Abstract
Embryonic stem (ES) cells are widely used for different purposes, including gene targeting, cell therapy, tissue repair, organ regeneration, and so on. However, studies and applications of ES cells are hindered by ethical issues regarding cell sources. To circumvent ethical disputes, great efforts have been taken to generate ES cell-like cells, which are not derived from the inner cell mass of blastocyst-stage embryos. In 2006, Yamanaka et al. first reprogrammed mouse embryonic fibroblasts into ES cell-like cells called induced pluripotent stem (iPS) cells. About one year later, Yamanaka et al. and Thomson et al. independently reprogrammed human somatic cells into iPS cells. Since the first generation of iPS cells, they have now been derived from quite a few different kinds of cell types. In particular, the use of peripheral blood facilitates research on iPS cells because of safety, easy availability, and plenty of cell sources. Now iPS cells have been used for cell therapy, disease modeling, and drug discovery. In this review, we describe the generations, applications, potential issues, and future perspectives of iPS cells.
Keywords: Induced pluripotent stem cells, Origin, Peripheral blood cells, Application, Potential issues
1. Introduction
Embryonic stem (ES) cells are pluripotent cells which were derived from the inner cell mass of blastocyst-stage embryos. Pluripotency is the potential that one type of cells can differentiate into various kinds of cells, such as muscle cells, neural cells, and even germ cells. Based on this property, ES cells can generate any type of cell to meet the requirements of different applications. ES cells are also capable of self-renewal; they can be semi-permanently cultured on feeder cells, which supply necessary growth factors for ES cells.
Mouse ES cells were established by Evans and Kaufman (1981). At present, most of gene-targeted and transgenic mice are generated via mouse ES cells. Basic research on gene functions can be carried out on these animal models. However, research and clinical applications on human ES cells, which were established by Thomson et al. (1998), have been restricted by ethical issues regarding cell sources and immunological rejection in cell therapy. Most debates on ethics are concentrated on the morality of destroying human embryos for the benefit of other people. Obtaining stem cells from oocytes and embryos is full of disputes regarding the onset of personhood and reproduction. Furthermore, immunizing antigens from different ES cells are obviously different. After transplanted into a recipient, somatic cells, which were derived from human ES cells, may be rejected by the recipient’s immune system. Therefore, it is necessary to make patient-specific pluripotent stem cells.
Scientists have attempted to reprogram somatic cells to develop a new kind of stem cell with self-renewal properties and pluripotency through many methods, such as nuclear transfer (Wilmut et al., 1997; Chesné et al., 2002) and cell fusion (Ying et al., 2002). However, all methods cannot do without the pluripotency of the cell nucleus in animals. As early as 1962, in fact, Gurdon (1962) found that the differentiation of animal cells is reversible. In the classic experiment, he replaced the nuclei in unfertilized frogs’ eggs with the nuclei from intestinal cells. The modified eggs finally still developed into normal tadpoles. This means that the DNA of adult cells in frogs still contains all the genetic information as would be found in the nucleus of the zygote. In 1996, the birth of Dolly indicated that a mammal could be successfully cloned from adult cells (Wilmut et al., 1997). In 2006, a Japanese group made a remarkable breakthrough. Takahashi and Yamanaka (2006) generated induced pluripotent stem (iPS) cells by over-expressing a few types of transcription factors. iPS cells without ethical arguments have self-renewal properties and pluripotency just like ES cells. In 2012, Gurdon and Yamanaka received the Nobel Prize in physiology or medicine for their researches.
Here we review the initial development of iPS cells and their applications in pharmacology and medicine, especially the usage of peripheral blood as an easily accessible source for deriving iPS cells. In addition, we also provide discussions on potential issues and future perspectives for iPS cells.
2. Generations of iPS cells
When mouse somatic cells are hybridized with mouse ES cells, their nuclei can be reprogrammed. The hybridized somatic cells have the ability of differentiating into endoderm, mesoderm, and ectoderm cells. These findings demonstrated that reprogramming factors expressed in ES cells could induce pluripotency in somatic cells. The most difficult part in reprogramming somatic cells is how to find these reprogramming factors which can convert somatic cells to pluripotent stem cells. Now this problem has been solved by the Fbx15-Neo reporter system. Fbx15 is a gene which was expressed specifically in ES cells. Normal fibroblasts cannot survive in the presence of Geneticin (G418), an analog of Neomycin (Neo) used for screening ES cells. Therefore, candidate reprogramming factors can be screened via fibroblasts with a Neo resistance gene in their Fbx15 locus. Fibroblast reprogrammed by the candidate reprogramming factors can activate the Fbx15 locus, which leads to the expression of the Neo resistance gene. Thereby, the fibroblasts can survive in the presence of G418.
Takahashi and Yamanaka (2006) selected 24 genes, which were important transcripts of ES cells and oncogenes as candidate reprogramming factors. Different combinations of these candidates were introduced into mouse embryonic fibroblasts in order to screen proper reprogramming factors via the Fbx15-Neo reporter system. If these candidate genes could reprogram the fibroblasts, G418-resistant stem cell-like colonies would appear about two weeks later. Finally, the 24 candidates were narrowed down to four transcription factor genes. After introduction of the retroviral mediated factors Oct3/4, Sox2, Klf4, and c-Myc, mouse embryonic fibroblasts were reprogrammed into ES cell-like cells called iPS cells. This was a revolutionary breakthrough that immediately sparked immense interest so that many scientists subsequently focused on this research. However, some key pluripotent genes were not fully activated by the four transcription factor genes. Therefore, the fibroblasts were only partially reprogrammed. When the iPS cells were injected into mouse blastocysts, they could not generate postnatal chimeras or contribute to the germline. In fact, pluripotent iPS cell lines were not established until 2007. Live chimeras and germline transmitted mice were derived from these iPS cells through blastocyst injection (Maherali et al., 2007; Okita et al., 2007; Wernig et al., 2007). Zhao et al. (2009) and Kang et al. (2009) used iPS cells to generate germline transmitted mice through tetraploid complementation. As mentioned above, research in different laboratories indicates that iPS cells, which are similar to ES cells, have the potential to differentiate into any cell type.
Takahashi et al. (2007) and Yu et al. (2007) independently reprogrammed human somatic cells to iPS cells. The former used Oct3/4, Sox2, Klf4, and c-Myc on human dermal fibroblasts when the latter used Oct3/4, Sox2, Nanog, and Lin28 on human somatic cells. Both researches demonstrated that human iPS cells resemble human ES cells in many aspects, such as morphology, proliferation, pluripotency markers, gene expression profiles, epigenetic status, and differentiation potential. These findings revealed that human iPS cells have the capability of replacing human ES cells. Human iPS cells provide the correct direction of addressing the ethical disputes over stem cell sources and immunological rejection in cell therapy.
Since the first iPS cell line was established by Yamanaka in 2006, scientists have made efforts to improve the safety and efficiency of the reprogramming process, including single (Si-Tayeb et al., 2010) and multiple transient transfections (Okita et al., 2008), non-integrating vectors (Stadtfeld et al., 2008a; Yu et al., 2009; Okita et al., 2011), excisable vectors (Kaji et al., 2009; Lacoste et al., 2009; Woltjen et al., 2009), direct protein transduction (Kim D. et al., 2009; Zhou et al., 2009; Cho et al., 2010), RNA-based Sendai viruses (SeVs) (Fusaki et al., 2009; Nishimura et al., 2010; Seki et al., 2010), mRNA-based transcription factor delivery (Warren et al., 2010; Yakubov et al., 2010), microRNA transfections (Maehr et al., 2009), and the use of chemical compounds (Desponts and Ding, 2010; Li and Ding, 2010).
Recently, small-molecule compounds have been used to generate mouse iPS cells from somatic cells (Hou et al., 2013). Small-molecule compounds have advantages over other inducers because they can be cell-permeable, nonimmunogenic, easily synthesized, and cost-effective. Moreover, their effects on inhibiting and activating the function of specific proteins are often reversible and can be reversed by varying the concentrations. It is a milestone in the field of iPS cells. In the future, this chemical reprogramming strategy will be hotspots for reprogramming different somatic cells.
3. Cell sources for deriving iPS cells
Moreover, many other cell sources are also used in research on iPS cells. Up to now, iPS cells have been derived from many different species, such as mice, humans, rats, marmosets, rhesus monkeys, pigs, and rabbits (Table 1). However, most iPS cell lines cannot generate live chimeras. Because of the successful reprogramming of the fibroblasts, many different cell types have been analyzed for their capacity to be reprogrammed. The cell types successfully reprogrammed contain hepatocytes, gastric epithelial cells, keratinocytes, stomach cells, mesenchymal cells, neural stem cells, pancreatic cells, B and T lymphocytes, blood progenitor cells, cord blood cells, peripheral blood cells, and so on (Table 1).
Table 1.
iPS cells derived from different species and somatic cell types
| Species | Cell type | Factor or chemical | Vector | Reference |
| Mouse | Fibroblast | OKSM or OKS | Retrovirus | Takahashi and Yamanaka, 2006; Nakagawa et al., 2008; Wernig et al., 2008 |
| Fibroblast | OSE or KSNr | Retrovirus | Feng et al., 2009; Heng et al., 2010 | |
| Fibroblast | mir302/367 cluster | Lentivirus | Anokye-Danso et al., 2011 | |
| Fibroblast | OKSM | PB transposon and 2A peptides | Kaji et al., 2009 | |
| Fibroblast | Proteins (OKSM) | Poly-arginine | Zhou et al., 2009 | |
| Fibroblast | OKSM | Plasmid or adenovirus | Okita et al., 2008; Stadtfeld et al., 2008a | |
| Dermal papilla | OKM or OK | Retrovirus | Tsai et al., 2010 | |
| Melanocyte | OKM | Drug-inducible lentivirus | Utikal et al., 2009 | |
| Mature B and T cell | OKSM | Retrovirus | Eminli et al., 2009 | |
| Myeloid progenitor | OKSM | Retrovirus | Eminli et al., 2009 | |
| Hematopoietic stem cell | OKSM | Retrovirus | Eminli et al., 2009 | |
| Pancreatic β cell | OKSM | Drug-inducible lentivirus | Stadtfeld et al., 2008b | |
| Intestinal epithelial cell | OKSM | Drug-inducible lentivirus | Wernig et al., 2008 | |
| Hepatocyte | OKS | Retrovirus | Aoi et al., 2008 | |
| Gastric epithelial cell | OKSM | Retrovirus | Aoi et al., 2008 | |
| Adipose stem cell | OKSM | Retrovirus | Sugii et al., 2010 | |
| Neural stem cell | OK or O | Retrovirus | Kim et al., 2008; 2009b | |
|
| ||||
| Human | Fibroblast | OKSM or OKS | Retrovirus | Takahashi et al., 2007; Nakagawa et al., 2008 |
| Fibroblast | OSLN | Lentivirus | Yu et al., 2007 | |
| Fibroblast | OKSM or OKS | Floxed lentivirus | Soldner et al., 2009 | |
| Fibroblast | OS and valproic acid | Retrovirus | Huangfu et al., 2008 | |
| Fibroblast | Proteins (OKSM) | Poly-arginine | Kim D. et al., 2009 | |
| Fibroblast | OKSM | Adenovirus | Zhou and Freed, 2009 | |
| HUVEC | OKSM | Retrovirus | Lagarkova et al., 2010 | |
| Peripheral blood cell | OKSM | Drug-inducible lentivirus | Loh et al., 2010; Staerk et al., 2010 | |
| Cord blood endothelial cell | OSLN | Lentivirus | Haase et al., 2009 | |
| Cord blood stem cell | OKSM or OS | Retrovirus | Eminli et al., 2009; Giorgetti et al., 2009 | |
| Adipose stem cell | OKSM | Lentivirus | Sun et al., 2009 | |
| Adipose stem cell | OKS | Retrovirus | Aoki et al., 2010 | |
| Amniotic cell | OKSM | Retrovirus | Li C. et al., 2009 | |
| Amniotic cell | OSN | Lentivirus | Zhao et al., 2010 | |
| Neural stem cell | O | Retrovirus | Kim J.B. et al., 2009a | |
| Marrow mesenchymal cell | OKSM or OK | Retrovirus | Park et al., 2008 | |
| Adipose stem cell | OSLN | Nonviral minicircle DNA | Park et al., 2008 | |
| Hepatocyte | OKSM | Retrovirus | Liu et al., 2010 | |
| Astrocyte | OKSM | Retrovirus | Ruiz et al., 2010 | |
| Keratinocyte | OKSM or OKS | Retrovirus | Aasen et al., 2008 | |
|
| ||||
| Pig | Fibroblast | OKSM | Drug-inducible lentivirus | Wu et al., 2009 |
|
| ||||
| Rabbit | Hepatocyte and stomach cell | OKSM | Lentivirus | Honda et al., 2010 |
|
| ||||
| Rat | Fibroblast | OKS | Retrovirus | Chang et al., 2010 |
| Fibroblast | OKSM | Lentivirus | Liao et al., 2009 | |
| Neural progenitor cell | OKS | Retrovirus | Chang et al., 2010 | |
| Liver progenitor cell | OKS | Retrovirus | Li W. et al., 2009 | |
|
| ||||
| Marmoset | Fibroblast | OKSM | Retrovirus | Wu et al., 2010 |
|
| ||||
| Rhesus monkey | Fibroblast | OKSM | Retrovirus | Liu et al., 2008 |
HUVEC: human umbilical vein endothelial cell; O: Oct3/4; S: Sox2; K: Klf4; M: c-Myc; E: Esrrb; L: Lin28; N: Nanog; Nr: Nr52a
4. Advantages of peripheral blood over other cell sources for iPS cells
The generation of patient-specific iPS cells is a critical step in cell therapy and other clinical applications. As shown in Table 1, human iPS cells are normally derived from dermal fibroblasts because of their accessibility and relatively high reprogramming efficiency. However, skin biopsy and a prolonged period of expansion in cell culture are required prior to using dermal fibroblasts. During skin biopsy, the exposure of the dermis to ultraviolet light might increase the risk for chromosomal aberrations. In addition, it cannot be ignored that patients would experience the pain and the risk of infection when obtaining dermal fibroblasts. These issues limit the wide application of iPS cells.
Blood cells are the most easily accessible source of patients’ tissues for reprogramming because it is not need to maintain cell cultures extensively prior to reprogramming experiments. Furthermore, the venipuncture is safer than skin biopsy. And numerous peripheral blood samples have already been frozen and stored in blood banks, so we can directly generate human iPS cells via these samples.
The reprogramming of peripheral blood cells started with research on mice in 2008. Hanna et al. (2008) utilized retroviral-mediated factors (Oct3/4, Klf4, Sox2, and c-Myc) to reprogram mouse B lymphocytes. In the experiment, they improved the reprogramming efficiency by either ectopic expression of the myeloid transcription factor CCAAT/enhancer-binding-protein-α or knockdown of the B cell transcription factor Pax5. Hong et al. (2009) generated iPS cells from mouse T lymphocytes by the introduction of Oct3/4, Sox2, Klf4, and c-Myc in a p53-null background.
After mouse peripheral blood cells were reprogrammed, Haase et al. (2009) generated human iPS cells from cord blood (CB). It is an advantage that CB can be obtained from public and commercial CB banks without any risk to donors. Ye Z. et al. (2009) derived human iPS cells from previously frozen CB and CD34+ cells of healthy adult donors. However, the use of CB is still limited because it can only be obtained from neonates.
Loh et al. (2010), Seki et al. (2010), and Staerk et al. (2010) independently derived iPS cells from human peripheral blood cells. Loh et al. (2010) separated mononuclear cells (PBMCs) and CD34+ cells (PBCD34+) from peripheral blood samples, which were then collected through venipuncture and Ficoll density centrifugation. After infection with lentiviruses expressing Klf4, Sox2, Oct3/4, and c-Myc, PBCD34+ cells showed a reprogramming efficiency of 0.002%, whereas PBMCs showed relatively low values of 0.0008% to 0.001%. Staerk et al. (2010) utilized a doxycycline-inducible lentivirus construct to derive iPS cells from T lymphocytes and myeloid cells which were cultured in IL-7 or G-CSF, GM-CSF, IL-3, and IL-6; this lentivirus construct could encode four reprogramming factors (Oct3/4, Sox2, Klf4, and c-Myc) into a polycistronic expression cassette (pHAGE2-TetOminiCMV-hSTEMCCA). Their results showed that the reprogramming efficiency of T lymphocytes was higher than that of myeloid cells. Because T lymphocytes exhibited a higher proliferation rate and had a better long-term growth potential in vitro than myeloid cells, Seki et al. (2010) induced T lymphocytes into iPS cells by a temperature-sensitive mutant SeV vector encoding human Oct3/4, Sox2, Klf4, and c-Myc with an efficiency of 0.1%. This SeV vector is a non-integrating type, and it could not proliferate at standard culture temperatures. So these characteristics significantly increase the safety for the generation of iPS cells. Chou et al. (2011) generated iPS cells from newborn CB and adult peripheral blood mononuclear cells with an improved EBNA1/OriP plasmid. By this new reprogramming vector, iPS cells were derived from peripheral blood cells within 14 d, instead of 28 to 30 d as in a previous work on fibroblasts.
The research and findings provide the evidence that human iPS cells from peripheral blood cells are comparable to human ES cells in terms of morphology, surface antigens, pluripotency gene expression, DNA methylation, and differentiation potential. iPS cells from mononuclear cells of peripheral blood can be considered reliable and safe. Therefore, methods of generating iPS cells from human peripheral blood cells will accelerate research on and promote clinical applications of iPS cells in the future.
Interestingly, human iPS cells can also differentiate into peripheral blood cells because of their pluripotency. Lei et al. (2012) made human iPS cells differentiate into both conventional and antigen-specific T lymphocytes for T cell-based immunotherapy by utilizing either in vitro or in vivo induction systems. The recently established human iPS cells by Ebihara et al. (2012) represent potentially unlimited safe sources of donor-free red blood cells for blood transfusion, as they can proliferate indefinitely in vitro without the potential for infectious disease via blood transfusion.
5. Applications of iPS cells
Similar to ES cells, mouse iPS cells are able to differentiate into any type of cell, and even have the capability of germline transmission (Maherali et al., 2007; Okita et al., 2007; Wernig et al., 2007; Kang et al., 2009; Zhao et al., 2009). This means that we can obtain various differentiated cells in large quantities for research and therapy. For example, Zhu et al. (2012) and Easley et al. (2012) independently investigated the differentiation potential of human and mouse iPS cells into spermatogonial stem cells and late-stage male germ cells. The derivation of male germ cells from iPS cells represents potential applications in the treatment of male infertility and provides a model for uncovering the molecular mechanisms underlying male germ cell development.
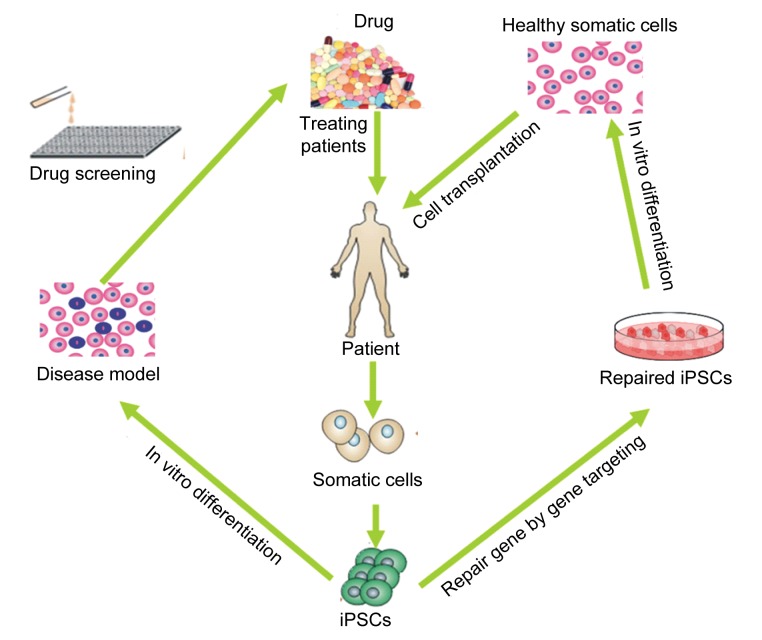
Up to now, iPS cells have had main applications in three major areas: human disease modeling (Zhang et al., 2012), regenerative medicine, and drug discovery (Fig. 1).
Fig. 1.
A schematic illustration for the applications of iPS cells (iPSCs) in treating human disease
Modified from Stadtfeld and Hochedlinger (2010)
5.1. Disease modeling
For many human genetic diseases, therapeutic research is hindered by problems regarding the source of experimental materials. iPS cells can overcome these issues by establishing disease-specific models. Disease-specific iPS cells can form cell lineages reflecting the defects caused by a certain disease in patients. Some human diseases for which models have been established by patient-specific iPS cells include amyotrophic lateral sclerosis (Dimos et al., 2008), spinal muscular atrophy (Ebert et al., 2009), Parkinson’s disease (Soldner et al., 2009), β-thalassemia (Ye L. et al., 2009), Rett syndrome (Hotta et al., 2009), adenosine deaminase deficiency-related severe combined immunodeficiency, Shwachman-Bodian-Diamond syndrome, Gaucher disease, Duchenne muscular dystrophy, Becker muscular dystrophy, Huntington’s disease, type 1 diabetes mellitus, Down syndrome, and Lesch-Nyhan syndrome (carriers) (Park et al., 2008). Disease models based on iPS cells can result in both insights into the mechanisms of pathogenesis and the development of new drugs. Patient- and disease-specific therapies represent the most valuable outcome of the whole area of iPS cells. Considering the generation of a wide variety of cell types from iPS cells, these provide the potential to treat disorders of virtually all tissue systems in the body.
5.2. Regenerative medicine
Immunological rejection is a major issue in organ transplantation and cell therapy, because long-term treatment with immunosuppressive drugs would produce serious side effects. Patient-specific iPS cells have the immune markers of the patient, so they solve the problem of immunological rejection. In addition, disease-causing mutations can also be restored by gene targeting in patient-specific iPS cells. Repaired cells can differentiate into targeted cells. After transplanted into the diseased area, they can alleviate disease symptoms. To illustrate this, using a mouse model, Hanna et al. (2007) proved that iPS cells can be used to cure sickle cell anemia, a genetic blood disorder that renders red blood cells nonfunctional. The disease-causing mutation was repaired in iPS cells generated from the mouse model via gene targeting. The repaired iPS cells then differentiated into blood-forming progenitor cells. These healthy progenitors were transplanted into an anemic mouse where they can proliferate and generate normal red blood cells, thereby curing the disease. In fact, the application of patient-specific iPS cells for tissue replacement and cell therapy indicates what the ultimate goal of regenerative medicine is. At present, however, many limitations still affect the possibility to apply this personalized medicine. The main limitations are related to technical issues, including the development of safe and efficient methods for iPS generation as well as the choice of the most appropriate cell type for reprogramming.
5.3. Drug discovery
Before using novel drugs for treatment, we need to obtain reliable data on their potential toxic effects on humans. In drug discovery, the effects and side effects of novel drugs are usually tested in laboratory animals, such as mice, dogs, and pigs. However, these tests are costly and humans and animals have relatively significant differences. Moreover, animal tests are not effectively standardized. Now we can efficiently test novel drugs on disease models generated from patient-specific iPS cells. This approach will greatly facilitate research on pharmacology and toxicology. Some drugs have already been tested on iPS cells derived from patients suffering from various diseases, such as spinal muscular atrophy (Ebert et al., 2009), familial dysautonomia (Lee et al., 2009), and LEOPARD syndrome (Carvajal-Vergara et al., 2010). The fact that novel drugs alleviate “symptoms” in patient-specific iPS cells demonstrates their therapeutic potential. This method now has been applied to many other diseases and will benefit many patients.
6. Potential issues regarding iPS cells
Crucial experiments based on iPS cell technology have shed light on human diseases at the cellular and molecular levels. The application of iPS cells in drug discovery can reduce costs and increase the chances of success. Above all, iPS cells circumvent ethical disputes on ES cells and patient-specific iPS cells may resolve issues of immunological rejection in cell therapy.
Currently, in the field of iPS cells, scientists have developed more efficient methods of deriving iPS cells from various cell sources, including those from patients who suffer from different diseases. More progress and new innovations regarding iPS cells will be made in the near future. However, some problems remain to be solved in the clinical application of iPS cells. Many genomic changes, including chromosomal aneuploidy, translocations, point mutations, megabase-scale duplications and/or deletions, and so on, have been observed in human iPS cells; these problems may limit the therapeutic potential of iPS cells. As suggested recently by Gore et al. (2011), in the exome sequencing of 22 human iPS cell lines obtained from seven laboratories by five different methods, they found 124 point mutations in the iPS cell lines, but no mutations in the parental cells. This study identified many missense mutations related to the protein function modification and point mutations in genes implicated in cancers. Although the reprogramming process itself might cause genomic anomalies, not all anomalies result from it, because genomic alterations have been identified in human iPS cells produced through different techniques, including non-integrating methods such as those that use synthetic mRNAs (Warren et al., 2010). In spite of the fact that the low efficiency of reprogramming is one of the hurdles that prevents the area from moving forward, compared with improving reprogramming efficiency, solving problems in chromosomal anomalies in iPS cells is more important. Thus, in the future, obtaining iPS cells with the fewest genomic alterations should be the focus in the field of iPS cells. The causes of chromosomal abnormalities during reprogramming somatic cells need to be investigated as well.
In addition, some other questions remain: What is the mechanism for iPS cell induction? What are the optimal reprogramming factors? How do we reduce risks of insertion mutagenesis in the genome of iPS cells? How do we achieve directed differentiation? How do we evaluate the safeness of iPS cells in clinical applications? Obtaining the answers to these questions requires thorough analyses of the induction process and the epigenetics of iPS cells. Furthermore, a reliable evaluation system on clinical trials needs to be established. The clarification of these questions might improve the application of iPS technology as well as the length and quality of life for people in the future.
7. Future perspectives
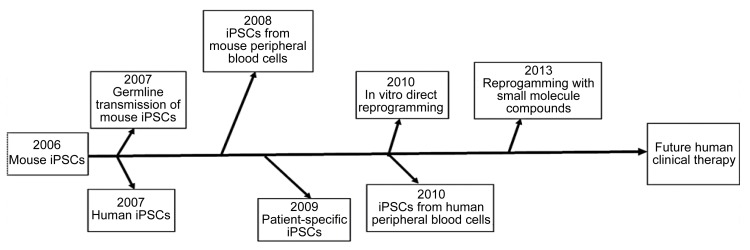
The generation of iPS cells is regarded as a milestone for life science (Fig. 2). In spite of the problems mentioned in the last section, the advantages of iPS cells cannot be ignored. iPS cells can avoid ethical disputes as well as immunological rejection in cell therapy. Moreover, disease models generated from iPS cells can be used to study the mechanisms of human genetic disorders and test the effects of novel drugs (Fig. 1). iPS cell biology has admittedly become a new field within stem cell research that covers various important and attractive scientific areas.
Fig. 2.
History and milestones of iPS cell (iPSC) research
Modified from Yamanaka (2012)
The whole area of iPS cells is a hot topic in biomedical research and is rapidly approaching its clinical utilization. The future clinical application of iPS cells needs a more comprehensive knowledge of the reprogramming process. Recent advancements, especially the iPS cells derived from peripheral blood and chemical reprogramming strategy, have increased their therapeutic potential. Along with the improvement of iPS cell technology, clinical therapy based on iPS cells will be put on the agenda in the foreseeable future.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30871436, 30973297, 31171194, and 31271534), the National Basic Research Program (973) of China (Nos. 2010CB945002 and 2014CB541703), the Shandong Provincial Science and Technology Key Program (No. 2009GG10003039), and the Independent Development Foundation of Shandong University (Nos. 2012JC019 and 2012ZD030), China
Compliance with ethics guidelines: Jing ZHAO, Wen-jie JIANG, Chen SUN, Cong-zhe HOU, Xiao-mei YANG, and Jian-gang GAO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 2.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 4.Aoki T, Ohnishi H, Oda Y, Tadokoro M, Sasao M, Kato H, Hattori K, Ohgushi H. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-Myc. Tissue Eng Part A. 2010;16(7):2197–2206. doi: 10.1089/ten.tea.2009.0747. [DOI] [PubMed] [Google Scholar]
- 5.Carvajal-Vergara X, Sevilla A, D′Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MY, Kim D, Kim CH, Kang HC, Yang E, Moon JI, Ko S, Park J, Park KS, Lee KA, et al. Direct reprogramming of rat neural precursors cells and fibroblasts into pluripotent stem cells. PLoS ONE. 2010;5(3):e9838. doi: 10.1371/journal.pone.0009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesné P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol. 2002;20(4):366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, Lee EJ, Roh TY, Chu IS, Leem SH, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116(3):386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 9.Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21(3):518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desponts C, Ding S. Using small molecules to improve generation of induced pluripotent stem cells from somatic cells. Methods Mol Biol. 2010;636:207–218. doi: 10.1007/978-1-60761-691-7_13. [DOI] [PubMed] [Google Scholar]
- 11.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 12.Easley CA, 4th, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2(3):440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert AD, Yu JY, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebihara Y, Ma F, Tsuji K. Generation of red blood cells from human embryonic/induced pluripotent stem cells for blood transfusion. Int J Hematol. 2012;95(6):610–616. doi: 10.1007/s12185-012-1107-9. [DOI] [PubMed] [Google Scholar]
- 15.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41(9):968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans MJ, Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 17.Feng B, Jiang J, Kraus P, Ng JH, Heng JC, Chan YS, Yaw LP, Zhang W, Loh YH, Han J, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11(2):197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 18.Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodríguez-Pizà I, Vassena R, Raya A, Boué S, Barrero MJ, Corbella BA, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2 . Cell Stem Cell. 2009;5(4):353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gore A, Li A, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurdon JB. The development capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Embryol Exp Morphol. 1962;10(4):622–640. [PubMed] [Google Scholar]
- 22.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5(4):434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 24.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133(2):250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, Ogura A. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem. 2010;285(41):31362–31369. doi: 10.1074/jbc.M110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotta A, Cheung AY, Farra N, Vijayaragavan K, Séguin CA, Draper JS, Pasceri P, Maksakova IA, Mager DL, Rossant J, et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6(5):370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- 29.Hou PP, Li YQ, Zhang X, Liu C, Guan JY, Li HG, Zhao T, Ye JQ, Zhao Y, Deng HK. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 30.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Melton induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2 . Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 31.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5(2):135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 35.Kim JB, Greber B, Araúzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Schöler HR. Direct reprogramming of human neural stem cells by OCT4 . Nature. 2009;461(7264):649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 36.Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Lacoste A, Berenshteyn F, Brivanlou AH. An efficient and reversible transposable system for gene delivery and lineage-specific differentiation in human embryonic stem cells. Cell Stem Cell. 2009;5(3):332–342. doi: 10.1016/j.stem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Lagarkova MA, Shutova MV, Bogomazova AN, Vassina EM, Glazov EA, Zhang P, Rizvanov AA, Chestkov IV, Kiselev SL. Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale. Cell Cycle. 2010;9(5):937–946. doi: 10.4161/cc.9.5.10869. [DOI] [PubMed] [Google Scholar]
- 39.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPS cells. Nature. 2009;461(7262):402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei F, Haque R, Xiong X, Song J. Directed differentiation of induced pluripotent stem cells towards T lymphocytes. J Vis Exp. 2012;63:e3986. doi: 10.3791/3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, Yu H, Jin S, Wei Z, Chen F, et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009;18(22):4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31(1):36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4(1):16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H, et al. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4(1):11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, Jiang W, Cai J, Liu M, Cui K, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3(6):587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Ye Z, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51(5):1810–1819. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7(1):15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. PNAS. 2009;106(37):15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura K, Sano M, Ohtaka M, Furuta B, Umemura Y, Nakajima Y, Ikehara Y, Kobayashi T, Segawa H, Takayasu S, et al. Development of defective and persistent sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2010;286(6):4760–4771. doi: 10.1074/jbc.M110.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 53.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 54.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 55.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz S, Brennand K, Panopoulos AD, Herrerías A, Gage FH, Izpisua-Belmonte JC. High-efficient generation of induced pluripotent stem cells from human astrocytes. PLoS ONE. 2010;5(12):e15526. doi: 10.1371/journal.pone.0015526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seki T, Yuasa S, Oda M, Eqashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7(1):11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Si-Tayeb K, Noto FK, Sepac A, Sedlic F, Bosnjak ZJ, Lough JW, Duncan SA. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev Biol. 2010;10(1):81. doi: 10.1186/1471-213X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic β cells into induced pluripotent stem cells. Curr Biol. 2008;18(12):890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7(1):20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugii S, Kida Y, Kawamura T, Suzuki J, Vassena R, Yin YQ, Lutz MK, Berggren WT, Izpisúa Belmonte JC, Evans RM. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. PNAS. 2010;107(8):3558–3563. doi: 10.1073/pnas.0910172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. PNAS. 2009;106(37):15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 68.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swierqiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 69.Tsai SY, Clavel C, Kim S, Ang YS, Grisanti L, Lee DF, Kelley K, Rendl M. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28(2):221–228. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- 70.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009;122(19):3502–3510. doi: 10.1242/jcs.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 73.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26(8):916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 75.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Zhang Y, Mishra A, Tardif SD, Hornsby PJ. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 2010;4(3):180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009;1(1):46–54. doi: 10.1093/jmcb/mjp003. [DOI] [PubMed] [Google Scholar]
- 78.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394(1):189–193. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 79.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10(6):678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Ye L, Chang JC, Lin C, Sun X, Yu J, Kan YW. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. PNAS. 2009;106(24):9826–9830. doi: 10.1073/pnas.0904689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, Dang VL, Spivak JL, Moliterno AR, Cheng L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114(27):5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416(6880):545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 83.Yu J, Vodyanik MK, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 84.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, Zhao J, Jiang WJ, Shan XW, Yang XM, Gao JG. Conditional gene manipulation: Cre-ating a new biological ear. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(7):511–524. doi: 10.1631/jzus.B1200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, Luo YN, Yin GW, Li Y, Wang J, et al. Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation. 2010;80(2-3):123–129. doi: 10.1016/j.diff.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 90.Zhu Y, Hu HL, Li P, Yang S, Zhang W, Ding H, Tian RH, Ning Y, Zhang LL, Guo XZ, et al. Generation of male germ cells from induced pluripotent stem cells (iPS cells): an in vitro and in vivo study. Asian J Androl. 2012;14(4):574–579. doi: 10.1038/aja.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]