNF-α and IL-6 gene expression was mediated partly through changes in gene transcription.231 The potential reasons for this differential effect are unclear but may be related to the differential expression and the sensitivity of the different p38 kinase isoforms or cell types. Indeed, 4 genes encode the known members of the p38 family, p38α,221 p38β,27,232 p38γ,233 and p38δ.234,235 Hale et al. have shown that the expression of p38 family members is not ubiquitous but is controlled during cell differentiation and in a lineage-specific fashion.236 Differential expression was most striking in monocytes that strongly expressed p38α, but did not express p38β or p38γ. As blood monocytes differentiated into macrophages, a striking induction of p38δ mRNA and protein occurred so that p38δ protein became at least as abundant as p38α.236 In neutrophils, only p38α and p38δ were detected.237 Although p38γ mRNA was present in endothelial cells, p38γ protein was not detected in any cell type.236 This is consistent with other reports that p38γ is a muscle-specific protein.233 Furthermore, in terms of regulation it has been shown that p38α is preferentially activated by MKK-3 in PC-12 cells, whereas p38α is predominantly activated by MKK-6 in monocytes cells, suggesting that p38 is activated by different MKKs in a cell type–dependent manner.238 Unlike p38α, p38δ was activated by MKK-3, -4, -6, and -7 in an approximately equal manner in 293 T cells, suggesting that the regulation of p38δ may be distinct from p38α. However, it is still not clear whether the regulation of p38δ depends on cell type.238 A role for p38δ in inflammation was suggested recently by Jiang et al.,235 who reported the activation of renal p38δ during glomerulonephritis in rats. The widely used pyridinyl imidazole inhibitors of p38, SB 203580 and SB 202190, are nearly equipotent against p38α and p38β, but do not inhibit p38γ or p38δ.27,234,235,239,240 Thus, if p38γ or p38δ are the predominant isoforms involved in the release of TNF-α in BAL fluid following LPS inhalational challenge, this could explain the apparent ineffectiveness of SB 203580. However, the development of inhibitors with greater selectivity for the different isoforms of p38 kinase will undoubtedly allow a more precise definition of the relative contribution of p38 kinases to airway inflammation and airway inflammatory diseases. Alternatively, it may indicate that the release of TNF-α may not be solely controlled via the p38 kinase signal transduction pathway and could be due to the activation of other signaling pathways. In fact, both p38 and ERK are involved in LPS-induced TNF-α production from macrophages in vitro.241 In conclusion, p38 MAPK appears to be involved in the release of IL-1β and the associated sustained phase of neutrophilia following aerosolized LPS. This may suggest a role for p38 inhibitors in the treatment of airway inflammatory diseases (e.g., septic shock, COPD, and acute respiratory distress syndrome) associated with neutrophilia of the airways.
Functional Role of p38 MAPK
Based on the above discussion, it is quite evident that p38 MAPK plays a critical role in several aspects of cancer cell biology: cell survival,45 cell death,115,174 cell differentiation/dedifferentiation,10 apoptosis,242 check-point control,243 overcoming dependence on addictive oncogenic pathways,244 drug resistance,78 actin cytoskeletal remodulation,30 cell migration,110 invasion,41 and metastasis.45,46 In addition, although not discussed in detail here, p38 MAPK as a key regulator of inflammation and immune modulation with respect to tumor progression cannot be undermined. The most common isoform, p38α, has a key role in the production of many cytokines, such as TNF-α, IL-1, and IL-6, which have pro-inflammatory, prosurvival, and angiogenic effects35 and also regulate cytokine expression by modulating transcription factors, such as NF-κB,245 or at the posttranscriptional level, by regulating mRNA stability and protein translation, which is mostly mediated by the downstream kinase MK2.246 Specific deletion of different forms of p38 in genetically engineered mouse models has provided in vivo evidence for the importance of this pathway in cytokine production and inflammatory responses (Table 1),247,248 whereas p38β does not seem to be required for acute or chronic inflammatory responses.249,250 p38α may also directly affect tumor invasion and angiogenesis independently of its role in inflammation by inducing the expression of the matrix metalloproteinases MMP1, MMP3, and MMP13, which regulate matrix remodeling and degradation by metastatic cancer cells, as well as vascular endothelial growth factor A (vEGFA), a potent inducer of tumor survival and angiogenesis.251 In addition, it is also reported that p38α can activate hypoxia inducible factor 1 (HIF1), involved in hypoxia-driven expression of angiogenic factors, at least partly through the stabilization of its α-subunit.252 p38γ has been associated with Ras-induced invasion253 and p38δ regulates squamous cell carcinoma invasion.254 By contrast, the p38 MAPK activator MKK6 has been reported to suppress metastasis255 or to have no effect,11 depending on the experimental model used. In addition to facilitating metastasis, p38α also regulates cancer.110,256,257 MK2 has an important role in cell migration downstream of p38α by remodeling the actin cytoskeleton. This cytoskeletal remodeling may be mediated by the phosphorylation of heat shock 27 kDa protein (HsP27), inducing its release from F-actin caps.258
Conclusions and Future Directions
Cells in solid tumors in general live in a hostile environment. They have to live in periods of deprived oxygen, altered pH, and deprived nutrition and avoiding consequences of additional barrage of the host responses, inflammation, and immune surveillance. Thus, it is imperative for their survival that these cells are able to tightly monitor the changes to their environment and adequately respond to such changes.259 Our discussion as listed above, suggests that mammalian p38 MAPK plays varying roles in normal conditions, but the role of this signaling pathway in solid tumor biology (cell survival, invasion and progression, and inflammatory response) may be critical determinants of tumor cell survival and metastasis, although the mechanisms involved seem to be more diverse and complex than previously anticipated. In addition to other roles, the p38 MAPK signaling pathway may be essential for mere survival of cancer cells in general especially during nutrient deficiency and hypoxia reoxygenation periods. There have been a large number of previous studies examining the presence of different isoforms of p38 MAPK in different types of tumors. Although several of these studies have suggested prognostic significance for different isoforms of p38 MAPK, few studies have described actual usefulness of the individual targets in different types of solid tumors. Moreover, none of the signal transduction pathways exist as individual entities, as such understanding of the interplay between p38 MAPK family members and the cross-talk with other signaling pathways in a context-specific response under normal physiological and pathological conditions is necessary for better understanding the role of this signaling pathway. Systems biology approaches and high-throughput genomic and proteomic technologies may provide valuable insights into these questions. Moreover, the use of genetically modified mice to modulate the activity of the p38 MAPK signaling pathway in a time- and tissue-specific manner as well as to express at physiological levels p38 MAPK proteins with particular mutations will be very useful to elucidate in vivo functions to test the effectiveness of p38 MAPK-inhibiting drugs. Irrespective of our understanding of the specific mechanisms, mere preponderance of evidence for the critical role of p38 MAP kinase in various aspects of tumor progression (hypoxic survival, reoxygenation injury, inflammation and immune response modulation, cell migration and invasion, to name a few aspects) would imply that this stress kinase pathway might offer potential therapeutic targets for intervention in solid tumors. Additional studies are warranted to evaluate the differential requirement of the p38 MAP kinase pathway for survival and metastasis of cancer cells, to fill the gaps that exist in our current understanding of the functional cosequences of p38 MAP kinase inhibition with respect to solid tumor biology.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Studies supported in part by Chair Commitment (H. Koul). H. Koul is supported in part by VA Merit Award 01BX001258, NIH R01 DK54084, and NIH/NCI R01CA161880.
References
- 1. Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482(7385):419-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sayama K, Hanakawa Y, Nagai H, et al. Transforming growth factor-beta-activated kinase 1 is essential for differentiation and the prevention of apoptosis in epidermis. J Biol Chem. 2006;281(31):22013-20 [DOI] [PubMed] [Google Scholar]
- 3. Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem. 2012;287(43):36593-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sayama K, Yamasaki K, Hanakawa Y, Shirakata Y, Tokumaru S, Ijuin T, Takenawa T, Hashimoto K. Phosphatidylinositol 3-kinase is a key regulator of early phase differentiation in keratinocytes. J Biol Chem 2002;277(43):40390-6 [DOI] [PubMed] [Google Scholar]
- 5. Lawson SK, Dobrikova EY, Shveygert M, Gromeier M. p38alpha mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by miR-124 and -128 in neurons. Mol Cell Biol. 2013;33(1):127-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu Y, Richardson DR. Cellular iron depletion stimulates the JNK and p38 MAPK signaling transduction pathways, dissociation of ASK1-thioredoxin, and activation of ASK1. J Biol Chem. 2011;286(17):15413-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299(5614):1859-63 [DOI] [PubMed] [Google Scholar]
- 8. Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276(5319):1702-5 [DOI] [PubMed] [Google Scholar]
- 9. Vassalli G, Milano G, Moccetti T. Role of mitogen-activated protein kinases in myocardial ischemia-reperfusion injury during heart transplantation. J Transplant. 2012;2012:928954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raingeaud J, Gupta S, Rogers JS, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270(13):7420-6 [DOI] [PubMed] [Google Scholar]
- 11. Vander Griend DJ, Kocherginsky M, Hickson JA, Stadler WM, Lin A, Rinker-Schaeffer CW. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65(23):10984-91 [DOI] [PubMed] [Google Scholar]
- 12. Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403-17 [DOI] [PubMed] [Google Scholar]
- 13. New L, Han J. The p38 MAP kinase pathway and its biological function. Trends Cardiovasc Med. 1998;8(5):220-8 [DOI] [PubMed] [Google Scholar]
- 14. Frazier WJ, Xue J, Luce WA, Liu Y. MAPK signaling drives inflammation in LPS-stimulated cardiomyocytes: the route of crosstalk to G-protein-coupled receptors. PLoS One. 2012;7(11):e50071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu YC, Ip MM. Conjugated linoleic acid-induced apoptosis in mouse mammary tumor cells is mediated by both G protein coupled receptor-dependent activation of the AMP-activated protein kinase pathway and by oxidative stress. Cell Signal. 2011;23(12):2013-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan-Hui PY, Weaver R. Human mitogen-activated protein kinase kinase kinase mediates the stress-induced activation of mitogen-activated protein kinase cascades. Biochem J. 1998;336(Pt 3):599-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ge B, Gram H, Di Padova F, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295(5558):1291-4 [DOI] [PubMed] [Google Scholar]
- 18. Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398(6724):252-6 [DOI] [PubMed] [Google Scholar]
- 19. Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6(11):1087-95 [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270(5244):2008-11 [DOI] [PubMed] [Google Scholar]
- 21. Ono K, Ohtomo T, Ninomiya-Tsuji J, Tsuchiya M. A dominant negative TAK1 inhibits cellular fibrotic responses induced by TGF-beta. Biochem Biophys Res Commun. 2003;307(2):332-7 [DOI] [PubMed] [Google Scholar]
- 22. Kim SI, Kwak JH, Zachariah M, He Y, Wang L, Choi ME. TGF-beta-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-beta1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am J Physiol Renal Physiol. 2007;292(5):F1471-8 [DOI] [PubMed] [Google Scholar]
- 23. Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J Biol Chem. 2005;280(27):25920-7 [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346-51 [DOI] [PubMed] [Google Scholar]
- 25. Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326(1):105-15 [DOI] [PubMed] [Google Scholar]
- 26. Yao J, Kim TW, Qin J, et al. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282(9):6075-89 [DOI] [PubMed] [Google Scholar]
- 27. Stein B, Yang MX, Young DB, et al. p38-2, a novel mitogen-activated protein kinase with distinct properties. J Biol Chem. 1997;272(31):19509-17 [DOI] [PubMed] [Google Scholar]
- 28. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807-69 [DOI] [PubMed] [Google Scholar]
- 29. Beyaert R, Cuenda A, Vanden Berghe W, et al. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15(8):1914-23 [PMC free article] [PubMed] [Google Scholar]
- 30. Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80(3):383-92 [DOI] [PubMed] [Google Scholar]
- 31. Zhu T, Lobie PE. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal re-organization and mitogenesis. J Biol Chem. 2000;275(3):2103-14 [DOI] [PubMed] [Google Scholar]
- 32. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911-2 [DOI] [PubMed] [Google Scholar]
- 33. Feng Y, Wen J, Chang CC. p38 Mitogen-activated protein kinase and hematologic malignancies. Arch Pathol Lab Med. 2009;133(11):1850-6 [DOI] [PubMed] [Google Scholar]
- 34. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55-72 [DOI] [PubMed] [Google Scholar]
- 35. Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2(9):717-26 [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Gibson TB, Robinson F, et al. MAP kinases. Chem Rev. 2001;101(8):2449-76 [DOI] [PubMed] [Google Scholar]
- 37. Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10(3):125-9 [DOI] [PubMed] [Google Scholar]
- 38. Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23(16):2838-49 [DOI] [PubMed] [Google Scholar]
- 39. Park JS, Carter S, Reardon DB, Schmidt-Ullrich R, Dent P, Fisher PB. Roles for basal and stimulated p21(Cip-1/WAF1/MDA6) expression and mitogen-activated protein kinase signaling in radiation-induced cell cycle checkpoint control in carcinoma cells. Mol Biol Cell. 1999;10(12):4231-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park JI, Lee MG, Cho K, et al. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene. 2003;22(28):4314-32 [DOI] [PubMed] [Google Scholar]
- 41. Khandrika L, Lieberman R, Koul S, et al. Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer. Oncogene. 2009;28(9):1248-60 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Maroni PD, Koul S, Meacham RB, Koul HK. Mitogen activated protein kinase signal transduction pathways in the prostate. Cell Commun Signal. 2004;2(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsai PW, Shiah SG, Lin MT, Wu CW, Kuo ML. Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa B signaling pathway. J Biol Chem. 2003;278(8):5750-9 [DOI] [PubMed] [Google Scholar]
- 44. Suarez-Cuervo C, Merrell MA, Watson L, et al. Breast cancer cells with inhibition of p38alpha have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin Exp Metastasis. 2004;21(6):525-33 [DOI] [PubMed] [Google Scholar]
- 45. Kumar B, Sinclair J, Khandrika L, Koul S, Wilson S, Koul HK. Differential effects of MAPKs signaling on the growth of invasive bladder cancer cells. Int J Oncol. 2009;34(6):1557-64 [DOI] [PubMed] [Google Scholar]
- 46. Kumar B, Koul S, Petersen J, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70(2):832-41 [DOI] [PubMed] [Google Scholar]
- 47. Iyoda K, Sasaki Y, Horimoto M, et al. Involvement of the p38 mitogen-activated protein kinase cascade in hepatocellular carcinoma. Cancer. 2003;97(12):3017-26 [DOI] [PubMed] [Google Scholar]
- 48. Greenberg AK, Basu S, Hu J, et al. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 2002;26(5):558-64 [DOI] [PubMed] [Google Scholar]
- 49. Elenitoba-Johnson KS, Jenson SD, Abbott RT, et al. Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. Proc Natl Acad Sci U S A. 2003;100(12):7259-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin Z, Crockett DK, Jenson SD, Lim MS, Elenitoba-Johnson KS. Quantitative proteomic and transcriptional analysis of the response to the p38 mitogen-activated protein kinase inhibitor SB203580 in transformed follicular lymphoma cells. Mol Cell Proteomics. 2004;3(8):820-33 [DOI] [PubMed] [Google Scholar]
- 51. Liu RY, Fan C, Liu G, Olashaw NE, Zuckerman KS. Activation of p38 mitogen-activated protein kinase is required for tumor necrosis factor-alpha -supported proliferation of leukemia and lymphoma cell lines. J Biol Chem. 2000;275(28):21086-93 [DOI] [PubMed] [Google Scholar]
- 52. Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12(1):27-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276(50):46707-13 [DOI] [PubMed] [Google Scholar]
- 54. Cheng TL, Symons M, Jou TS. Regulation of anoikis by Cdc42 and Rac1. Exp Cell Res. 2004;295(2):497-511 [DOI] [PubMed] [Google Scholar]
- 55. Bulavin DV, Demidov ON, Saito S, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31(2):210-5 [DOI] [PubMed] [Google Scholar]
- 56. Brancho D, Tanaka N, Jaeschke A, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17(16):1969-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bulavin DV, Fornace AJ., Jr. p38 MAP kinase’s emerging role as a tumor suppressor. Adv Cancer Res. 2004;92:95-118 [DOI] [PubMed] [Google Scholar]
- 58. Timofeev O, Lee TY, Bulavin DV. A subtle change in p38 MAPK activity is sufficient to suppress in vivo tumorigenesis. Cell Cycle. 2005;4(1):118-20 [DOI] [PubMed] [Google Scholar]
- 59. Ellinger-Ziegelbauer H, Kelly K, Siebenlist U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol Cell Biol. 1999;19(5):3857-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pruitt K, Pruitt WM, Bilter GK, Westwick JK, Der CJ. Raf-independent deregulation of p38 and JNK mitogen-activated protein kinases are critical for Ras transformation. J Biol Chem. 2002;277(35):31808-17 [DOI] [PubMed] [Google Scholar]
- 61. Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272(33):20490-4 [DOI] [PubMed] [Google Scholar]
- 62. She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61(4):1604-10 [PubMed] [Google Scholar]
- 63. Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5(8):824-8 [DOI] [PubMed] [Google Scholar]
- 64. Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ., Jr. Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol. 2003;23(11):3859-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23(1):40-5 [DOI] [PubMed] [Google Scholar]
- 66. Nelson PS, Montgomery B. Cell type-specific analyses for identifying prostate cancer biomarkers. Curr Urol Rep. 2006;7(1):57-63 [DOI] [PubMed] [Google Scholar]
- 67. Cronauer MV, Schulz WA, Burchardt T, et al. The androgen receptor in hormone-refractory prostate cancer: relevance of different mechanisms of androgen receptor signaling. Int J Oncol. 2003;23(4):1095-102 [DOI] [PubMed] [Google Scholar]
- 68. Guan M, Fousek K, Chow WA. Nelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancer. FEBS J. 2012;279(13):2399-411 [DOI] [PubMed] [Google Scholar]
- 69. Magi-Galluzzi C, Montironi R, Cangi MG, Wishnow K, Loda M. Mitogen-activated protein kinases and apoptosis in PIN. Virchows Arch. 1998;432(5):407-13 [DOI] [PubMed] [Google Scholar]
- 70. Royuela M, Arenas MI, Bethencourt FR, Sanchez-Chapado M, Fraile B, Paniagua R. Regulation of proliferation/apoptosis equilibrium by mitogen-activated protein kinases in normal, hyperplastic, and carcinomatous human prostate. Hum Pathol. 2002;33(3):299-306 [DOI] [PubMed] [Google Scholar]
- 71. Uzgare AR, Kaplan PJ, Greenberg NM. Differential expression and/or activation of P38MAPK, erk1/2, and jnk during the initiation and progression of prostate cancer. Prostate. 2003;55(2):128-39 [DOI] [PubMed] [Google Scholar]
- 72. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tuohimaa P, Lyakhovich A, Aksenov N, et al. Vitamin D and prostate cancer. J Steroid Biochem Mol Biol. 2001;76(1-5):125-34 [DOI] [PubMed] [Google Scholar]
- 74. Mehta PB, Robson CN, Neal DE, Leung HY. Keratinocyte growth factor activates p38 MAPK to induce stress fibre formation in human prostate DU145 cells. Oncogene. 2001;20(38):5359-65 [DOI] [PubMed] [Google Scholar]
- 75. Kim J, Adam RM, Freeman MR. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62(5):1549-54 [PubMed] [Google Scholar]
- 76. Grasso AW, Wen D, Miller CM, Rhim JS, Pretlow TG, Kung HJ. ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene. 1997;15(22):2705-16 [DOI] [PubMed] [Google Scholar]
- 77. Stein B, Brady H, Yang MX, Young DB, Barbosa MS. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271(19):11427-33 [DOI] [PubMed] [Google Scholar]
- 78. Lin JC, Chang SY, Hsieh DS, Lee CF, Yu DS. Modulation of mitogen-activated protein kinase cascades by differentiation-1 protein: acquired drug resistance of hormone independent prostate cancer cells. J Urol. 2005;174(5):2022-6 [DOI] [PubMed] [Google Scholar]
- 79. Ricote M, Royuela M, Garcia-Tunon I, Bethencourt FR, Paniagua R, Fraile B. Pro-apoptotic tumor necrosis factor-alpha transduction pathway in normal prostate, benign prostatic hyperplasia and prostatic carcinoma. J Urol. 2003;170(3):787-90 [DOI] [PubMed] [Google Scholar]
- 80. Ricote M, Garcia-Tunon I, Bethencourt FR, Fraile B, Paniagua R, Royuela M. Interleukin-1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer. 2004;100(7):1388-96 [DOI] [PubMed] [Google Scholar]
- 81. Ranganathan P, Rangnekar VM. Regulation of cancer cell survival by Par-4. Ann N Y Acad Sci. 2005;1059:76-85 [DOI] [PubMed] [Google Scholar]
- 82. Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br J Pharmacol. 2006;147(Suppl 2):S144-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278(36):33753-62 [DOI] [PubMed] [Google Scholar]
- 84. Zoumpourlis V, Papassava P, Linardopoulos S, Gillespie D, Balmain A, Pintzas A. High levels of phosphorylated c-Jun, Fra-1, Fra-2 and ATF-2 proteins correlate with malignant phenotypes in the multistage mouse skin carcinogenesis model. Oncogene. 2000;19(35):4011-21 [DOI] [PubMed] [Google Scholar]
- 85. Recio JA, Noonan FP, Takayama H, et al. Ink4a/arf deficiency promotes ultraviolet radiation-induced melanomagenesis. Cancer Res. 2002;62(22):6724-30 [PubMed] [Google Scholar]
- 86. Huynh H. Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins. Int J Oncol. 2002;20(6):1297-303 [PubMed] [Google Scholar]
- 87. Xiao D, Qu X, Weber HC. GRP receptor-mediated immediate early gene expression and transcription factor Elk-1 activation in prostate cancer cells. Regul Pept. 2002;109(1-3):141-8 [DOI] [PubMed] [Google Scholar]
- 88. Ricote M, Garcia-Tunon I, Bethencourt F, et al. The p38 transduction pathway in prostatic neoplasia. J Pathol. 2006;208(3):401-7 [DOI] [PubMed] [Google Scholar]
- 89. Chen L, He HY, Li HM, et al. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett. 2004;215(2):239-47 [DOI] [PubMed] [Google Scholar]
- 90. Shimada K, Nakamura M, Ishida E, Kishi M, Konishi N. Roles of p38- and c-jun NH2-terminal kinase-mediated pathways in 2-methoxyestradiol-induced p53 induction and apoptosis. Carcinogenesis. 2003;24(6):1067-75 [DOI] [PubMed] [Google Scholar]
- 91. Permpongkosol S, Wang JD, Takahara S, et al. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer. 2002;98(2):167-72 [DOI] [PubMed] [Google Scholar]
- 92. Eandi JA, Yang JC, Evans CP. Signal transduction-mediated regulation of urokinase gene expression in human prostate cancer. Biochem Biophys Res Commun. 2001;288(3):521-7 [DOI] [PubMed] [Google Scholar]
- 93. Gao N, Jiang BH, Leonard SS, et al. p38 Signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J Biol Chem. 2002;277(47):45041-8 [DOI] [PubMed] [Google Scholar]
- 94. Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13(3):902-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 2003;63(7):1684-95 [PubMed] [Google Scholar]
- 96. Skjoth IH, Issinger OG. Profiling of signaling molecules in four different human prostate carcinoma cell lines before and after induction of apoptosis. Int J Oncol. 2006;28(1):217-29 [PubMed] [Google Scholar]
- 97. Koul S, Huang M, Chaturvedi L, Meacham RB, Koul HK. p42/p44 Mitogen-activated protein kinase signal transduction pathway regulates interleukin-6 expression in PC3 cells, a line of hormone-refractory prostate cancer cells. Ann N Y Acad Sci. 2004;1030:253-7 [DOI] [PubMed] [Google Scholar]
- 98. Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25(21):2987-98 [DOI] [PubMed] [Google Scholar]
- 99. Denkert C, Siegert A, Leclere A, Turzynski A, Hauptmann S. An inhibitor of stress-activated MAP-kinases reduces invasion and MMP-2 expression of malignant melanoma cells. Clin Exp Metastasis. 2002;19(1):79-85 [DOI] [PubMed] [Google Scholar]
- 100. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747-52 [DOI] [PubMed] [Google Scholar]
- 101. del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40(1):79-84 [DOI] [PubMed] [Google Scholar]
- 102. Janda E, Lehmann K, Killisch I, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156(2):299-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith AP, Verrecchia A, Faga G, et al. A positive role for Myc in TGFbeta-induced Snail transcription and epithelial-to-mesenchymal transition. Oncogene. 2009;28(3):422-30 [DOI] [PubMed] [Google Scholar]
- 104. Yu M, Smolen GA, Zhang J, et al. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev. 2009;23(15):1737-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21(12):2907-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Horn G, Gaziel A, Wreschner DH, Smorodinsky NI, Ehrlich M. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Exp Cell Res. 2009;315(8):1490-504 [DOI] [PubMed] [Google Scholar]
- 107. Lee MK, Pardoux C, Hall MC, et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26(17):3957-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Takekawa M, Tatebayashi K, Itoh F, Adachi M, Imai K, Saito H. Smad-dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF-beta. EMBO J. 2002;21(23):6473-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25(5):1375-82 [PubMed] [Google Scholar]
- 110. Kim MS, Lee EJ, Kim HR, Moon A. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63(17):5454-61 [PubMed] [Google Scholar]
- 111. Yong HY, Hwang JS, Son H, et al. Identification of H-Ras-specific motif for the activation of invasive signaling program in human breast epithelial cells. Neoplasia. 2011;13(2):98-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nick JA, Avdi NJ, Young SK, et al. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99(5):975-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang Y, Neo SY, Han J, Yaw LP, Lin SC. RGS16 attenuates galphaq-dependent p38 mitogen-activated protein kinase activation by platelet-activating factor. J Biol Chem. 1999;274(5):2851-7 [DOI] [PubMed] [Google Scholar]
- 114. Haagenson KK, Wu GS. The role of MAP kinases and MAP kinase phosphatase-1 in resistance to breast cancer treatment. Cancer Metastasis Rev. 2010;29(1):143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou JY, Liu Y, Wu GS. The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death. Cancer Res. 2006;66(9):4888-94 [DOI] [PubMed] [Google Scholar]
- 116. Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386(6622):296-9 [DOI] [PubMed] [Google Scholar]
- 117. Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272(5266):1347-9 [DOI] [PubMed] [Google Scholar]
- 118. Yang SH, Galanis A, Sharrocks AD. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19(6):4028-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003;22(6):1313-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Song H, Ki SH, Kim SG, Moon A. Activating transcription factor 2 mediates matrix metalloproteinase-2 transcriptional activation induced by p38 in breast epithelial cells. Cancer Res. 2006;66(21):10487-96 [DOI] [PubMed] [Google Scholar]
- 121. Campbell J, Ciesielski CJ, Hunt AE, et al. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004;173(11):6928-37 [DOI] [PubMed] [Google Scholar]
- 122. Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem. 1999;274(43):30858-63 [DOI] [PubMed] [Google Scholar]
- 123. Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94(8):1154-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Morooka H, Bonventre JV, Pombo CM, Kyriakis JM, Force T. Ischemia and reperfusion enhance ATF-2 and c-Jun binding to cAMP response elements and to an AP-1 binding site from the c-jun promoter. J Biol Chem. 1995;270(50):30084-92 [DOI] [PubMed] [Google Scholar]
- 125. Zhao M, New L, Kravchenko VV, et al. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19(1):21-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huang S, New L, Pan Z, Han J, Nemerow GR. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem. 2000;275(16):12266-72 [DOI] [PubMed] [Google Scholar]
- 127. Han YC, Zeng XX, Wang R, Zhao Y, Li BL, Song M. Correlation of p38 mitogen-activated protein kinase signal transduction pathway to uPA expression in breast cancer. Ai Zheng. 2007;26(1):48-53 [PubMed] [Google Scholar]
- 128. Han Q, Leng J, Bian D, et al. Rac1-MKK3-p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem. 2002;277(50):48379-85 [DOI] [PubMed] [Google Scholar]
- 129. Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67(8):3752-8 [DOI] [PubMed] [Google Scholar]
- 130. Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res. 2005;308(1):135-45 [DOI] [PubMed] [Google Scholar]
- 131. Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23(11):2469-76 [DOI] [PubMed] [Google Scholar]
- 132. Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22(16):5835-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Underwood DC, Osborn RR, Kotzer CJ, et al. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther. 2000;293(1):281-8 [PubMed] [Google Scholar]
- 134. Shin I, Kim S, Song H, Kim HR, Moon A. H-Ras-specific activation of Rac-MKK3/6-p38 pathway: its critical role in invasion and migration of breast epithelial cells. J Biol Chem. 2005;280(15):14675-83 [DOI] [PubMed] [Google Scholar]
- 135. Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64(2):327-36 [DOI] [PubMed] [Google Scholar]
- 136. Johansson N, Ala-aho R, Uitto V, et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113(Pt 2):227-35 [DOI] [PubMed] [Google Scholar]
- 137. Simon C, Simon M, Vucelic G, et al. The p38 SAPK pathway regulates the expression of the MMP-9 collagenase via AP-1-dependent promoter activation. Exp Cell Res. 2001;271(2):344-55 [DOI] [PubMed] [Google Scholar]
- 138. Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58(6):1135-9 [PubMed] [Google Scholar]
- 139. Reunanen N, Li SP, Ahonen M, Foschi M, Han J, Kahari VM. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277(35):32360-8 [DOI] [PubMed] [Google Scholar]
- 140. Rust W, Kingsley K, Petnicki T, Padmanabhan S, Carper SW, Plopper GE. Heat shock protein 27 plays two distinct roles in controlling human breast cancer cell migration on laminin-5. Mol Cell Biol Res Commun. 1999;1(3):196-202 [DOI] [PubMed] [Google Scholar]
- 141. Yao J, Xiong S, Klos K, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20(56):8066-74 [DOI] [PubMed] [Google Scholar]
- 142. Nguyen TH, Mustafa FB, Pervaiz S, Ng FS, Lim LH. ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int J Oncol. 2008;33(1):81-92 [PubMed] [Google Scholar]
- 143. Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Res. 2003;63(10):2483-91 [PubMed] [Google Scholar]
- 144. Davidson B, Konstantinovsky S, Nielsen S, et al. Altered expression of metastasis-associated and regulatory molecules in effusions from breast cancer patients: a novel model for tumor progression. Clin Cancer Res. 2004;10(21):7335-46 [DOI] [PubMed] [Google Scholar]
- 145. Konstantinovsky S, Nielsen S, Vyberg M, et al. Angiogenic molecule expression is downregulated in effusions from breast cancer patients. Breast Cancer Res Treat. 2005;94(1):71-80 [DOI] [PubMed] [Google Scholar]
- 146. Davidson B, Reich R, Lazarovici P, Ann Florenes V, Nielsen S, Nesland JM. Altered expression and activation of the nerve growth factor receptors TrkA and p75 provide the first evidence of tumor progression to effusion in breast carcinoma. Breast Cancer Res Treat. 2004;83(2):119-28 [DOI] [PubMed] [Google Scholar]
- 147. Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60(3):261-310 [DOI] [PubMed] [Google Scholar]
- 148. Sengupta N, Siddiqui E, Mumtaz FH. Cancers of the bladder. J R Soc Promot Health. 2004;124(5):228-9 [DOI] [PubMed] [Google Scholar]
- 149. Oosterlinck W, Lobel B, Jakse G, et al. Guidelines on bladder cancer. Eur Urol. 2002;41(2):105-12 [DOI] [PubMed] [Google Scholar]
- 150. Tolley DA, Parmar MK, Grigor KM, et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol. 1996;155(4):1233-8 [PubMed] [Google Scholar]
- 151. Feng GJ, Goodridge HS, Harnett MM, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163(12):6403-12 [PubMed] [Google Scholar]
- 152. Scherle PA, Jones EA, Favata MF, et al. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161(10):5681-6 [PubMed] [Google Scholar]
- 153. Valledor AF, Comalada M, Xaus J, Celada A. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000;275(10):7403-9 [DOI] [PubMed] [Google Scholar]
- 154. Shan X, Hu A, Veler H, et al. Regulation of Toll-like receptor 4-induced proasthmatic changes in airway smooth muscle function by opposing actions of ERK1/2 and p38 MAPK signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L324-33 [DOI] [PubMed] [Google Scholar]
- 155. Yokoyama T, Komori A, Nakamura M, et al. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK signaling pathways. Liver Int. 2006;26(4):467-76 [DOI] [PubMed] [Google Scholar]
- 156. Seguchi T, Yokokawa K, Sugao H, Nakano E, Sonoda T, Okuyama A. Interleukin-6 activity in urine and serum in patients with bladder carcinoma. J Urol. 1992;148(3):791-4 [DOI] [PubMed] [Google Scholar]
- 157. Cardillo MR, Sale P, Di Silverio F. Heat shock protein-90, IL-6 and IL-10 in bladder cancer. Anticancer Res. 2000;20(6B):4579-83 [PubMed] [Google Scholar]
- 158. Okamoto M, Hattori K, Oyasu R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. Int J Cancer. 1997;72(1):149-54 [DOI] [PubMed] [Google Scholar]
- 159. Horwood NJ, Page TH, McDaid JP, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176(6):3635-41 [DOI] [PubMed] [Google Scholar]
- 160. Davies B, Waxman J, Wasan H, et al. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53(22):5365-9 [PubMed] [Google Scholar]
- 161. Nutt JE, Durkan GC, Mellon JK, Lunec J. Matrix metalloproteinases (MMPs) in bladder cancer: the induction of MMP9 by epidermal growth factor and its detection in urine. BJU Int. 2003;91(1):99-104 [DOI] [PubMed] [Google Scholar]
- 162. Ozdemir E, Kakehi Y, Okuno H, Yoshida O. Role of matrix metalloproteinase-9 in the basement membrane destruction of superficial urothelial carcinomas. J Urol. 1999;161(4):1359-63 [PubMed] [Google Scholar]
- 163. Cohen M, Meisser A, Haenggeli L, Bischof P. Involvement of MAPK pathway in TNF-alpha-induced MMP-9 expression in human trophoblastic cells. Mol Hum Reprod. 2006;12(4):225-32 [DOI] [PubMed] [Google Scholar]
- 164. Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278(2):484-92 [DOI] [PubMed] [Google Scholar]
- 165. Alam J, Wicks C, Stewart D, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275(36):27694-702 [DOI] [PubMed] [Google Scholar]
- 166. Keum YS, Yu S, Chang PP, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66(17):8804-13 [DOI] [PubMed] [Google Scholar]
- 167. Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64(5-6):765-70 [DOI] [PubMed] [Google Scholar]
- 168. Buytaert E, Callewaert G, Hendrickx N, et al. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006;20(6):756-8 [DOI] [PubMed] [Google Scholar]
- 169. Assefa Z, Vantieghem A, Declercq W, et al. The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J Biol Chem. 1999;274(13):8788-96 [DOI] [PubMed] [Google Scholar]
- 170. Hendrickx N, Volanti C, Moens U, et al. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J Biol Chem. 2003;278(52):52231-9 [DOI] [PubMed] [Google Scholar]
- 171. Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15(1):11-8 [DOI] [PubMed] [Google Scholar]
- 172. Lee TJ, Sartor O, Luftig RB, Koochekpour S. Saposin C promotes survival and prevents apoptosis via PI3K/Akt-dependent pathway in prostate cancer cells. Mol Cancer. 2004;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31(4):319-24 [DOI] [PubMed] [Google Scholar]
- 174. Kocanova S, Buytaert E, Matroule JY, et al. Induction of heme-oxygenase 1 requires the p38MAPK and PI3K pathways and suppresses apoptotic cell death following hypericin-mediated photodynamic therapy. Apoptosis. 2007;12(4):731-41 [DOI] [PubMed] [Google Scholar]
- 175. Bostrom PJ, Ravanti L, Reunanen N, et al. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int J Cancer. 2000;88(3):417-23 [PubMed] [Google Scholar]
- 176. Buytaert E, Matroule JY, Durinck S, et al. Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene. 2008;27(13):1916-29 [DOI] [PubMed] [Google Scholar]
- 177. Romanenko A, Morimura K, Wanibuchi H, et al. Urinary bladder lesions induced by persistent chronic low-dose ionizing radiation. Cancer Sci. 2003;94(4):328-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Capon DJ, Chen EY, Levinson AD, Seeburg PH, Goeddel DV. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983;302(5903):33-7 [DOI] [PubMed] [Google Scholar]
- 179. Aaltonen V, Bostrom PJ, Soderstrom KO, et al. Urinary bladder transitional cell carcinogenesis is associated with down-regulation of NF1 tumor suppressor gene in vivo and in vitro. Am J Pathol. 1999;154(3):755-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Larrea E, Garcia N, Qian C, Civeira MP, Prieto J. Tumor necrosis factor alpha gene expression and the response to interferon in chronic hepatitis C. Hepatology. 1996;23(2):210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kaplanski G, Farnarier C, Payan MJ, Bongrand P, Durand JM. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42(11):2277-84 [DOI] [PubMed] [Google Scholar]
- 182. Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274(5290):1194-7 [DOI] [PubMed] [Google Scholar]
- 183. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326-31 [DOI] [PubMed] [Google Scholar]
- 184. Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90-4 [DOI] [PubMed] [Google Scholar]
- 185. Chen Z, Seimiya H, Naito M, et al. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18(1):173-80 [DOI] [PubMed] [Google Scholar]
- 186. MacFarlane M, Cohen GM, Dickens M. JNK (c-Jun N-terminal kinase) and p38 activation in receptor-mediated and chemically-induced apoptosis of T-cells: differential requirements for caspase activation. Biochem J. 2000;348(Pt 1):93-101 [PMC free article] [PubMed] [Google Scholar]
- 187. Sakamoto H, Mashima T, Sato S, Hashimoto Y, Yamori T, Tsuruo T. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res. 2001;7(8):2513-8 [PubMed] [Google Scholar]
- 188. Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380(6569):75-9 [DOI] [PubMed] [Google Scholar]
- 189. Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273(3):1741-8 [DOI] [PubMed] [Google Scholar]
- 190. Butterfield L, Storey B, Maas L, Heasley LE. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J Biol Chem. 1997;272(15):10110-6 [DOI] [PubMed] [Google Scholar]
- 191. Zanke BW, Boudreau K, Rubie E, et al. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6(5):606-13 [DOI] [PubMed] [Google Scholar]
- 192. Brenner B, Koppenhoefer U, Weinstock C, Linderkamp O, Lang F, Gulbins E. Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J Biol Chem. 1997;272(35):22173-81 [DOI] [PubMed] [Google Scholar]
- 193. Goillot E, Raingeaud J, Ranger A, et al. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci U S A. 1997;94(7):3302-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491-501 [DOI] [PubMed] [Google Scholar]
- 195. Farley N, Pedraza-Alva G, Serrano-Gomez D, et al. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol. 2006;26(6):2118-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hatai T, Matsuzawa A, Inoshita S, et al. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275(34):26576-81 [DOI] [PubMed] [Google Scholar]
- 197. Teng DH, Perry WL, 3rd, Hogan JK, et al. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57(19):4177-82 [PubMed] [Google Scholar]
- 198. Kang JS, Kim HM, Choi IY, et al. DBM1285 suppresses tumor necrosis factor alpha production by blocking p38 mitogen-activated protein kinase/mitogen-activated protein kinase-activated protein kinase 2 signaling pathway. J Pharmacol Exp Ther. 2010;334(2):657-64 [DOI] [PubMed] [Google Scholar]
- 199. Shin EC, Shin JS, Park JH, Kim JJ, Kim H, Kim SJ. Expression of Fas-related genes in human hepatocellular carcinomas. Cancer Lett. 1998;134(2):155-62 [DOI] [PubMed] [Google Scholar]
- 200. Loda M, Capodieci P, Mishra R, et al. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149(5):1553-64 [PMC free article] [PubMed] [Google Scholar]
- 201. Kikuchi K, Kitamura K, Kakinoki Y, et al. Gene expressions and activities of protein phosphatases 1 alpha, 2A and 2C in hepatocarcinogenesis and regeneration after partial hepatectomy. Cancer Detect Prev. 1997;21(1):36-43 [PubMed] [Google Scholar]
- 202. Seufferlein T, Seckl MJ, Schwarz E, et al. Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br J Cancer. 2002;86(7):1188-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wu J, Suzuki H, Akhand AA, Zhou YW, Hossain K, Nakashima I. Modes of activation of mitogen-activated protein kinases and their roles in cepharanthine-induced apoptosis in human leukemia cells. Cell Signal. 2002;14(6):509-15 [DOI] [PubMed] [Google Scholar]
- 204. Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors—mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82(2-3):389-97 [DOI] [PubMed] [Google Scholar]
- 205. Barnes PJ. Novel approaches and targets for treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S72-9 [DOI] [PubMed] [Google Scholar]
- 206. Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47(2-3):185-201 [DOI] [PubMed] [Google Scholar]
- 207. Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50(4):515-96 [PubMed] [Google Scholar]
- 208. Tamura DY, Moore EE, Johnson JL, Zallen G, Aiboshi J, Silliman CC. p38 mitogen-activated protein kinase inhibition attenuates intercellular adhesion molecule-1 up-regulation on human pulmonary microvascular endothelial cells. Surgery. 1998;124(2):403-7 [PubMed] [Google Scholar]
- 209. Scott PH, Paul A, Belham CM, et al. Hypoxic stimulation of the stress-activated protein kinases in pulmonary artery fibroblasts. Am J Respir Crit Care Med. 1998;158(3):958-62 [DOI] [PubMed] [Google Scholar]
- 210. Hashimoto S, Matsumoto K, Gon Y, Nakayama T, Takeshita I, Horie T. Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 1999;159(2):634-40 [DOI] [PubMed] [Google Scholar]
- 211. Kankaanranta H, De Souza PM, Barnes PJ, Salmon M, Giembycz MA, Lindsay MA. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils. J Pharmacol Exp Ther. 1999;290(2):621-8 [PubMed] [Google Scholar]
- 212. Underwood DC, Osborn RR, Bochnowicz S, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L895-902 [DOI] [PubMed] [Google Scholar]
- 213. Marshall LA, Hansbury MJ, Bolognese BJ, Gum RJ, Young PR, Mayer RJ. Inhibitors of the p38 mitogen-activated kinase modulate IL-4 induction of low affinity IgE receptor (CD23) in human monocytes. J Immunol. 1998;161(11):6005-13 [PubMed] [Google Scholar]
- 214. Bracke M, Coffer PJ, Lammers JW, Koenderman L. Analysis of signal transduction pathways regulating cytokine-mediated Fc receptor activation on human eosinophils. J Immunol. 1998;161(12):6768-74 [PubMed] [Google Scholar]
- 215. Ulich TR, Yin SM, Guo KZ, del Castillo J, Eisenberg SP, Thompson RC. The intratracheal administration of endotoxin and cytokines. III. The interleukin-1 (IL-1) receptor antagonist inhibits endotoxin- and IL-1-induced acute inflammation. Am J Pathol. 1991;138(3):521-4 [PMC free article] [PubMed] [Google Scholar]
- 216. Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42(4):640-51 [PubMed] [Google Scholar]
- 217. Koh Y, Hybertson BM, Jepson EK, Repine JE. Tumor necrosis factor induced acute lung leak in rats: less than with interleukin-1. Inflammation. 1996;20(5):461-9 [DOI] [PubMed] [Google Scholar]
- 218. Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA. Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993;142(6):1739-49 [PMC free article] [PubMed] [Google Scholar]
- 219. Lee JC, Adams JL. Inhibitors of serine/threonine kinases. Curr Opin Biotechnol. 1995;6(6):657-61 [DOI] [PubMed] [Google Scholar]
- 220. Lee JC, Badger AM, Griswold DE, et al. Bicyclic imidazoles as a novel class of cytokine biosynthesis inhibitors. Ann N Y Acad Sci. 1993;696:149-70 [DOI] [PubMed] [Google Scholar]
- 221. Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739-46 [DOI] [PubMed] [Google Scholar]
- 222. Young P, McDonnell P, Dunnington D, Hand A, Laydon J, Lee J. Pyridinyl imidazoles inhibit IL-1 and TNF production at the protein level. Agents Actions. 1993;39 Spec No:C67-9. [DOI] [PubMed] [Google Scholar]
- 223. Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42(4):459-67 [DOI] [PubMed] [Google Scholar]
- 224. Prichett W, Hand A, Sheilds J, Dunnington D. Mechanism of action of bicyclic imidazoles defines a translational regulatory pathway for tumor necrosis factor alpha. J Inflamm. 1995;45(2):97-105 [PubMed] [Google Scholar]
- 225. O’Leary EC, Marder P, Zuckerman SH. Glucocorticoid effects in an endotoxin-induced rat pulmonary inflammation model: differential effects on neutrophil influx, integrin expression, and inflammatory mediators. Am J Respir Cell Mol Biol. 1996;15(1):97-106 [DOI] [PubMed] [Google Scholar]
- 226. Ulich TR, Yin S, Remick DG, Russell D, Eisenberg SP, Kohno T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol. 1993;142(5):1335-8 [PMC free article] [PubMed] [Google Scholar]
- 227. Wesselius LJ, Smirnov IM, O’Brien-Ladner AR, Nelson ME. Synergism of intratracheally administered tumor necrosis factor with interleukin-1 in the induction of lung edema in rats. J Lab Clin Med. 1995;125(5):618-25 [PubMed] [Google Scholar]
- 228. Fuchs HJ, Debs R, Patton JS, Liggitt HD. The pattern of lung injury induced after pulmonary exposure to tumor necrosis factor-alpha depends on the route of administration. Diagn Microbiol Infect Dis. 1990;13(5):397-404 [DOI] [PubMed] [Google Scholar]
- 229. Kolls JK, Lei D, Nelson S, Summer WR, Greenberg S, Beutler B. Adenovirus-mediated blockade of tumor necrosis factor in mice protects against endotoxic shock yet impairs pulmonary host defense. J Infect Dis. 1995;171(3):570-5 [DOI] [PubMed] [Google Scholar]
- 230. Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276(5 Pt 1):L715-27 [DOI] [PubMed] [Google Scholar]
- 231. Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Respir Cell Mol Biol. 1999;20(4):751-8 [DOI] [PubMed] [Google Scholar]
- 232. Jiang Y, Chen C, Li Z, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J Biol Chem. 1996;271(30):17920-6 [DOI] [PubMed] [Google Scholar]
- 233. Lechner C, Zahalka MA, Giot JF, Moller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci U S A. 1996;93(9):4355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wang XS, Diener K, Manthey CL, et al. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272(38):23668-74 [DOI] [PubMed] [Google Scholar]
- 235. Jiang Y, Gram H, Zhao M, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272(48):30122-8 [DOI] [PubMed] [Google Scholar]
- 236. Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162(7):4246-52 [PubMed] [Google Scholar]
- 237. Nick JA, Avdi NJ, Young SK, et al. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest. 1999;103(6):851-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Hu MC, Wang YP, Mikhail A, Qiu WR, Tan TH. Murine p38-delta mitogen-activated protein kinase, a developmentally regulated protein kinase that is activated by stress and proinflammatory cytokines. J Biol Chem. 1999;274(11):7095-102 [DOI] [PubMed] [Google Scholar]
- 239. Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem Biophys Res Commun. 1997;235(3):533-8 [DOI] [PubMed] [Google Scholar]
- 240. Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160(2):920-8 [PubMed] [Google Scholar]
- 241. Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179(4):939-44 [DOI] [PubMed] [Google Scholar]
- 242. Hui L, Bakiri L, Stepniak E, Wagner EF. p38alpha: a suppressor of cell proliferation and tumorigenesis. Cell Cycle. 2007;6(20):2429-33 [DOI] [PubMed] [Google Scholar]
- 243. Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5(1):44-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Tortora G, Bianco R, Daniele G, et al. Overcoming resistance to molecularly targeted anticancer therapies: rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist Updat. 2007;10(3):81-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Clark AR, Dean JL, Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546(1):37-44 [DOI] [PubMed] [Google Scholar]
- 247. Kim C, Sano Y, Todorova K, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9(9):1019-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Kang YJ, Chen J, Otsuka M, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180(7):5075-82 [DOI] [PubMed] [Google Scholar]
- 249. Beardmore VA, Hinton HJ, Eftychi C, et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25(23):10454-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. O’Keefe SJ, Mudgett JS, Cupo S, et al. Chemical genetics define the roles of p38alpha and p38beta in acute and chronic inflammation. J Biol Chem. 2007;282(48):34663-71 [DOI] [PubMed] [Google Scholar]
- 251. Pourgholami MH, Morris DL. Inhibitors of vascular endothelial growth factor in cancer. Cardiovasc Hematol Agents Med Chem. 2008;6(4):343-7 [DOI] [PubMed] [Google Scholar]
- 252. Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol. 2010;177(2):984-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci. 2008;13:3581-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Junttila MR, Ala-Aho R, Jokilehto T, et al. p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene. 2007;26(36):5267-79 [DOI] [PubMed] [Google Scholar]
- 255. Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66(4):2264-70 [DOI] [PubMed] [Google Scholar]
- 256. Dreissigacker U, Mueller MS, Unger M, et al. Oncogenic K-Ras down-regulates Rac1 and RhoA activity and enhances migration and invasion of pancreatic carcinoma cells through activation of p38. Cell Signal. 2006;18(8):1156-68 [DOI] [PubMed] [Google Scholar]
- 257. McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem. 2005;280(22):20995-1003 [DOI] [PubMed] [Google Scholar]
- 258. Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25(4):713-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Koul H, Khandrika L. Signaltransduction targets in prostate cancer. Curr Signal Transduction Ther. 2008;3(2):112-28 [Google Scholar]
- 260. Adams RH, Porras A, Alonso G, et al. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6(1):109-16 [PubMed] [Google Scholar]
- 261. Mudgett JS, Ding J, Guh-Siesel L, et al. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000;97(19):10454-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Sabio G, Arthur JS, Kuma Y, et al. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24(6):1134-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Sumara G, Formentini I, Collins S, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136(2):235-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Schindler EM, Hindes A, Gribben EL, et al. p38delta Mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Res. 2009;69(11):4648-55 [DOI] [PubMed] [Google Scholar]
- 265. Demuth T, Reavie LB, Rennert JL, et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6(4):1212-22 [DOI] [PubMed] [Google Scholar]
- 266. Esteva FJ, Sahin AA, Smith TL, et al. Prognostic significance of phosphorylated P38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer. 2004;100(3):499-506 [DOI] [PubMed] [Google Scholar]
- 267. Pomerance M, Quillard J, Chantoux F, Young J, Blondeau JP. High-level expression, activation, and subcellular localization of p38-MAP kinase in thyroid neoplasms. J Pathol. 2006;209(3):298-306 [DOI] [PubMed] [Google Scholar]
- 268. Xu C, Shen G, Yuan X, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27(3):437-45 [DOI] [PubMed] [Google Scholar]
- 269. Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1(12):1059-66 [PubMed] [Google Scholar]
- 270. Maeda H, Hori S, Nishitoh H, et al. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61(14):5432-40 [PubMed] [Google Scholar]
- 271. Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62(13):3615-9 [PubMed] [Google Scholar]
- 272. Murillo H, Schmidt LJ, Tindall DJ. Tyrphostin AG825 triggers p38 mitogen-activated protein kinase-dependent apoptosis in androgen-independent prostate cancer cells C4 and C4-2. Cancer Res. 2001;61(20):7408-12 [PubMed] [Google Scholar]
- 273. Cho SD, Ahn NS, Jung JW, et al. Critical role of the c-JunNH2-terminal kinase and p38 mitogen-activated protein kinase pathways on sodium butyrate-induced apoptosis in DU145 human prostate cancer cells. Eur J Cancer Prev. 2006;15(1):57-63 [DOI] [PubMed] [Google Scholar]
- 274. Ricote M, Garcia-Tunon I, Fraile B, et al. P38 MAPK protects against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer cells. Apoptosis. 2006;11(11):1969-75 [DOI] [PubMed] [Google Scholar]