Abstract
Objective:
To examine the underlying pathophysiology of mild traumatic brain injury through changes in gray matter diffusion and atrophy during the semiacute stage.
Methods:
Fifty patients and 50 sex-, age-, and education-matched controls were evaluated with a clinical and neuroimaging battery approximately 14 days postinjury, with 26 patients returning for follow-up 4 months postinjury. Clinical measures included tests of attention, processing speed, executive function, working memory, memory, and self-reported postconcussive symptoms. Measures of diffusion (fractional anisotropy [FA], mean diffusivity) and atrophy were obtained for cortical and subcortical structures to characterize effects of injury as a function of time.
Results:
Patients reported more cognitive, somatic, and emotional complaints during the semiacute injury phase, which were significantly reduced 4 months postinjury. Patients showed evidence of increased FA in the bilateral superior frontal cortex during the semiacute phase, with the left superior frontal cortex remaining elevated 4 months postinjury. There were no significant differences between patients and matched controls on neuropsychological testing or measures of gray matter atrophy/mean diffusivity at either time point.
Conclusions:
Increased cortical FA is largely consistent with an emerging animal literature of gray matter abnormalities after neuronal injury. Potential mechanistic explanations for increased FA include cytotoxic edema or reactive gliosis. In contrast, there was no evidence of cortical or subcortical atrophy in the current study, suggesting that frank neuronal or neuropil loss does not occur early in the chronic disease course for patients with typical mild traumatic brain injury.
Although numerous diffusion tensor imaging studies have explored axonal integrity after mild traumatic brain injury (mTBI),1,2 the effects of mTBI on gray matter are more poorly characterized. Previous studies reported nonsignificant trends3 and reduced4 anisotropic diffusion after mTBI. However, both studies were conducted with chronically symptomatic and/or mixed patient populations. Both variables contribute to neurobehavioral sequelae5 and white matter integrity,1 indicating the need for a well-powered study of gray matter diffusion metrics in patients with more typical mTBI. Additionally, recent animal models indicate increased anisotropy within the thalamus and hippocampus during acute and more chronic injury phases.6,7
Although evidence of atrophy has been found as early as 1 to 3 weeks postinjury in moderate to severe TBI,8,9 it becomes more prevalent at 6 to 12 months,10–13 even in the absence of macroscopically detectable lesions.14,15 Studies of patients with complicated16 and symptomatic mild to moderate17 TBI indicate atrophy as a function of disease progression approximately 6 months16 or 1 year17 postinjury. To our knowledge, no studies have directly assessed cortical thickness changes prospectively after mTBI.
In this study, we investigated potentially time-dependent biomarkers of gray matter injury in 50 patients with mTBI imaged both within 21 days and approximately 4 months (n = 26) after injury. We hypothesized that measures of anisotropy would capture gray matter injury in semiacutely injured patients while atrophy would more likely be present during the early chronic phases of mTBI, if at all.
METHODS
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from all participants according to University of New Mexico guidelines.
Participants.
Data from 51 patients with mTBI (25 females; aged 27.86 ± 9.24 years; 13.12 ± 2.21 years of education) and 51 sex-, age-, and education-matched healthy controls (HCs) (25 females; aged 27.40 ± 8.94 years; 13.90 ± 2.09 years of education) were examined. One patient and one HC were identified as motion outliers on diffusion data and eliminated from future analyses. We have previously reported on white matter metrics of diffusion for a similar cohort in a separate publication.18
All patients were evaluated clinically (clinical examination = 14.00 ± 4.89 days postinjury) and with neuroimaging (imaging examination = 13.98 ± 5.25 days postinjury) within 21 days of injury (table e-1 on the Neurology® Web site at www.neurology.org). Inclusion criteria for mTBI were based on the American Congress of Rehabilitation Medicine (Glasgow Coma Scale score of 13–15, loss of consciousness [if present] <30 minutes, posttraumatic amnesia [if present] <24 hours). All participants minimally experienced an alteration in mental status and were seen in local hospitals. Diagnosis was confirmed by a licensed neuropsychologist through interview and medical record review. Participants were excluded if there was a history of neurologic disease, psychiatric disturbance, other head injuries with more than 5 minutes’ loss of consciousness, other head injuries within the last year, learning disorder, attention-deficit/hyperactivity disorder, or recent history of substance/alcohol abuse.
Whenever possible, clinical measures were converted to T-scores (mean = 50, SD = 10) using published age-specific norms and then averaged to provide an overall composite score. Composite indices were calculated for the major cognitive domains based on the following neuropsychological tests: attention (Trails A, Paced Auditory Serial Addition Test, Stroop, and Wechsler Adult Intelligence Scale–III Digit Span), working memory (letter number sequence, arithmetic, and digits backward), processing speed (grooved pegboard and digit symbol coding), executive function (Wisconsin Card Sort, Trails B, and Fluency [FAS]), and memory (California Verbal Learning Test–II). The Neurobehavioral Symptom Inventory, a modified version of the Rivermead Questionnaire, the Beck Depression Inventory, and State Trait Anxiety Index were also given to measure self-reported postconcussive symptoms and emotional sequelae. The Wechsler Test of Adult Reading provided an estimate of overall premorbid cognitive functioning. The Test of Memory Malingering allowed assessment of participant effort.
Imaging.
The current study examined whole-brain measures of gray matter diffusion and atrophy using both region-of-interest (ROI) and traditional whole-brain (i.e., vertex- or voxel-wise) analyses. High-resolution T1- and T2-weighted anatomical images were collected on a 3T Siemens Trio scanner (Siemens Medical Solutions, Malvern, PA). Susceptibility-weighted images were collected on a subset of 24 patients with mTBI to better characterize petechial hemorrhages. Diffusion tensor imaging data (fractional anisotropy [FA] and mean diffusivity [MD] scalars) were collected and processed through a procedure fully described in previous publications.18,19 See e-Methods for image acquisition details.
Data analyses.
The FreeSurfer reconstruction pipeline (version 5.1) was used to generate cortical thickness values and all ROIs based on standard labels.20 All results were visually inspected for accuracy. The FreeSurfer longitudinal pipeline was used for all visit 2 data to maximize between-visit image registration. Volumetric metrics were normalized by total intracranial volume and transformed by the square root. As a result of individual differences in cortical folding, the analysis streams were divided into separate pipelines for cortical (data projected onto a surface space) and subcortical regions (figure e-1). Our a priori predictions were that gray matter abnormalities would be more universally present within the thalamus and hippocampus21 as a result of the accumulation of shear stresses in these regions,22 as well as within the medial and lateral frontotemporal cortices as a result of skull morphology.14,23
Cortical analyses.
Principal analyses focused on frontotemporal cortical regions most susceptible to injury.14 A priori cortical ROIs were defined by FreeSurfer labels and included the rostral and caudal anterior cingulate gyrus, entorhinal cortex, inferior temporal gyrus, lateral orbitofrontal cortex, medial orbitofrontal cortex, middle temporal gyrus, pars orbitalis, pars triangularis, rostral middle frontal gyrus, superior temporal gyrus, frontal pole, and temporal pole. All ROIs were multiplied by respective diffusion maps (FA and MD) to obtain regional diffusion estimates. The diffusion measures exhibited a variable covariance structure (r's ranged from 0.00 to 0.81), but most showed evidence of moderate covariance. Therefore, to reduce the number of tests and control for false positives, a multivariate analysis of covariance (MANCOVA) approach was adopted for both the right and left hemisphere for principal regions. In addition, diffusion metrics and thickness estimates were also projected onto surface vertices of the FreeSurfer average template. Vertex-wise analyses (general linear model) were run with group as factor and estimates of premorbid intelligence as a covariate, corrected for multiple comparisons at p < 0.05 using Monte Carlo simulations.
Subcortical and hippocampi analyses.
Principal analyses examining subcortical diffusion (FA and MD) and atrophy (volume) were restricted to the thalami and hippocampi, and evaluated with MANCOVA on a per-hemisphere basis. Similar to cortical analyses, ROIs were bilaterally defined based on FreeSurfer segmentation labels and multiplied by respective diffusion maps. Voxel-wise analyses were also conducted on the subcortical volumes for exploratory purposes. To reduce redundancy with surface-based cortical analyses, a study-specific mask was created based on the subcortical FreeSurfer labels. Individual subject subcortical masks were summed, and only voxels in which there was 75% overlap in subjects' masks were subjected to further analyses. General linear model analyses were then conducted on FA and MD using group as a between-subjects factor and estimates of premorbid intelligence as a covariate, corrected for false positives at p < 0.05.
Effect sizes for various gray matter measures are presented for all cortical (table e-2) and subcortical (table e-3) FreeSurfer ROIs to facilitate future meta-analyses.
RESULTS
Clinical results.
There were no differences between the patients with mTBI and matched controls (p > 0.10) on key demographic variables (see table 1), including hand preference.24 Independent samples t tests indicated that HCs achieved higher estimates of premorbid intellectual functioning (t1,98 = 3.45, p = 0.001) despite educational matching. Patients showed a nonsignificant trend toward better performance on the Test of Memory Malingering (t1,66.86 = 1.855, p = 0.068), although both groups performed normally.
Table 1.
Neuropsychological and clinical summary measures for visit 1
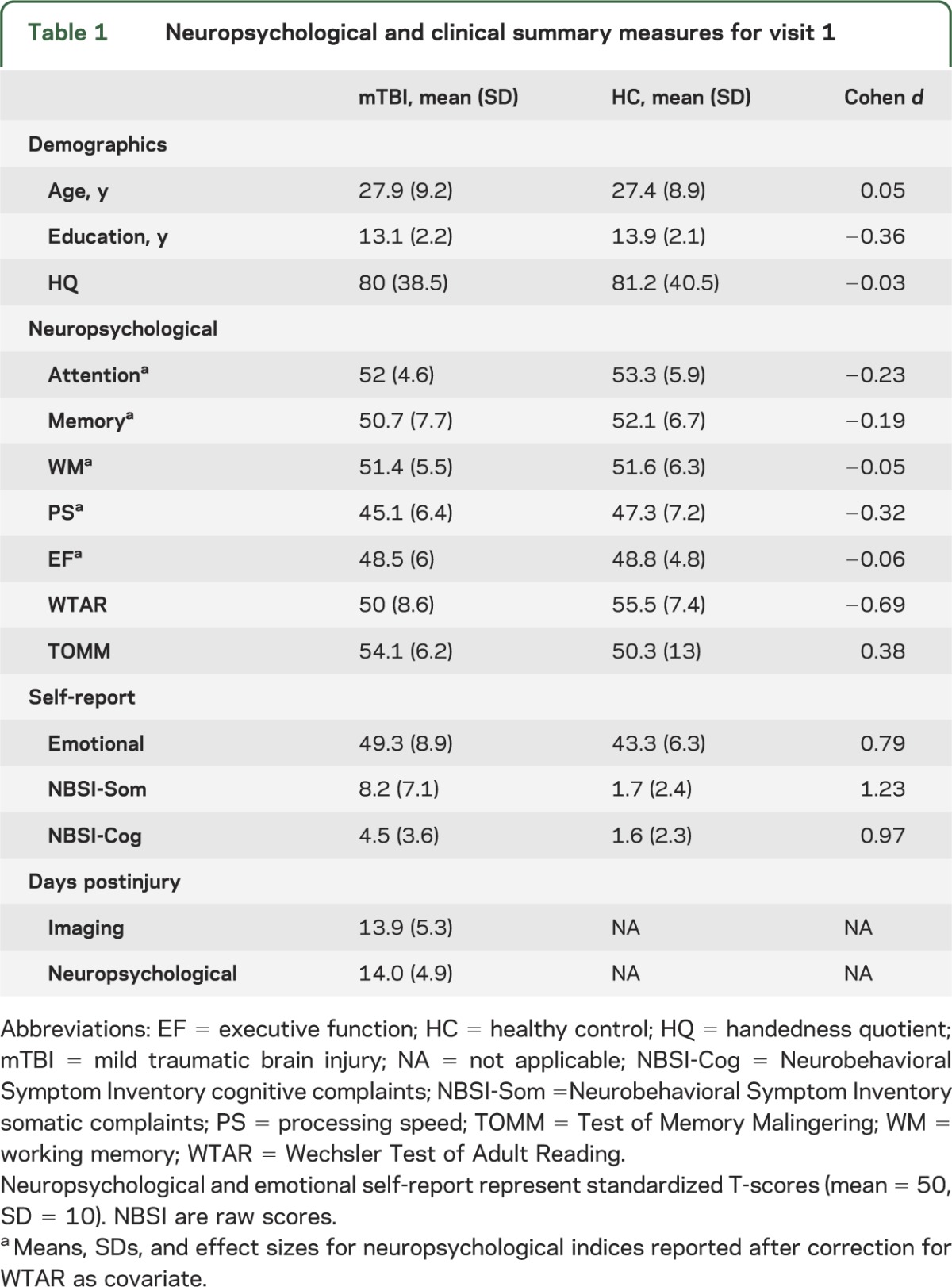
A MANCOVA comparing the domains of attention, processing speed, working memory, executive functioning, and memory with premorbid intelligence as a covariate did not reveal any group differences in cognition (p > 0.10) with small to medium effect sizes (table 1). A MANOVA indicated that patients with mTBI reported more cognitive (F1,97 = 23.62, p < 0.001), somatic (F1,97 = 37.49, p < 0.001), and emotional (F1,97 = 15.39, p < 0.001) complaints during the semiacute injury phase.
Visit 1 imaging results.
A total of 9 patients were identified as likely exhibiting trauma-induced pathology on CT (5/38 patients with mTBI) or anatomical (T1-, T2-, or susceptibility-weighted images) MRI (4/50 patients) scans by board-certified neuroradiologists (blinded for all MRI reads). However, there were no gross lesions, and findings were spatially variable across the 9 patients with complicated mTBI (table e-4).
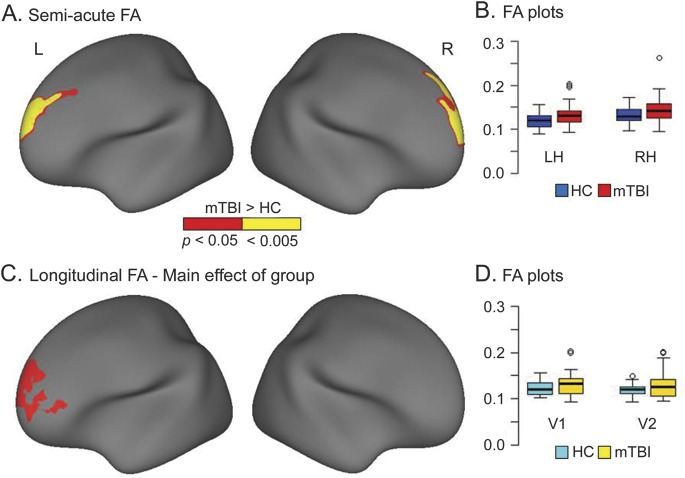
MANCOVAs conducted on both right- and left-hemisphere frontotemporal ROIs did not indicate any significant differences between the 2 groups (p > 0.10) for either FA or MD. Effect sizes (see table e-2) for all principal regions were typically in the small range. In contrast, results of the vertex-based analyses of diffusion metrics indicated regions of increased FA (mTBI > HC) in the bilateral superior frontal cortex (figure 1). Additional analyses confirmed that patients with complicated mTBI were not primary contributors to increased FA within these regions (see e-Results). Large effect sizes were also noted in the left occipital lobe for FA, but this cluster did not survive false-positive correction (figure e-2A).
Figure 1. Semiacute and longitudinal group differences in FA.
(A) Regions showing increased fractional anisotropy (FA) for patients with mild traumatic brain injury (mTBI) relative to healthy controls (HCs) during the semiacute injury phase after correction for multiple comparison (α = 0.05). The magnitude of significance is denoted by color (red, corrected p < 0.05; yellow, p < 0.005), and data are presented for the right hemisphere (RH) and left hemisphere (LH). Regions included the left (Montreal Neurological Institute [MNI] coordinates = −16.5, 49.8, 27.3) and right (MNI coordinates = 12.3, 62.2, 15.8) superior frontal cortex. (B) Box-and-whisker plots used to denote the distribution of the FA within these regions for both patients with mTBI (red boxplots) and controls (blue boxplots). (C) Regions that exhibited a main effect of group in the FreeSurfer longitudinal pipeline analyses. Increased FA was again observed within the left (MNI coordinates = −16.3, 60.9, 6.5) superior frontal cortex for patients with mTBI. (D) Box-and-whisker plots used to denote the longitudinal changes in distribution of the FA within the left superior frontal cortex for returning patients with mTBI (n = 26; yellow boxplots) and matched controls (cyan boxplots) at both visits 1 (V1) and 2 (V2).
MANCOVAs examining MD (ROI and vertex-based) and cortical thickness (vertex-based) were not significant for group differences. The current sample was well powered (>0.80) to detect clinically meaningful effect sizes (>0.60), but effect sizes were typically in the small range for both MD and thickness (figure e-2, B and C).
Both ROI (MANCOVAs; p >0.10) and voxel-wise tests of subcortical diffusion metrics (FA and MD) were also negative after correction for false positives. There were no significant differences in volumes within the bilateral hippocampi or thalami. Voxel-wise maps of effect sizes are presented for FA and MD (figure e-3) to illustrate that these negative results were not secondary to power.
A series of multiple regressions investigated the relationship between FA in the superior frontal regions and self-reported cognitive, emotional, or somatic symptomatology (mTBI group only). However, FA in these regions was not associated with significant variance in self-reported symptomatology (p > 0.10).
Supplemental results.
Additional supplementary analyses were conducted to evaluate other frequently used metrics of atrophy given our null results. Specifically, group-wise analyses were conducted examining ventricular volumes (lateral, inferior-lateral, third, fourth, fifth; MANCOVA) as well as total brain volume (analysis of covariance [ANCOVA]). All results were negative (p > 0.10). Given the potential heterogeneity of injury after mTBI, ROI analyses on diffusion metrics were also conducted for the remainder of FreeSurfer labels using ANCOVAs. Results (see tables e-2 and e-3) indicated that the majority of effect sizes were small for all ROIs for both diffusion measurements.
Longitudinal analyses.
A total of 27 subjects with mTBI returned for a follow-up visit, although imaging data from one patient was not usable. Reasons for loss to follow-up included inability to contact patients (e.g., phone number no longer valid, 79.17%), patient no-shows (12.50%), and inability of patient to complete second visit because of personal circumstances (8.33%). Importantly, there were no significant differences (p > 0.10) on either subjective (self-report) or objective (formal cognitive testing) neurobehavioral sequelae for returning vs nonreturning patients. Forty HCs returned for a second visit, and 26 of these subjects were selected on sex, age, and education to best match returning patients. The individual subject matches remained consistent across visit 1 and visit 2 data when possible. One of the matched HCs was unable to complete the neuropsychological testing during visit 2 and was not included in clinical analyses. To limit the number of tests, principal longitudinal analyses were restricted to measures that exhibited group differences during the semiacute injury phase.
Three 2 × 2 (group × time) mixed-measures ANOVAs examined changes in self-reported symptomatology as a function of recovery. A 2 × 2 analytic design was selected to reliably measure change in the mTBI group relative to controls. The group × time interaction was significant for somatic (F1,49 = 6.24, p = 0.016), cognitive (F1,49 = 6.43, p = 0.014), and emotional (F1,49 = 4.34, p = 0.042) complaints, with simple effects testing indicating that the level of complaints decreased as a function of time (visit 1 to visit 2) for patients with mTBI (all p < 0.05) but not HCs (all p > 0.10).
A 2 × 2 (group × time) ANCOVA examined changes in superior frontal FA values as a function of time postinjury. Neither the interaction term nor the main effect of time was significant, although the main effect of group remained significant for the left superior frontal cortex. A multiple regression investigating the potential relationship between persistent postconcussive symptoms (self-reported cognitive, emotional, or somatic symptomatology) at visit 2 and FA in the left superior frontal cortex (patients with mTBI only) was conducted. However, results from this analysis were negative.
Supplemental 2 × 2 (group × time) ANCOVA analyses were also conducted to examine the effects of mTBI on voxel-wise subcortical diffusion metrics and vertex-wise cortical thickness measurements in light of previous results from patients with chronic mTBI.3,4 However, no significant effects were detected for any analyses following appropriate corrections for false positives. Finally, a recent publication25 identified significant longitudinal differences (within-subject) in regional brain volume over a 1-year period in patients with mTBI using paired t tests. Longitudinal regional volumetric changes were assessed for the mTBI cohort using paired t tests on all 263 from the standard FreeSurfer 5.1 pipeline parcellations,26,27 including white matter (45 subcortical regions, 70 white matter, and 148 cortical regions). However, results did not show significant longitudinal differences for the patient cohort (uncorrected p < 0.001).
DISCUSSION
Similar to previous reports,28 patients with mTBI in the current study self-reported increased cognitive, emotional, and somatic complaints during the semiacute injury phase, and then showed evidence of decreased symptoms approximately 4 months postinjury. In contrast, there was no evidence of objective deficits on formal neuropsychological testing between patients and controls semiacutely (approximately 14 days postinjury). There is growing literature demonstrating that effect sizes obtained during formal cognitive testing decrease dramatically as a function of days postinjury,5,29,30 suggesting that early testing may be required to document the subtle cognitive deficits known to characterize single-episode mTBI.
Current results indicated increased FA within bilateral superior frontal cortex during the semiacute injury phase, as well as increased FA within the left superior frontal cortex approximately 4 months postinjury. Although the frontal cortex has previously been shown to be susceptible to injury,14 mechanistic explanations for gray matter changes in FA are less clear. Anisotropic diffusion is much lower in gray than white matter because of the amorphous nature of gray matter cells (e.g., pyramidal and stellate cells) and the lack of an overarching cellular organization (e.g., relative to a fiber bundle). Cytotoxic edema is frequently cited as a potential explanation for increased FA in mTBI,18 although the differential diffusion between intracellular and extracellular tortuosity is likely to be less in gray than white matter.
In animal models, increases in gray matter FA have been proposed to result from the palisading of protoplasmic astrocytes and the subsequent formation of glial scars, which occur asymmetrically in the direction of the lesion.6,31 Specifically, the normal morphology of protoplasmic astrocytes (i.e., stellate-shaped with minimal interdigitation of processes) may be altered after injury to become hypertrophic and/or asymmetric with overlapping processes.32,33 Although reactive processes peak 4 to 7 days postinjury,34,35 gliotic scarring continues for prolonged periods in animal models.6 This time course may therefore explain both short-term (approximately 14 days) and long-term (approximately 4 months) findings of increased diffusion in the current sample.
Previous studies have reported reduced FA in the thalamus during chronic phases of mTBI.3,4 However, injury chronicity (patients more than 1 year postinjury), the mixing of patients with different levels of injury severity (mild, moderate, and severe), and the increased likelihood of impairment in self-selected treatment-seeking mTBI samples likely contribute to the differences observed across previous and current results. For example, approximately 90% of patients in the Grossman study reported significant neurobehavioral sequelae when they were examined more than 1 year postinjury, with an additional 60% of patients identified as having objective impairment on neuropsychological testing.4 Thus, similar to the white matter diffusion literature, the direction of diffusion abnormalities in gray matter may also be dependent on injury severity (reduced FA in more severe injuries), presence of comorbid symptoms, as well as measurement time postinjury.1,2
In contrast to FA findings, proxy measures of gray matter atrophy (ventricular volume, brain volume, thickness, and MD) were nonsignificant during the semiacute and early chronic injury phases of mTBI. Atrophy has been observed within weeks of injury in more severe TBI.8,9 Previous work with symptomatic (81.3% with posttraumatic stress disorder), mixed injury cohorts (mild and moderate TBI) reported longitudinal differences 1 to 2 years postinjury in whole-brain parenchyma as well as cerebral white matter.17 Others reported atrophic whole-brain changes for patients with complicated mTBI 6 months postinjury, with typical mTBI patients showing no evidence of atrophy.16 Significant differences in brain volume have also been observed longitudinally after typical mTBI, with cross-sectional comparisons indicating differences between patients and controls within these structures at the chronic (mean = 13 months postinjury) but not semiacute (mean = 23 days postinjury) scan dates.25 Thus, current and previous findings suggest that atrophy due to frank neuronal or neuropil loss may occur later in the course of mTBI,25 may be limited to patients with lesions,16 or may be limited to chronically symptomatic patients.17 The long-term consequences of mTBI on gray matter remain to be elucidated, especially for repeat injuries. Emerging evidence from both professional athletes36,37 and military personnel36 indicates atrophy of the frontal and medial temporal lobes in individuals with histories of multiple concussive events.
There are several limitations to the current study. First, current analyses were based on the assumption that different initial injury conditions (see table e-1) produce spatially homogeneous injuries. It is increasingly recognized that novel approaches for classifying heterogeneous lesion locations may be necessary in mTBI research, especially for voxel-based analyses.18 However, the underlying assumptions of these approaches are still being validated and are likely to be dependent on the statistical properties of the data (e.g., sample size and distribution properties). Second, frontal regions are also highly susceptible to various imaging artifacts, indicating the need for replication of reported diffusion abnormalities in an independent sample. Third, current findings of increased FA were dependent on methodology (vertex-wise analyses vs ROI), which was likely a result of the size of the region over which data were averaged. Finally, even though we did not observe significant neurobehavioral differences in returning and nonreturning patients, the impact of sample attrition on longitudinal findings is unknown.
In summary, current results indicate increased anisotropic diffusion in cortical gray matter during both the semiacute and early chronic injury phases along with a decrease in neurobehavioral symptoms over a 4-month interval. Collectively, these findings suggest potentially different recovery courses for neurobehavioral (more rapidly resolving) and physiologic (more slowly resolving) signs of mTBI. In contrast, there was no evidence of cortical or subcortical atrophy in the current study, suggesting that frank neuronal loss may only occur in patients with atypical mTBI (e.g., complicated or chronically symptomatic), potentially occur later in the disease course, or occur as a function of repetitive injury. Importantly, current and previous results highlight the potential difficulties of relying on a single measure as metric of recovery from any mTBI sample.
Supplementary Material
ACKNOWLEDGMENT
Special thanks to Diana South and Cathy Smith for assistance with data collection.
GLOSSARY
- ANCOVA
analysis of covariance
- FA
fractional anisotropy
- HC
healthy control
- MANCOVA
multivariate analysis of covariance
- MD
mean diffusivity
- mTBI
mild traumatic brain injury
- ROI
region of interest
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Josef M. Ling: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data, statistical analysis. Stefan Klimaj: acquisition of data, statistical analysis. Trent Toulouse: statistical analysis. Andrew R. Mayer: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision or coordination, obtaining funding.
STUDY FUNDING
Supported by the NIH (R24-HD050836, R21-NS064464-01A1, and 3 R21 NS064464-01S1 to A.R.M.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010;25:241–255 [DOI] [PubMed] [Google Scholar]
- 2.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010;74:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman EJ, Ge Y, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012;29:2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belanger HG, Spiegel E, Vanderploeg RD. Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc 2010;16:262–267 [DOI] [PubMed] [Google Scholar]
- 6.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 2011;134:2248–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S, Zhuo J, Racz J, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J Neurotrauma 2011;28:2091–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blatter DD, Bigler ED, Gale SD, et al. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR Am J Neuroradiol 1997;18:1–10 [PMC free article] [PubMed] [Google Scholar]
- 9.Wright MJ, McArthur DL, Alger JR, et al. Early metabolic crisis-related brain atrophy and cognition in traumatic brain injury. Brain Imaging Behav Epub 2013 May 1 [DOI] [PMC free article] [PubMed]
- 10.Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci 1998;18:5663–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005;20:76–94 [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Chang E, Igarashi T, Noble LJ. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Res 2001;917:45–54 [DOI] [PubMed] [Google Scholar]
- 13.Warner MA, Youn TS, Davis T, et al. Regionally selective atrophy after traumatic axonal injury. Arch Neurol 2010;67:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav 2012;6:108–136 [DOI] [PubMed] [Google Scholar]
- 15.Palacios EM, Sala-Llonch R, Junque C, et al. Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 2013;49:646–657 [DOI] [PubMed] [Google Scholar]
- 16.Hofman PA, Stapert SZ, van Kroonenburgh MJ, Jolles J, de Kruijk J, Wilmink JT. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. AJNR Am J Neuroradiol 2001;22:441–449 [PMC free article] [PubMed] [Google Scholar]
- 17.Ross DE, Ochs AL, Seabaugh JM, et al. Progressive brain atrophy in patients with chronic neuropsychiatric symptoms after mild traumatic brain injury: a preliminary study. Brain Inj 2012;26:1500–1509 [DOI] [PubMed] [Google Scholar]
- 18.Ling JM, Pena A, Yeo RA, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 2012;135:1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp 2012;33:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980 [DOI] [PubMed] [Google Scholar]
- 21.Maxwell WL, Dhillon K, Harper L, et al. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol 2003;62:272–279 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Yang KH, King AI. A proposed injury threshold for mild traumatic brain injury. J Biomech Eng 2004;126:226–236 [DOI] [PubMed] [Google Scholar]
- 23.McAllister TW, Stein MB. Effects of psychological and biomechanical trauma on brain and behavior. Ann NY Acad Sci 2010;1208:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Cogn Neuropsychol 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Kierans A, Kenul D, et al. Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 2013;267:880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010;53:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 28.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003;290:2556–2563 [DOI] [PubMed] [Google Scholar]
- 29.Frencham KA, Fox AM, Maybery MT. Neuropsychological studies of mild traumatic brain injury: a meta-analytic review of research since 1995. J Clin Exp Neuropsychol 2005;27:334–351 [DOI] [PubMed] [Google Scholar]
- 30.Pertab JL, James KM, Bigler ED. Limitations of mild traumatic brain injury meta-analyses. Brain Inj 2009;23:498–508 [DOI] [PubMed] [Google Scholar]
- 31.Zhuo J, Xu S, Proctor JL, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 2012;59:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci 2007;27:6473–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberheim NA, Tian GF, Han X, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci 2008;28:3264–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol 2003;182:87–102 [DOI] [PubMed] [Google Scholar]
- 35.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007;27:11869–11876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005;57:128–134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.