Abstract
Objective:
Our objective was to determine the association of early brain maturation with neurodevelopmental outcome in premature neonates.
Methods:
Neonates born between 24 and 32 weeks’ gestation (April 2006 to August 2010) were prospectively studied with MRI early in life and again at term-equivalent age. Using diffusion tensor imaging and magnetic resonance spectroscopic imaging, fractional anisotropy (FA) (microstructure) and N-acetylaspartate (NAA)/choline (metabolism) were measured from the basal nuclei, white matter tracts, and superior white matter. Brain maturation is characterized by increasing FA and NAA/choline from early in life to term-equivalent age. In premature neonates, systemic illness and critical care therapies have been linked to abnormalities of these measures. Of the 177 neonates in this cohort, 5 died and 157 (91% of survivors) were assessed at 18 months’ corrected age (adjusted for prematurity) using the Bayley Scales of Infant and Toddler Development III motor, cognitive, and language composite scores (mean = 100, SD = 15).
Results:
Among these 157 infants, white matter injury was seen in 48 (30%). Severe white matter injury, in 10 neonates (6%), was associated with a decrease in motor (−18 points; p < 0.001) and cognitive (−8 points; p = 0.085) scores. With greater severity of adverse neurodevelopmental outcomes, slower increases in FA and NAA/choline were observed in the basal nuclei and brain white matter regions as neonates matured to term-equivalent age, independent of the presence of white matter injury.
Conclusions:
In the preterm neonate, abnormal brain maturation evolves through the period of neonatal intensive care and is associated with adverse neurodevelopmental outcomes.
In children born prematurely, a spectrum of white matter injury (WMI) is recognized with diagnostic MRI, but it does not fully account for the burden of neurodevelopmental disability frequently recognized in these infants.1–3 WMI is associated with widespread abnormalities of brain maturation, even in areas remote from WMI.4–6 Diffusion tensor imaging (DTI) and magnetic resonance spectroscopic imaging (MRSI) now provide a simple paradigm for evaluating microstructural and metabolic brain development.5,7–9
Although a large body of work has linked advanced magnetic resonance parameters of brain development at term-equivalent age to long-term outcomes,10−13 little is known about the trajectory of development from early in preterm life, the period when intensive care interventions are occurring. When preterm neonates are imaged at term-equivalent age, decreased cortical and subcortical gray matter volumes and abnormal white matter microstructure are associated with neurodevelopmental disabilities.13−15 In preterm neonates scanned serially with MRI, the rate of cerebral cortical growth predicted cognitive, but not motor, skills in childhood.16 Despite emerging links between systemic illness17−21 and critical care therapies22 and impaired white matter and subcortical brain microstructure and metabolism, the impact of early abnormal maturation of these structures on neurodevelopmental outcomes needs to be determined.
The objective of this prospective cohort study of preterm neonates studied with serial brain MRI was to determine the association of brain microstructural and metabolic maturation with neurodevelopment at 18 months’ corrected age. We hypothesized that adverse neurodevelopmental outcomes are associated with impaired brain maturation in the white matter and basal nuclei.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of British Columbia Clinical Research Ethics Board. After informed consent was obtained from parents, study participants were recruited prospectively from April 2006 to August 2010 at the British Columbia Women's Hospital, the major provincial tertiary-level neonatal intensive care unit.
Study population.
Newborns were eligible if they were born between 24 and 32 weeks’ gestation. Exclusion criteria comprised 1) congenital malformation or syndrome, 2) antenatal congenital infection, or 3) ultrasound evidence of a large parenchymal venous hemorrhagic infarction (>2 cm). All parents of eligible infants were approached if their child was admitted for more than 1 week and if they accepted to meet with a study team member. Of the parents approached (figure e-1 on the Neurology® Web site at www.neurology.org), 188 consented to participate in this study; 177 of them had at least one MRI scan. Of this cohort, 5 newborns (3.5%) died and 157 (91% of survivors) came to follow-up. This mortality rate is similar to other Canadian neonatal intensive care units.23 Enrolled patients were younger (median: 27.0 vs 29.0 weeks’ gestation; p = 0.0001) and smaller (median: 991 vs 1,165 g; p = 0.0001) at birth, compared with the nonparticipating newborns.
Clinical data collection.
Systematic detailed chart reviews were performed to collect clinical information about pregnancy, labor, delivery, and perinatal course (table e-1; see e-Methods for clinical definitions).
MRI studies.
With a magnetic resonance–compatible isolette (Lammers Medical Technology, Lubeck, Germany) and specialized neonatal head coil (Advanced Imaging Research, Cleveland, OH), newborns were studied twice with MRI: once within the first few weeks after birth when clinically stable and again at term-equivalent age. Detailed imaging methods applied in this cohort have been described previously.5,19 MRI studies were performed without pharmacologic sedation on a Siemens 1.5T Avanto scanner (Erlangen, Germany) with 3-dimensional coronal volumetric T1-weighted images and axial fast spin echo T2-weighted images.5,19 An experienced neuroradiologist (K.P.), blinded to the newborn's medical history, rated the severity of WMI, intraventricular hemorrhage (IVH), ventriculomegaly, and cerebellar hemorrhage using scores that have high intraobserver reliability.1,5,24 The severity of WMI was scored as minimal (≤3 lesions of <2 mm), moderate (>3 lesions or lesions >2 mm, but involving no more than 5% of the hemisphere), or severe (>5% of hemispheric involvement).1
Using DTI (see e-Methods), we measured fractional anisotropy (FA) from 7 white matter regions and 5 gray matter regions as reported previously.5,19 With white matter development, FA increases in parallel with the maturation of the oligodendrocyte lineage and early myelination.7 For analyses, regions of interest were grouped as follows: 1) basal nuclei (caudate, lentiform nuclei, and thalamus); 2) white matter tracts (genu and splenium of corpus callosum, posterior limb of the internal capsule and optic radiations); and 3) superior white matter (anterior, central, and posterior white matter). These regions of interest were grouped to reflect similarities in anatomical connectivity or proximity.
MRSI (see e-Methods) assesses brain regional metabolism by measuring the relative concentration of certain metabolites. The N-acetylaspartate (NAA)/choline ratio reflects neuronal integrity and metabolism, and increases with brain maturation.8 NAA/choline was measured from 4 gray matter regions and 3 white matter regions.5,19 As for DTI, these regions were grouped as gray matter (group 1) and white matter (groups 2 and 3). In previous studies examining the relationship between early brain injury and systemic illness with brain maturation, FA and NAA/choline demonstrated the most robust associations.5,19,21
Neurodevelopmental outcomes at 18 months of age.
At 18 months’ corrected age, 154 children underwent a neurodevelopmental assessment conducted by examiners who were unaware of the MRI findings (median 18.7 months’ corrected age; interquartile range [IQR] 18.3–19.2 months). Three children missed their 18-month follow-up appointment, but were assessed around 33 months of age for a total of 157 infants with follow-up data. Of these 157 children who came to follow-up, all were scanned at least once and 140 were scanned twice. The examiners assessed neurodevelopment using the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III), with separate composite scores (mean 100 ± SD 15) for cognition, language (receptive and expressive), and motor skills (fine and gross). Given previous findings that the Bayley-III seems to overestimate neurodevelopment,25 for statistical analysis purposes, the cohort was divided into 4 outcome categories: 1) normal (>100), 2) low normal (85–100), 3) mild impairment (70–84), and 4) significant impairment (<70). One patient was too delayed to be reliably tested and was assigned a score of 49 for each domain of the Bayley-III. Maternal education was described as a measure of socioeconomic status.26
Data analysis.
Statistical analysis was performed using Stata 11 software (StataCorp, College Station, TX). Clinical characteristics of the newborns were compared using 2-tailed Fisher exact test and Kruskal-Wallis test for categorical and continuous data, respectively. Linear regression was used to evaluate the association of brain injury (e.g., WMI) with Bayley-III domains at each scan. Using generalized estimating equation modeling for repeated measures, adjusting for gestational age at birth, postmenstrual age at MRI scan, and multiple regions of interest, mean values of FA and NAA/choline ratios from both scans were compared across outcome groups. To address our primary hypothesis, interaction terms for outcome groups by postmenstrual age at MRI were examined to determine whether parameters of brain maturation evolved differently across outcome groups.
RESULTS
Clinical characteristics of the newborns.
The total cohort comprised 177 preterm newborns (90 males) delivered at a median age of 27.6 weeks’ gestation (range 24.0–32.0; IQR 25.9–29.7 weeks). Patients had their first MRI scan at a median age of 32.1 weeks’ gestation (range 27.1–46.3; IQR 30.5–33.9 weeks). Of those, 141 had a second scan at 40.3 weeks (range 32.0–46.4; IQR 38.7–42.1 weeks). Five children died before 18 months of age. The clinical features of children lost to follow-up are provided in table e-1.
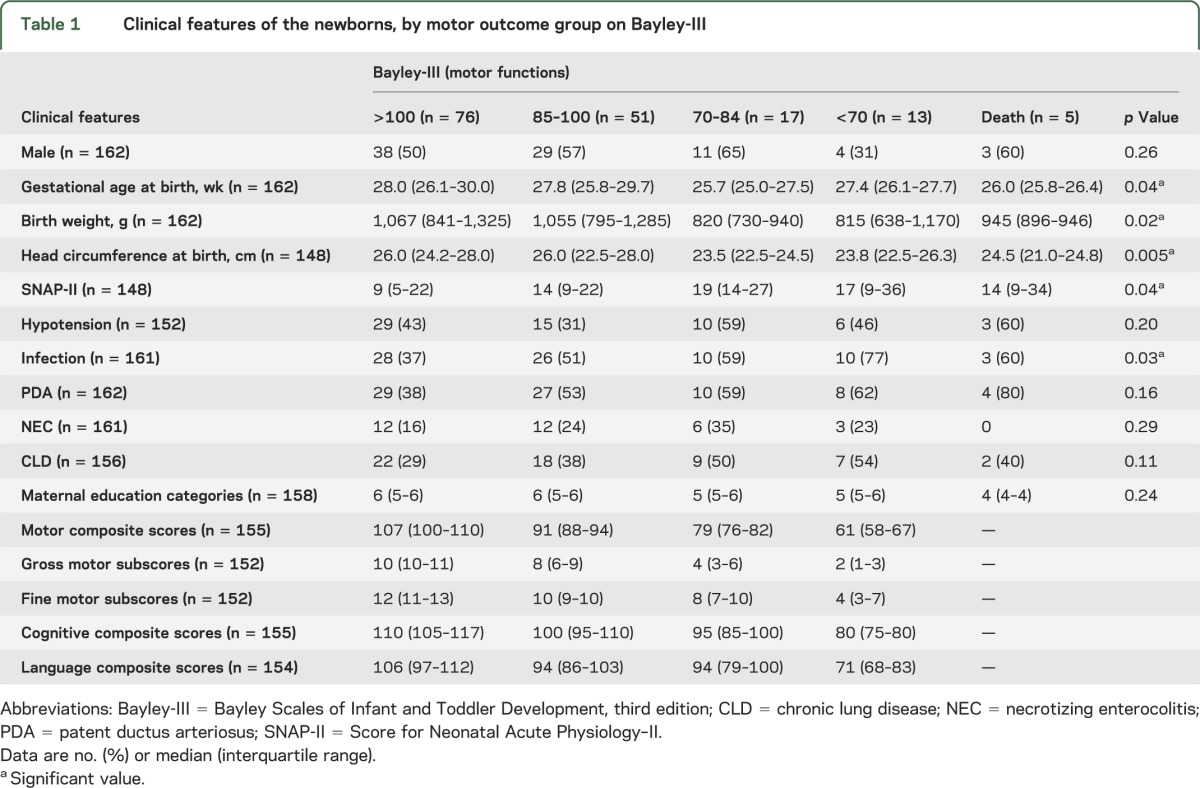
In general, motor scores were lower than cognitive and language scores (table 1). The children in each outcome group had similar clinical characteristics, except those with significant motor delay (scores <70) were born at lower gestational age, were smaller at birth, required more support in the first day of life, and were more likely to have postnatal infection. Maternal education categories (Hollingshead) were similar across the motor outcome groups.
Table 1.
Clinical features of the newborns, by motor outcome group on Bayley-III
Brain injury and neurodevelopmental outcomes.
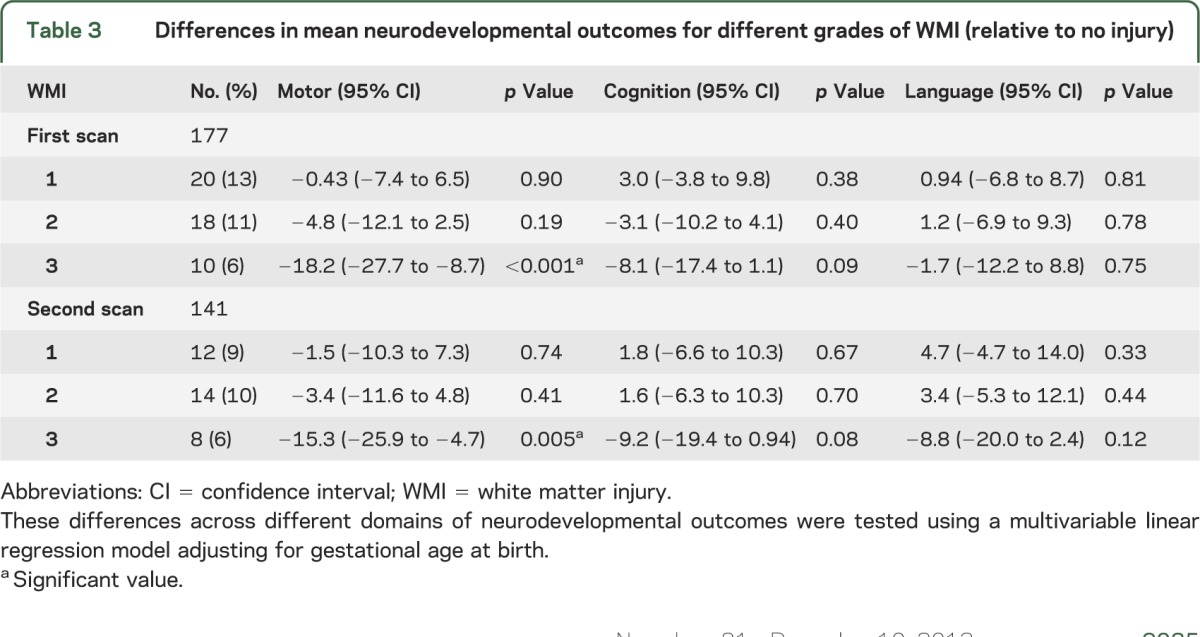
Brain MRI scans were normal in only 56 (34%) of the newborns (tables 2 and 3). On diagnostic MRI, only WMI was associated with motor, cognitive, or language outcomes. In linear regression models adjusting for gestational age at birth, severe WMI (grade 3) on both scans was significantly associated with abnormal motor outcomes (table 3), but not the range of IVH or cerebellar hemorrhage observed in this cohort. Cystic periventricular leukomalacia was seen in 3 infants; another 4 newborns were found to have hemorrhagic WMI, which may evolve into cystic periventricular leukomalacia. Interestingly, 18 newborns without evidence of WMI on the first MRI eventually were found to have some motor delay, including 8 children without overt IVH, ventriculomegaly, or cerebellar hemorrhage on MRI (table 2). Of these 18 newborns, only one infant developed mild WMI on the second scan (IVH grade 2 on first MRI). Of the 8 newborns with completely normal scans, most had mild motor delay but no cerebral palsy. Only 1 of the 8 was severely delayed and hypotonic; this child was considered to have a genetic syndrome.
Table 2.
Brain injuries as seen on the first MRI scan, by motor outcome group on Bayley-III
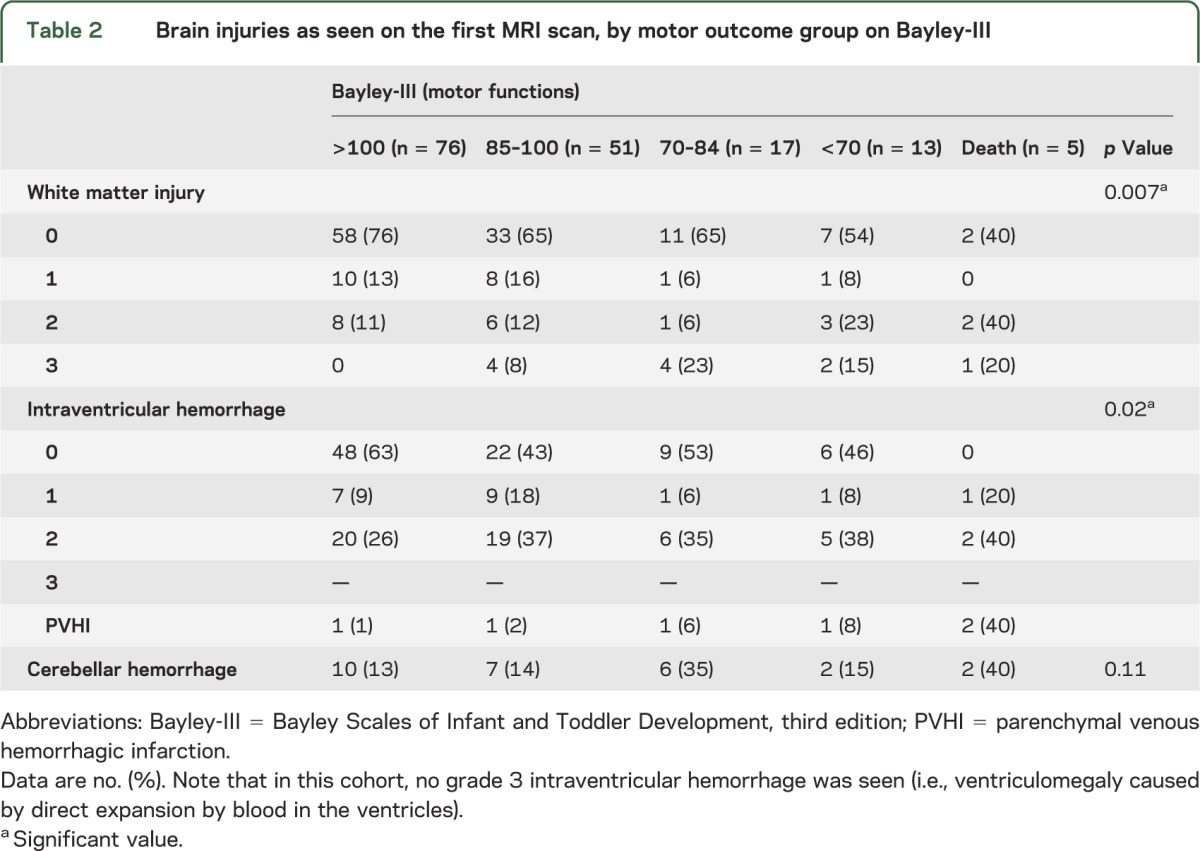
Table 3.
Differences in mean neurodevelopmental outcomes for different grades of WMI (relative to no injury)
Basal ganglia injury was seen in 4 newborns, which was concurrent with grade 3 WMI or small parenchymal venous hemorrhagic infarction. Of these, 2 died within a few months of life, one had low normal motor outcome (mild atrophy in the posterior body of the caudate), and one had spastic quadriparesis at her 18-month follow-up (signal abnormality in the lentiform nuclei bilaterally).
Brain maturation and neurodevelopmental outcomes.
Motor outcomes.
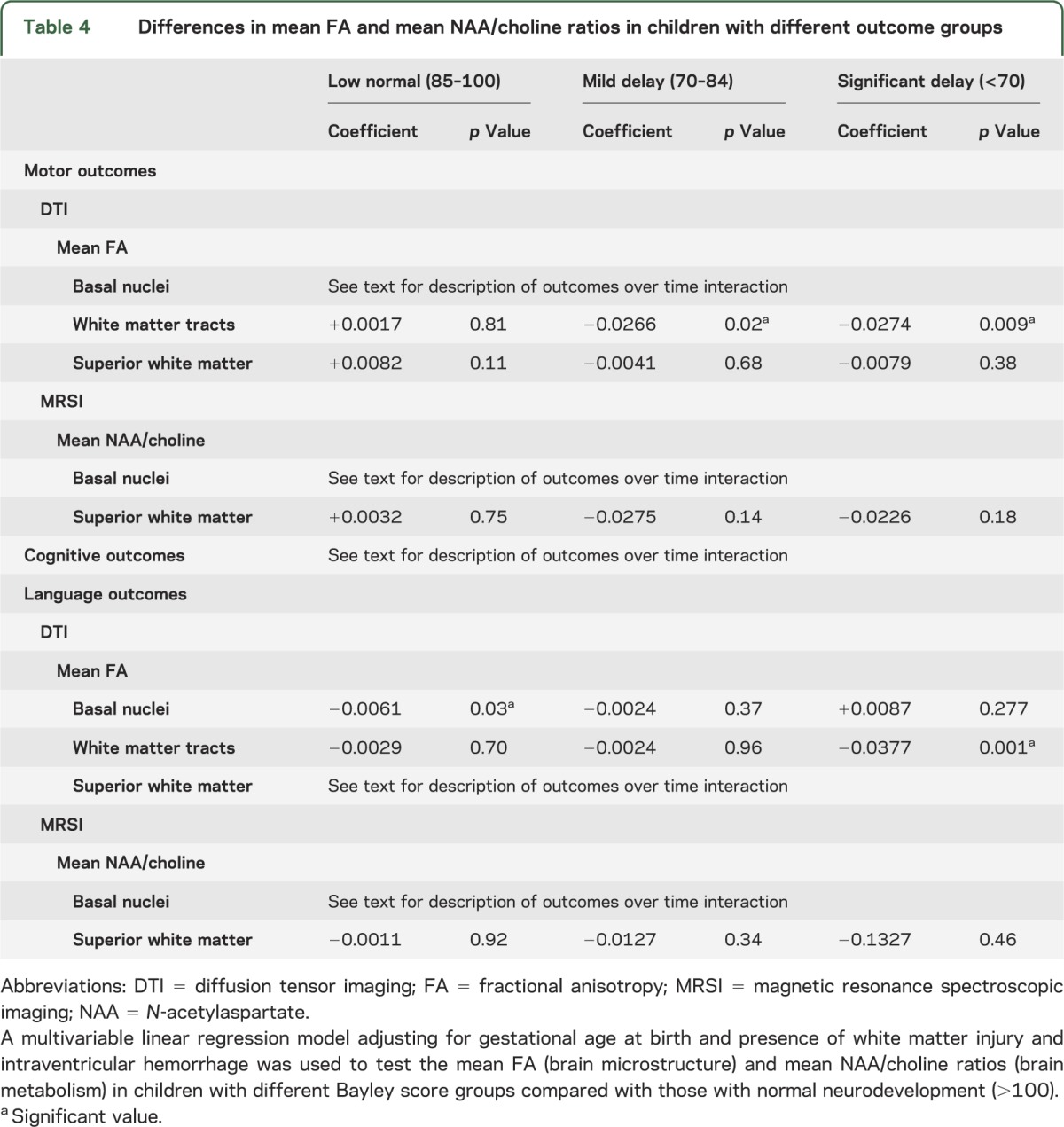
In basal nuclei, mean FA showed a slower increase across motor outcome groups (overall interaction p = 0.11), especially in children with significant delay (p = 0.037); and NAA/choline augmented less rapidly with increasing severity of adverse motor outcomes (overall interaction p = 0.02) (figure 1A). In white matter tracts, FA did not show an interaction across the motor outcome groups. However, FA was lower in the children with mild (p = 0.02) and significant (p = 0.009) motor delay. In superior white matter, FA and NAA/choline were not associated with outcomes, independent of age at birth and WMI (table 4). When these analyses were done separately for gross motor and fine motor functions, these findings persisted for the gross motor functions only.
Figure 1. Nonadjusted scatter plots of mean NAA/choline ratios and mean fractional anisotropy on the Bayley-III.
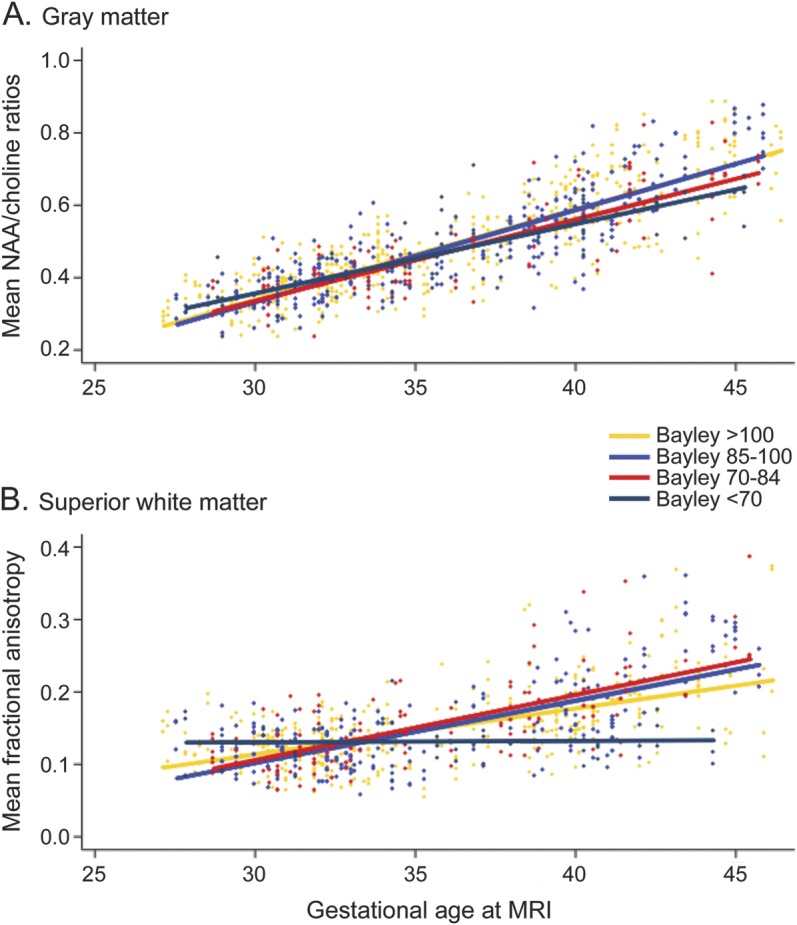
These nonadjusted scatter plots, by gestational age at MRI scan and measured on Bayley-III, show (A) the mean NAA/choline ratios in the basal nuclei for motor outcomes, and (B) the mean fractional anisotropy in the superior white matter for language outcomes. Bayley-III = Bayley Scales of Infant and Toddler Development, third edition; NAA = N-acetylaspartate.
Table 4.
Differences in mean FA and mean NAA/choline ratios in children with different outcome groups
Cognitive outcomes.
With increasingly adverse cognitive outcomes, FA showed a slower increase in the basal nuclei (overall interaction p = 0.002), white matter tracts (p = 0.05), and superior white matter (p = 0.03). Similarly, NAA/choline increased less rapidly in the gray and white matter (both p < 0.0001).
Language outcomes.
In basal nuclei, FA was not associated with language outcomes, but NAA/choline ratios increased more slowly (overall interaction p = 0.03), especially in the children with significant language delay (p = 0.04). In white matter tracts, FA values did not show an interaction with age across the language outcome groups. However, the values were lower in the children with significant (p = 0.001) language delay. In superior white matter, FA values showed a slower increase with increasing adverse language outcomes (overall p < 0.0001; figure 1B). However, NAA/choline ratios in white matter did not show a significant association (overall p = 0.82).
In all outcome domains (motor, cognitive, and language), microstructural abnormalities, reflected in FA, were due to impairment of radial (λ2 and λ3) rather than axial (λ1) diffusivity, mostly in white matter tracts and to a lesser extent in superior white matter (table e-2). Sex did not alter the associations noted in these statistical models.
DISCUSSION
In this prospective cohort of preterm newborns, studied serially with DTI and MRSI, we demonstrate that abnormal brain maturation detected early in life is associated with abnormal neurodevelopmental outcomes at 18 months’ corrected age. This novel finding builds on previous observations that brain injury on diagnostic MRI in premature newborns, both before1 and at term-equivalent age,2,3 is associated with adverse early neurodevelopment. In the current study, we find that while significant WMI on MRI is associated with adverse motor outcomes, impaired maturation of brain microstructure and metabolism from early in life is even more robustly associated with early cognitive and language outcomes. Given previous findings linking abnormalities of brain structure at term-equivalent age with adverse neurodevelopmental outcomes, our findings stress the importance of early-life brain health for functional neurodevelopmental outcomes.
Despite current efforts to understand the neurobiology and outcomes of children born preterm, there is wide variation in functional outcomes among preterm infants, even those born at the same gestational age. Some preterm children ultimately exhibit neurodevelopmental disability or cerebral palsy in the absence of visible brain lesions on diagnostic imaging.2 It is increasingly apparent that a number of clinically important systemic conditions are associated with abnormal early brain maturation.2,13−15,17−22 Data from the current study indicate that both early brain maturation and structural brain lesions are critical pathways from systemic illness to adverse outcomes. Because MRI has limited sensitivity for some aspects of WMI,27 these observations underscore the fact that apparent injuries on MRI, whether in early life1 or at term-equivalent age,2 are only one dimension of the detectable brain abnormalities prevalent in the preterm newborn.
Given the focus on WMI in the preterm newborn, it is important to note that impairments of microstructure and metabolism in the basal nuclei were strongly associated with adverse outcomes in all developmental domains. The observation of microstructural and metabolic development abnormalities in the basal nuclei in the preterm neonate preceding adverse neurodevelopmental outcome is consistent with a previous observation of disrupted thalamocortical connections in preterm newborns with WMI,12 resulting in significant motor delay and cognitive challenges. Other studies have found that preterm birth is associated with smaller regional brain volumes, including the cerebral cortex, thalamus, and cerebellum,28−30 and that reduced brain volumes in preterm children (measured in late childhood, i.e., at 8 years of age) are associated with abnormal cognitive outcomes.28,31 In line with Volpe's concept of “encephalopathy of prematurity,”32 the abnormalities in brain microstructure and metabolism detected in our study from early in life are neither transient nor limited to the white matter.
Together with recent experimental findings, our observations call for a refocus of the paradigm of brain injury in the premature newborns as a nonprogressive lesion to that of a dynamic process that evolves over the weeks newborns are cared for in the neonatal intensive care unit. Beyond the visible lesion seen on MRI, there are microstructural and metabolic abnormalities that seem to evolve over time. WMI has been linked to vulnerability of the oligodendrocyte lineage injury.6 However, recent experimental and human autopsy data indicate that myelination failure primarily relates to arrested maturation of the oligodendrocyte lineage at the preoligodendrocyte stage, rather than depletion of the preoligodendrocyte pool.6 The persistence of this “susceptible” cell population also maintains white matter vulnerability to recurrent insults.33 These experimental findings of impaired oligodendrocyte maturation are supported in this study, with the major differences in FA being attributable to components of radial (glial), as opposed to axial (axonal), diffusivity. Because the spectrum of brain abnormalities extends well beyond the boundaries of multifocal WMI and evolves over a considerable period of time, efforts to reduce exposure to hypoxia-ischemia and inflammation may prevent brain injury and optimize brain maturation. Furthermore, efforts to reduce systemic illnesses associated with abnormal early brain maturation, such as nosocomial infections, may provide new avenues to improve the brain health and neurodevelopmental outcome of the preterm newborn.
There are a number of limitations to our study. The lack of association of IVH and cerebellar hemorrhage with 18-month outcomes likely reflects the mild nature of these abnormalities in the context of this cohort's sample size. Nevertheless, the mortality rate and patient characteristics of our cohort are similar to those of other Canadian neonatal intensive care units.23 Furthermore, the limitations of MRI at clinical field strengths need to be acknowledged. In the preterm fetal sheep model of WMI, high-field MRI demonstrated promise for early detection of microscopic necrosis and gliosis associated with preoligodendrocyte maturation arrest.27 While scanning at clinical field strengths may lack the resolution required to detect microscopic necrosis, FA nevertheless provides a robust marker of the early progression of the oligodendrocyte lineage and the vulnerable cell lineage in the preterm brain,7 and ratios of NAA to choline provide a clinically relevant measure of neuronal metabolism. Our ability to probe the full spectrum of higher order neurocognitive disabilities evident only later in childhood34,35 is also limited by the age of this cohort. Even in preterm children with broadly normal intelligence, processing impairments can be seen and may include attention deficit and executive dysfunctions (e.g., cognitive flexibility, inhibitory control, working memory),35,36 and vulnerability of visually based information processing.35,37 Recent data with fMRI38 and magnetoencephalography39 point to altered cortical connectivity and synchronization during cognitive tasks relative to full-term controls, even in the absence of significant disability. Alterations in thalamocortical connectivity are now detectable in the neonatal period in the preterm population.12,22,40 Together, these findings suggest that while altered brain maturation begins in early life in neonates born very preterm, these children may compensate for early brain adversity by recruiting different patterns of cortical activation. How this compensation is affected by abnormal brain maturation in the neonatal period, particularly of the basal nuclei, is now a critical question and will be addressed with long-term follow-up of this cohort. Furthermore, as this cohort matures through childhood we will be able to test the hypothesis that the maturation of individual brain regions or pathways predicts specific neuropsychological functions.
In this prospective cohort of preterm neonates studied serially with MRI and high follow-up rates to 18 months’ corrected age, abnormal brain maturation as measured by DTI and MRSI from early in life to term-equivalent age is associated with adverse neurodevelopmental outcome. Therefore, brain abnormalities in preterm newborns related to adverse neurodevelopmental outcomes are not limited to structural brain lesions. These widespread abnormalities in brain maturation are not static or limited to the visible white matter lesions, but rather an evolving process that occurs during a period of intensive care, and possibly even beyond. Potentially modifiable risk factors for abnormal brain maturation include postnatal infections, lung disease, and pain.17−22 Establishing the link between abnormal brain maturation and adverse neurodevelopmental outcome now points to new opportunities to improve the brain health of preterm newborns.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the families for their participation, as well as the staff in the Neonatal Follow-Up Program of BC Children's & Women's Hospitals for their valuable contribution in assessing these children.
GLOSSARY
- Bayley-III
Bayley Scales of Infant and Toddler Development, third edition
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N-acetylaspartate
- WMI
white matter injury
Footnotes
Editorial, page 2062
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
V. Chau: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, determination of outcome variables. A. Synnes and R.E. Grunau: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, obtaining funding, determination of outcome variables. K.J. Poskitt: acquisition of data, critical revision of the manuscript for important intellectual content, determination of outcome variables. R. Brant: statistical analysis, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. S.P. Miller: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, obtaining funding, determination of outcome variables.
STUDY FUNDING
Supported by Canadian Institutes for Health Research (CIHR) operating grants MOP-79262 (S.P.M.) and MOP-86489 (R.E.G.). S.P.M. is currently the Bloorview Children's Hospital Chair in Pediatric Neuroscience and was supported by a Tier 2 Canada Research Chair in Neonatal Neuroscience, and Michael Smith Foundation for Health Research Scholar Award. Both R.E.G. and R.B. hold Senior Scientist Salary Awards from the Child & Family Research Institute.
DISCLOSURE
V. Chau: no disclosures to report. A. Synnes: funding from CIHR. R. Grunau: funding from CIHR, NICHD/NIH, Child & Family Research Institute. K. Poskitt: no disclosures to report. R. Brant: Child & Family Research Institute. S. Miller: funding from CIHR, SickKids Foundation, NeuroDevNet, NIH; prior support from Canada Research Chair, Michael Smith Foundation for Health Research, March of Dimes Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005;147:609–616 [DOI] [PubMed] [Google Scholar]
- 2.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–694 [DOI] [PubMed] [Google Scholar]
- 3.Mirmiran M, Barnes PD, Keller K, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics 2004;114:992–998 [DOI] [PubMed] [Google Scholar]
- 4.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153:170–175 [DOI] [PubMed] [Google Scholar]
- 5.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol 2009;66:155–164 [DOI] [PubMed] [Google Scholar]
- 6.Buser JR, Maire J, Riddle A, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 2012;71:93–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 2005;25:5988–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2002;48:949–958 [DOI] [PubMed] [Google Scholar]
- 9.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007;357:1928–1938 [DOI] [PubMed] [Google Scholar]
- 10.Hüppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 1998;43:224–235 [DOI] [PubMed] [Google Scholar]
- 11.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics 2007;120:e604–e609 [DOI] [PubMed] [Google Scholar]
- 12.Ball G, Boardman JP, Rueckert D, et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex 2012;22:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol 2012;33:188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115:286–294 [DOI] [PubMed] [Google Scholar]
- 15.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol 2007;49:745–750 [DOI] [PubMed] [Google Scholar]
- 16.Rathbone R, Counsell SJ, Kapellou O, et al. Perinatal cortical growth and childhood neurocognitive abilities. Neurology 2011;77:1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball G, Counsell SJ, Anjari M, et al. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 2010;53:94–102 [DOI] [PubMed] [Google Scholar]
- 18.Keunen K, Kersbergen KJ, Groenendaal F, Isgum I, de Vries LS, Benders MJ. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome—a systematic review. J Matern Fetal Neonatal Med 2012;25(suppl 1):89–100 [DOI] [PubMed] [Google Scholar]
- 19.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res 2012;71:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinall J, Grunau RE, Brant R, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med 2013;5:168ra8. [DOI] [PubMed] [Google Scholar]
- 21.Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol 2012;71:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol 2011;70:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CNN The Canadian neonatal network 2011 annual report [online]. Available at: http://www.canadianneonatalnetwork.org/portal/. Accessed July 7, 2013
- 24.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr 2010;156:882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acton BV, Biggs WS, Creighton DE, et al. Overestimating neurodevelopment using the Bayley-III after early complex cardiac surgery. Pediatrics 2011;128:e794–e800 [DOI] [PubMed] [Google Scholar]
- 26.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975 [Google Scholar]
- 27.Riddle A, Dean J, Buser JR, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol 2011;70:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 2000;284:1939–1947 [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 2007;119:759–765 [DOI] [PubMed] [Google Scholar]
- 30.Tam EW, Ferriero DM, Xu D, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res 2009;66:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr 2008;152:513–520.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 2008;63:520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saigal S, den Ouden L, Wolke D, et al. School-age outcomes in children who were extremely low birth weight from four international population-based cohorts. Pediatrics 2003;112:943–950 [DOI] [PubMed] [Google Scholar]
- 35.Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med 2002;156:615–620 [DOI] [PubMed] [Google Scholar]
- 36.Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 2004;114:50–57 [DOI] [PubMed] [Google Scholar]
- 37.Saavalainen P, Luoma L, Bowler D, et al. Spatial span in very prematurely born adolescents. Dev Neuropsychol 2007;32:769–785 [DOI] [PubMed] [Google Scholar]
- 38.Gozzo Y, Vohr B, Lacadie C, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage 2009;48:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doesburg SM, Ribary U, Herdman AT, et al. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage 2011;54:2330–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20:2852–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


