Abstract
Alkaloids are plant secondary metabolites that are widely distributed in Nicotiana species and contribute greatly to the quality of tobacco leaves. Some alkaloids, such as nornicotine and myosmine, have adverse effects on human health. To reduce the content of harmful alkaloids in tobacco leaves through conventional breeding, a genetic study of the alkaloid variation among different genotypes is required. In this study, alkaloid profiles in leaves of five Nicotiana tabacum cultivars and Nicotiana tomentosiformis were investigated. Six alkaloids were identified from all six genotypes via gas chromatograph-mass spectrometry (GC-MS). Significant differences in alkaloid content were observed both among different leaf positions and among cultivars. The contents of nornicotine and myosmine were positively and significantly correlated (R 2=0.881), and were also separated from those of other alkaloids by clustering. Thus, the genotype plays a major role in alkaloid accumulation, indicating a high potential for manipulation of alkaloid content through traditional breeding.
Keywords: Alkaloid, Genetic effects, Nicotiana
1. Introduction
Tobacco includes any plant of the genus Nicotiana of the nightshade family (Solanaceae). Cultivated tobacco (Nicotiana tabacum) is the most widely planted species and is grown worldwide for the production of tobacco leaf. The presence of alkaloids, such as nicotine, nornicotine, anabasine, and anatabine, is characteristic of Nicotiana species. Nicotine is synthesized in the tobacco roots and transported in the xylem to the shoots (Hibi et al., 1994; Shi et al., 2006). Nicotine is synthesized from the polyamine putrescine, which is produced either directly from ornithine or indirectly from arginine. The first step in nicotine biosynthesis is the conversion of putrescine to N-methylputrescine, catalysed by putrescine N-methyltransferase (Hashimoto and Yamada, 1994). N-methylputrescine is then oxidized by N-methylputrescine oxidase and cyclized to form a pyrrolidine ring, while quinolinic acid phosphoribosyl transferase serves in the pyridine ring synthesis that provides nicotinic acid (Hibi et al., 1994; Shoji et al., 2010). Nicotine is formed by a condensation of N-methylpyrrolinium and nicotinic acid, and can be further metabolized to form other alkaloids, such as nornicotine, nicotyrine, and myosmine (Leete and Chedekel, 1974; Häkkinen et al., 2007; Shoji et al., 2010).
The chemical composition of tobacco leaves is extremely complex, with one alkaloid, nicotine, being the most characteristic constituent of tobacco and responsible for its addictive nature (Cai et al., 2003). Therefore, the composition and content of alkaloids in leaves are critical to tobacco quality (Lewis, 2006). However, some types of alkaloids including nornicotine and myosmine, have adverse effects on human health (Dickerson and Janda, 2002; Wilp et al., 2002; Hecht, 2003; Brogan et al., 2005). Nornicotine is a biochemical precursor of N′-nitrosonornicotine, a tobacco-specific nitrosamine that has been shown to exhibit carcinogenic properties in laboratory animals (Hecht, 2003). In addition, upon nitrosation, myosmine can yield 4-hydroxy-1-(3-pyridyl)-1-butanone and N′-nitrosonornicotine, which are strong carcinogens (Wilp et al., 2002). Currently, there is no efficient approach for selectively reducing the amount of harmful alkaloids in tobacco leaves. The analysis of genetic effects is one useful method to improve nutrient quality traits, and has been applied in many crops, such as rice and cotton (Shi et al., 1996; Feng et al., 2011). Many previous studies have shown obvious differences in alkaloid profiles among tobacco cultivars (Shi et al., 2001; Lin et al., 2002; Lian et al., 2008), but there have been few reports on the effects of genetic variation on alkaloid accumulation in tobacco leaves.
Plant metabolism is controlled by genetic and environmental factors (Yan and Chen, 2007). Tobacco cultivation in China is widely dispersed. The differences in geographical and climatic conditions might be the main factors related to different plant metabolite levels. To exclude environmental interference, cultivars originating from different countries were grown in Yunnan Province, one of the best areas for tobacco cultivation in China. In this study, we used leaves from various positions on plants of five common tobacco cultivars and Nicotiana tomentosiformis grown at the same location, to investigate genetic variation in alkaloid accumulation in Nicotiana leaves. The study will lay the theoretical basis for selectively reducing the levels of harmful alkaloids through traditional breeding at the given location.
2. Materials and methods
2.1. Plant materials
Five commercial tobacco cultivars, including two Chinese (Cuibiyihao and Honghuadajinyuan), one American (NC297), and two Zimbabwean cultivars (KRK26 and T66), and Nicotiana tomentosiformis, were chosen and used in this study. In Feb. 2011, sterilized seeds were germinated and planted in a floating system in the World Tobacco Species Garden, Yuxi City, Yunnan, China. The seedlings were grown in a controlled environment greenhouse equipped with supplemental lighting providing a 14 h/10 h light/dark cycle, and at an average temperature of 25 °C during the day and 20 °C at night. Two hundred seedlings with 7–9 true leaves were transplanted into an agricultural field with 60 cm between plants within rows and 120 cm between rows. The field design was completely random. Water, fertilizer, and pesticides were applied, as and when required.
Plant material (free of any insects and mechanical damage) was obtained from three different leaf positions [upper (No. 17), middle (No. 12), and lower (No. 5) leaves)] at the mature leaf stage. For sampling, leaves from five tobacco plants were collected as a replicate, and three independent replicates were taken for analysis. After harvest, the leaf samples were immediately frozen in liquid nitrogen, lyophilized to dryness, and ground to a coarse powder with a coffee grinder.
2.2. Sample preparation
Alkaloid was extracted from lyophilized samples and analyzed as previously described by Cai et al. (2011) with minor modification. A 300-mg sample of leaf powder was added to 2.0 ml of 5% NaOH (0.05 g/ml) in a 50 ml conical flask until mixed, and then incubated for 15 min at room temperature. Alkaloids were extracted by addition of 20 ml extraction solution [180 mg/L 2-methylquinoline and 10 mg/L 2,4′-bipyridyl (Tokyo Chemical Industry Co., Ltd., Tokyo) dissolved in 0.01% triethylamine (Fluka, USA)/chloroform (Merck, Germany)] and ultrasonically extracted for 15 min at room temperature. Following phase separation, an aliquot of the organic phase was filtered through a column filled with anhydrous sodium sulfate. The filtrate (2 ml) was transferred to a sample vial for further analysis.
2.3. Gas chromatograph-mass spectrometry (GC-MS) analysis
GC-MS analysis of the alkaloids in the tobacco leaves was carried out using an Agilent 7890A GC interfaced to an Agilent 5975C mass-selective detector (Agilent, USA), controlled by an Agilent G1701EA GC-MSD ChemStation. Chromatographic separations were achieved in an HP-35 (30 m×0.250 mm, 0.25 μm film thickness) capillary column (Agilent Technologies Inc., USA). The column temperature was set to 100 °C for 3 min, and programmed to 260 °C at 8 °C/min and kept at this temperature for 10 min. The column flow, using helium as the carrier gas, was held constant at 1.0 ml/min. The injector temperature was 250 °C, and the injector was set in split mode (10:1) with an injection volume of 2 μl.
The ion source temperature was 230 °C and the interface temperature 280 °C. Mass spectra were recorded at 70 eV, scanned from 30 to 500 amu, and compared to authentic standards (retention time and mass spectra) for verification. Quantitative analysis was carried out in selected ion monitoring (SIM) mode. Ions were acquired in SIM mode with a solvent cut time of 8.0 min (Cai et al., 2011).
2.4. Statistical analysis
The genetic model, including genotype×environment interactions, was used for the analysis of inheritance (Zhu, 1996). The model used for the analysis was
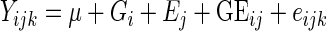 , ,
|
where Yijk is the phenotypic mean of the cross of cultivar i and leaf position j in the kth block, μ is the population mean, Gi is the cultivar effect, Ej is the leaf position effect, GEij is the cultivar×leaf position effect, and eijk is the residual error.
The TestR Model was analyzed using the MINQUE method in QGAStation 2.0 software (http://ibi.zju.edu.cn/software/qga/v2.0/index_c.htm) for estimating variances and covariances and further calculating the ratios of genetic variance to phenotypic variance. The adjusted unbiased prediction method was adopted to estimate different effects (Zhu and Weir, 1996). The Jackknife resampling method (Miller, 1974) was used to calculate the standard errors of the estimated values for t-test, and the significance of differences was tested by t-test.
TIGR MeV software (version 4.1) was used for t-test clustering of significant differences in alkaloid level. The clustering of alkaloids by degree of similarity (or dissimilarity) was computed using K-mean and Euclidian distance. Data normalization was calculated using the formula x=(value−average)/standard deviation (SD) (Scherling et al., 2009).
Statistical analysis was performed using the SPSS package program version 11.5 (SPSS Inc., Chicago, IL, USA). Data were analyzed with a one-way analysis of variance (ANOVA) model. The variables were cultivars and leaf positions. The means were compared through the least significant difference (LSD) test at a significance level of 0.05. The values were reported as mean±SD.
3. Results
3.1. Composition and content of alkaloids in Nicotiana leaves
The leaves of five commercial tobacco cultivars and N. tomentosiformis were evaluated to determine variation in the amounts and types of alkaloids present in samples grown under identical conditions using the same analytical procedure. Six alkaloids (nicotine, nornicotine, myosmine, anabasine, anatabine, and cotinine) were identified via GC-MS (Table 1). A typical total ion chromatogram (TIC) of the alkaloids in the tobacco leaves is shown in Fig. 1.
Table 1.
Data from GC-MS analysis of alkaloids in Nicotiana leaves
| Peak No. | Alkaloid | RT (min) | Quantitative ion m/z | Qualitative ion m/z |
| 1 | Nicotine | 10.44 | 84 | 133 |
| 2 | Nornicotine | 12.39 | 119 | 147 |
| 3 | Myosmine | 12.71 | 146 | 118 |
| 4 | Anabasine | 13.31 | 84 | 105 |
| 5 | Anatabine | 14.13 | 160 | 105 |
| 6 | Cotinine | 17.86 | 98 | 176 |
| IS1 | 2-Methylquinoline | 10.27 | 143 | 128 |
| IS2 | 2,4′-Bipyridyl | 14.71 | 156 | 129 |
IS: internal standard; RT: retention time
Fig. 1.
Total ion chromatogram (TIC) of alkaloids in Nicotiana leaves with 2-methylquinoline and 2,4′-bipyridyl as internal standards 1 and 2 (IS1 and IS2), respectively
Six alkaloids were detected in the collection of tobacco leaves studied (Table 2), and significant differences were noted in the contents of total alkaloids and of all individual alkaloids from different leaf positions and cultivars. Nicotine was the predominant alkaloid in all the analysed cultivars, followed by anatabine, nornicotine and anabasine. Nornicotine was the abundant alkaloid in N. tomentosiformis, and its content was significantly higher than that of other alkaloids. In our work, the highest total alkaloids level [(38.76±2.18) mg/g] was observed in the upper leaves from NC297, while the lowest level [(2.66±0.11) mg/g] was observed in the upper leaves from N. tomentosiformis (Table 2).
Table 2.
Alkaloid composition and content in Nicotiana leaves (dry weight) from different leaf positions
| Leaf position | Nicotine (mg/g) | Nornicotine (μg/g) | Myosmine (μg/g) | Anabasine (μg/g) | Anatabine (μg/g) | Cotinine (μg/g) | Total alkaloids (mg/g) |
| Cuibiyihao | |||||||
| Upper | 20.83±0.44e | 665.85±11.55h–j | 18.60±0.76h | 238.94±0.87e–g | 958.70±36.98f | 15.24±0.23g | 22.73±0.48e |
| Middle | 14.69±0.37g | 608.17±1.30jk | 20.37±2.55f–h | 227.36±1.17g–i | 779.79±11.27gh | 16.17±1.14fg | 16.34±0.39g |
| Lower | 14.79±0.91g | 608.93±56.39jk | 19.48±1.77gh | 223.01±1.83h–j | 686.62±25.53i | 14.91±0.81gh | 16.34±0.99g |
| Honghuadajinyuan | |||||||
| Upper | 32.69±0.48b | 830.65±14.79f | 22.94±0.73d–g | 307.82±2.42a | 1798.32±30.28a | 19.48±0.08bc | 35.67±0.51b |
| Middle | 18.72±1.31f | 611.56±10.24jk | 21.93±3.50e–h | 241.77±3.41ef | 1006.91±44.06f | 18.22±1.75c–f | 20.62±1.37f |
| Lower | 18.05±0.22f | 619.61±13.72i–k | 20.68±1.68f–h | 230.90±1.52f–h | 830.23±7.38g | 16.69±1.29e–g | 19.77±0.22f |
| NC297 | |||||||
| Upper | 35.55±2.06a | 1164.99±29.61d | 25.49±3.70de | 310.28±7.56a | 1684.47±79.75b | 20.92±1.44ab | 38.76±2.18a |
| Middle | 25.02±0.23c | 840.64±12.75f | 20.66±0.20f–h | 266.69±1.52c | 1099.48±14.75e | 15.85±0.33g | 27.27±0.24c |
| Lower | 23.27±0.88d | 706.88±22.17gh | 22.92±1.79d–g | 228.88±3.20g–i | 743.81±30.06hi | 16.75±1.04d–g | 24.99±0.93d |
| KRK26 | |||||||
| Upper | 31.28±2.05b | 1070.39±37.34e | 21.58±1.99f–h | 293.40±6.64b | 1780.93±101.30a | 18.50±0.81c–e | 34.47±2.20b |
| Middle | 25.99±1.00c | 887.37±30.60f | 25.66±3.18d | 255.20±2.22cd | 1419.29±47.25c | 21.82±1.33a | 28.60±1.08c |
| Lower | 15.68±0.52g | 652.75±10.06h–j | 22.04±2.63d–h | 226.23±3.22hi | 790.94±15.00gh | 16.63±1.38e–g | 17.39±0.54g |
| T66 | |||||||
| Upper | 14.60±0.57g | 679.78±16.30hi | 18.65±0.93h | 253.79±4.27d | 1149.09±37.92e | 15.44±0.66g | 16.72±0.63g |
| Middle | 9.79±0.20h | 584.79±1.80k | 20.06±0.26gh | 256.19±26.50cd | 808.81±19.47g | 19.85±2.61a–c | 11.48±0.19h |
| Lower | 22.96±0.77d | 752.82±16.43g | 24.03±1.44d–f | 247.00±2.54de | 1309.79±46.94d | 18.83±0.77b–d | 25.31±0.82d |
| Nicotiana tomentosiformis | |||||||
| Upper | 0.73±0.05i | 1244.27±47.64c | 69.41±3.24c | 211.39±2.29j | 393.65±7.24k | 11.70±0.67i | 2.66±0.11j |
| Middle | 0.76±0.11i | 2260.34±94.86b | 136.12±3.26a | 219.39±2.84h–j | 507.41±8.44j | 12.99±1.92hi | 3.90±0.13ij |
| Lower | 0.35±0.03i | 3342.53±95.38a | 111.51±2.08b | 217.30±1.50ij | 493.27±9.66j | 11.23±0.14i | 4.52±0.13i |
Data are expressed mean±SD, n=3. Values in the same column followed by the same letter are not significantly different at P<0.05
Evaluation of alkaloid levels showed significant differences between leaf positions in each tobacco cultivar. In four N. tabacum cultivars, the highest nicotine and total alkaloids levels were found in the upper leaves (Table 2). However, there were some exceptions. For example, the highest level of alkaloid in T66 was in the lower leaves. The variation in the alkaloid content among leaf positions in N. tomentosiformis was also determined, and results indicated that the alkaloid levels increased gradually from the upper to the lower leaves (Table 2).
Considering N. tabacum cultivars, the highest levels of total alkaloids were found in NC297, with a mean value of 30.34 mg/g, followed by KRK26 (26.82 mg/g) and Honghuadajinyuan (25.35 mg/g). N. tomentosiformis accumulated nornicotine as the major alkaloid, with a 3.72-fold higher level than nicotine (Table 2). Although the nornicotine level in N. tomentosiformis was more than twice that of found in the N. tabacum cultivars, the nicotine content was less than 4% of that found in the N. tabacum cultivars.
3.2. Correlations between the contents of different alkaloids in Nicotiana leaves
The phenotypic and genotypic correlation coefficients between pairs of alkaloids were extremely significant at the 0.01 level (Table 3). According to their values, the correlation coefficients were divided into two categories: nornicotine and myosmine contents were negatively correlated to those of the five other alkaloids, and the contents of the other alkaloids were positively correlated among themselves. The highest negative phenotypic and genotypic correlations (−0.724 and −0.729, respectively) were found between myosmine and nicotine, while the highest positive phenotypic and genotypic correlations (0.998 and 0.998, respectively) were found between nicotine and the total alkaloids content (Table 3). The values of phenotypic correlation in our study were almost lower than those of the genotypic correlations. This is probably because the phenotypic correlations include the correlations of the interactions between the genotypes and other factors.
Table 3.
Correlation coefficients between pairs of alkaloids in Nicotiana leaves
| Trait | Nicotine | Nornicotine | Myosmine | Anabasine | Anatabine | Cotinine | Total alkaloids |
| Nicotine | −0.540** | −0.724** | 0.810** | 0.891** | 0.744** | 0.998** | |
| −0.544** | −0.729** | 0.827** | 0.891** | 0.793** | 0.998** | ||
| Nornicotine | −1.000** | 0.881** | −0.275** | −0.364** | −0.556** | −0.488** | |
| 0.000 | 0.883** | −0.283** | −0.367** | −0.598** | −0.492** | ||
| 0.468** | |||||||
| Myosmine | −0.969** | 1.000** | −0.443** | −0.544** | −0.629** | −0.684** | |
| −1.000 | 0.000 | −0.456** | −0.549** | −0.695** | −0.689** | ||
| 0.439** | 0.637** | ||||||
| Anabasine | 1.000** | −1.000** | −1.000** | 0.927** | 0.642** | 0.829** | |
| 0.991** | 0.000 | −1.000 | 0.944** | 0.725** | 0.846** | ||
| 0.759** | 0.696** | 0.513** | |||||
| Anatabine | 0.940** | −1.000** | −1.000** | 0.981** | 0.731** | 0.906** | |
| 0.975** | 0.000 | −1.000 | 1.000** | 0.782** | 0.906** | ||
| 0.955** | 0.642** | 0.519** | 0.879** | ||||
| Cotinine | 0.938** | −1.000** | −1.000** | 1.000** | 1.000** | 0.735** | |
| −0.988 | 0.000 | −0.214 | 1.000* | 0.629* | 0.783** | ||
| 0.692** | 0.237* | 0.211** | 0.636** | 0.620** | |||
| Total alkaloids | 1.000** | −1.000** | −0.969** | 1.000** | 0.927** | 0.937** | |
| 1.000** | 0.000 | −1.000 | 0.992** | 0.973** | −1.000 | ||
| 0.996** | 0.543** | 0.481** | 0.794** | 0.972** | 0.680** |
The phenotypic correlation coefficient and genotypic correlation coefficient were in the upper and lower lines in the upper right corner of the table, respectively. The variety correlation coefficient, leaf position correlation coefficient, and variety×leaf position interaction correlation coefficient were in the upper, middle, and lower lines in the lower left corner of the table, respectively
indicates significance at 0.05 probability level in the same column
indicates significance at 0.01 probability level in the same column
To further elucidate the correlation between pairs of alkaloids, different genetic effects were taken into consideration and the genetic correlation coefficient was divided into component parts (Table 3). The correlation coefficients of cultivar and cultivar×leaf position interaction in our work were significant, whereas fewer than half of the leaf position correlations were significant. As in the case of the phenotypic and genotypic correlation coefficients, nornicotine and myosmine were compared with the other alkaloids. The correlations with other alkaloids based on cultivars were negative and extremely significant, with R 2 values close to −1.000. Interestingly, the leaf position correlation coefficients for nornicotine and other alkaloids were zero, while the values of myosmine and other alkaloids (except nornicotine) were close to −1.000 and not significant. However, the corresponding correlation coefficients for the cultivar×leaf position interaction were positive and highly significant. The highest positive cultivar correlation coefficients were those among nicotine, anabasine, and total alkaloids, while nornicotine was negatively correlated with nicotine and total alkaloids, and myosmine was negatively correlated with anabasine and anatabine.
The variation in the alkaloid levels of tobacco leaves among leaf positions and cultivars was visualized by t-test clustering (Fig. 2). Significant variation was found among the six alkaloids. Nornicotine and myosmine were separated from the other alkaloids by clustering (Fig. 2). In addition, the percentages of nornicotine and myosmine were significantly lower in commercial tobacco than in N. tomentosiformis.
Fig. 2.

Significant differences in the alkaloid levels of Nicotiana leaves from different positions on the plant, illustrated by t-test clustering
‘A–F’ stand for the cultivars, as follows: Cuibiyihao, Honghuadajinyuan, NC297, KRK26, T66, and Nicotiana tomentosiformis, respectively. Numbers ‘1–3’ stand for the three replicates of the upper leaves, ‘4–6’ stand for the three replicates of the middle leaves, ‘7–9’ stand for the three replicates of the lower leaves. The clustering visualizes the abundance of the alkaloids in each of the samples ranging from high (red) to average (black) to low (green). Data normalization results from the formula x=(value−average)/SD (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
3.3. Variance analysis of genetic effects
Analysis of the results using the TestR Model in QGAStation showed that total and individual alkaloid levels were markedly affected at the 0.01 level by cultivar and cultivar×leaf position interaction (Table 4). Apart from anabasine and anatabine, the alkaloid concentrations were primarily determined by the cultivar effect, with the genetic component accounting for more than 50% of the variance. In particular, the variance of the cultivar effect on myosmine was as high as 85.0%, which was about six times the genetic interaction variance. Unlike the other alkaloids, although the cultivar variances for anabasine and anatabine were significant, the values were smaller than those of their interaction effects. In addition, the leaf position effects on anabasine and anatabine were equal to their genetic variance components at 32.0% and 22.7%, respectively. However, the leaf position variances of other alkaloids were much lower and not significant (Table 4). Taken together, the data demonstrated that the cultivar effect played the major role in determining the accumulation of alkaloids in the tobacco leaves, but for anabasine and anatabine the effect of the cultivar×leaf position interaction could not be ignored.
Table 4.
Estimated proportions of variance components for alkaloids in Nicotiana leaves
| Parameter | Nicotine | Nornicotine | Myosmine | Anabasine | Anatabine | Cotinine | Total alkaloids |
| V C/V P | 0.686** | 0.580** | 0.850** | 0.249** | 0.343** | 0.544** | 0.633** |
| V L/V P | 0.085** | 0.000 | 0.001 | 0.320** | 0.227** | 0.004 | 0.089** |
| V CL/V P | 0.223** | 0.418** | 0.145** | 0.385** | 0.423** | 0.313** | 0.271** |
| V e/V P | 0.006* | 0.003 | 0.004* | 0.046** | 0.007* | 0.139** | 0.007* |
V C/V P: ratio of cultivar variance to phenotypic variance; V L/V P: ratio of leaf position variance to phenotypic variance; V CL/V P: ratio of cultivar×leaf position interaction variance to phenotypic variance; V e/V P: ratio of error variance to phenotypic variance
indicates the significance at 0.05 probability level in the same column
indicates the significance at 0.01 probability level in the same column
4. Discussion
4.1. Alkaloid content
In this study, we found significant differences in alkaloid content among different cultivars and leaf positions. The highest level of total alkaloids in N. tabacum was found in NC297, with a mean value of 30.34 mg/g. The lowest content was found in T66, which had less than 60% of the total alkaloids content found in NC297. From a random examination of 152 cultivars of Nicotiana tabacum, Djordjevic and Doran (2009) found a range of alkaloid contents between 1.7 and 49.3 mg/g. Our results showed that the total alkaloids content in N. tabacum was obviously higher than that in N. tomentosiformis (Table 2). N. tabacum is a natural allotetraploid derived from the interspecific hybridization of ancestral N. sylvestris and N. tomentosiformis, while N. tomentosiformis is a diploid species (Gavilano et al., 2007; Zhao et al., 2011). The differences in species and ploidy between N. tomentosiformis and commercial tobacco (N. tabacum) may be the cause of the differences in alkaloid accumulation between them. In addition, the alkaloid content in the upper leaves was evidently higher than those of the middle and lower leaves of N. tabacum (Table 2). Similar results have been reported previously (Djordjevic et al., 1989; Shi et al., 2001; Lin et al., 2002). Djordjevic et al. (1989) found that in NC95, the contents of nicotine, nornicotine, and anatabine from upper stalk positions exceeded those from lower and middle stalk positions.
In this study, all five N. tabacum cultivars and N. tomentosiformis were grown at the same location. Different from our results, Lin et al. (2002) found that the total alkaloids contents of upper, middle, and lower leaves of plants grown in Cuibiyihao in Fujian Province, Southeast China, were 51.35, 41.19, and 28.06 mg/g, respectively. Yunnan Province is located in Southwest China and is influenced by a low latitude plateau, mountainous terrain, a monsoon climate with strong ultraviolet light, long total sun exposure time, relatively stable average temperature, and distinct, alternating dry and rainy seasons (Zhang et al., 2013). The huge differences between locations in temperature, illumination, water, and soil conditions might be responsible for the large differences found in the alkaloid contents from the same cultivars and leaf positions.
In most previous studies, baked tobacco leaves were used (Shi et al., 2001; Lin et al., 2002; Lian et al., 2008). However, each step in the processing of tobacco leaves that affects plant metabolism may result in changes in the alkaloid content to a certain degree (Djordjevic and Doran, 2009). Djordjevic et al. (1989) found that the contents of nicotine, nornicotine, anatabine, and anabasine increased in NC95 from harvesting through curing. By using fresh leaves, we were able to exclude the effects of various curing methods on alkaloid content.
4.2. Genetic variation in alkaloid accumulation
Nicotine is synthesized in the roots of tobacco plants and is transported via the xylem to the shoots (Hibi et al., 1994; Shi et al., 2006). Besides biosynthesis, transportation, phytohormones, and other unknown regulatory factors also influence the levels of alkaloids in tobacco leaves. All these factors vary between cultivars and give rise to genotype variation. Therefore, analysis of genetic effects on alkaloid accumulation in tobacco leaves is needed. In the present study, total alkaloids levels were significantly affected by a cultivar effect, and the different individual alkaloids varied in their response to the cultivar effect (Table 4). The highest variance ratio for the cultivar effect (V C/V P, ratio of cultivar variance to phenotypic variance) was for one of the carcinogenic alkaloids, myosmine, which had a concentration as high as 85.0% and for which the variance ratio was more than three times that of anabasine (V C/V P=0.249). Such differences indicate a high potential for improvement of the composition and content of alkaloids.
The analysis of genetic effects is widely used to improve the quality of many crops. Shi et al. (1996) investigated genetic effects in indica rice, and reported that nutrient quality traits were controlled by cytoplasmic and maternal effects, and by direct seed effects. Thus, they proposed that selection could be applied to these traits in early generations. Significant negative correlations between fibre color and lint percentage, fibre length, and elongation, indicated that the lint percentage and fibre qualities of brown cotton could be improved by taking advantage of environmental variation (Feng et al., 2011). Therefore, it is important to evaluate genetic effects on alkaloid accumulation in tobacco leaves. In our study, the alkaloid profiles varied among different cultivars and leaf positions of plants grown in Yunnan Province. Using the alkaloid content data, the genetic effects of alkaloid accumulation were analyzed for the given location.
The results showed that the contents of nornicotine and myosmine were significantly and positively correlated (Table 3), and that they clustered separately from those of other alkaloids (Fig. 2). Previous studies indicating that nornicotine is converted by nicotine in the leaf, were confirmed by 2′-14C-labeled alkaloid feeding experiments (Zador and Jones, 1986). Siminszky et al. (2005) found that the demethylation of nicotine to nornicotine was catalyzed by the cytochrome P450 enzyme CYP82E4. Nornicotine was also shown by an analytical method to be the possible precursor of myosmine (Leete and Chedekel, 1974; Zwickenpflug et al., 1998). These observations might explain why the phenotypic and genotypic correlations for nornicotine and myosmine were so different from those of other alkaloids.
Because of their potential carcinogenic properties, the accumulation of nornicotine and myosmine in tobacco plants is undesirable. Previous studies have shown that the nornicotine content can be reduced by molecular biotechnology (Gavilano et al., 2006) and hybrid technology (Shi et al., 2007). However, these methods were focused mainly on burley converter plants, not burley non-converters or other kinds of tobacco, such as flue-cured tobacco. In addition, there was no associated decline in the contents of other alkaloids. In our study, highly positive correlations between nornicotine and myosmine indicated that they could be reduced simultaneously. Our genetic analysis implied that some particular cultivars (for instance, Cuibiyihao and NC297) could be selected to breed new cultivars that contain a normal content of nicotine, but low contents of nornicotine and myosmine. Therefore, our results suggest that there is good potential to manipulate alkaloid content via conventional breeding.
5. Conclusions
In summary, six alkaloids were separated and identified via GC-MS, and significant differences were noted among different leaf positions and cultivars in both total alkaloid content and individual alkaloid profiles. Analysis of genetic correlations showed that the correlation between nornicotine and myosmine content was extremely significant and positive. Nornicotine and myosmine contents were also separated from those of other alkaloids by t-test clustering. In summary, it is feasible to reduce the content of harmful alkaloids in specific cultivars by conventional breeding based on the genetic analysis of parental lines.
Acknowledgments
The authors thank Xiao-yu WANG and Ya-qiong QIN from Zhengzhou Tobacco Research Institute (Zhengzhou, China) for their helpful suggestions on GC-MS analysis, and Jian-sheng WANG from Zhejiang Academy of Agricultural Sciences (Hangzhou, China) for great technical help in this research.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31000137), the China National Tobacco Company and Zhengzhou Tobacco Research Institute Science and Technology Project (No. 122009CZ0410), and the PhD Programs Foundation of Ministry of Education of China (No. 2012M510149)
Compliance with ethics guidelines: Bo SUN, Fen ZHANG, Guo-jun ZHOU, Guo-hai CHU, Fang-fang HUANG, Qiao-mei WANG, Li-feng JIN, Fu-cheng LIN, and Jun YANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Brogan AP, Dickerson TJ, Boldt GE, Janda KD. Altered retinoid homeostasis catalyzed by a nicotine metabolite: implications in macular degeneration and normal development. PNAS. 2005;102(30):10433–10438. doi: 10.1073/pnas.0504721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai JB, Liu BZ, Lin P, Su QD. Fast analysis of nicotine related alkaloids in tobacco and cigarette smoke by megabore capillary gas chromatography. J Chromatogr A. 2003;1017(1):187–193. doi: 10.1016/j.chroma.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Cai JL, Zhao G, Yin YQ, et al. Tobacco and Tobacco Products—Determination of Nicotine, Nornicotine, Anatabine, Myosmine and Anabasine—GC-MSD method. Beijing, China: China Standard Press; 2011. pp. 1–12. (in Chinese) [Google Scholar]
- 4.Dickerson TJ, Janda KD. A previously undescribed chemical link between smoking and metabolic disease. PNAS. 2002;99(23):15084–15088. doi: 10.1073/pnas.222561699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djordjevic MV, Doran KA. Nicotine Content and Delivery across Tobacco Products. Nicotine Psychopharmacology; 2009. pp. 61–82. [DOI] [PubMed] [Google Scholar]
- 6.Djordjevic MV, Gay SL, Bush LP, Chaplin JF. Tobacco-specific nitrosamine accumulation and distribution in flue-cured tobacco alkaloid isolines. J Agric Food Chem. 1989;37:752–756. doi: 10.1021/jf00087a040. [DOI] [Google Scholar]
- 7.Feng HJ, Sun JL, Wang J, Jia YH, Zhang XY, Pang BY, Sun J, Du XM. Genetic effects and heterosis of the fibre colour and quality of brown cotton (Gossypium hirsutum) Plant Breed. 2011;130(4):450–456. doi: 10.1111/j.1439-0523.2010.01842.x. [DOI] [Google Scholar]
- 8.Gavilano LB, Coleman NP, Burnley LE, Bowman ML, Kalengamaliro NE, Hayes A, Bush L, Siminszky B. Genetic engineering of Nicotiana tabacum for reduced nornicotine content. J Agric Food Chem. 2006;54(24):9071–9078. doi: 10.1021/jf0610458. [DOI] [PubMed] [Google Scholar]
- 9.Gavilano LB, Coleman NP, Bowen SW, Siminszky B. Functional analysis of nicotine demethylase genes reveals insights into the evolution of modern tobacco. J Biol Chem. 2007;282(1):249–256. doi: 10.1074/jbc.M609512200. [DOI] [PubMed] [Google Scholar]
- 10.Häkkinen ST, Tilleman S, Šwiątek A, de Sutter V, Rischer H, Vanhoutte I, van Onckelen H, Hilson P, Inzé D, Oksman-Caldentey K, et al. Functional characterization of genes involved in pyridine alkaloid biosynthesis in tobacco. Phytochemistry. 2007;68(22-24):2773–2785. doi: 10.1016/j.phytochem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Yamada Y. Alkaloid biogenesis: molecular aspects. Annu Rev Plant Physiol Plant Mol Biol. 1994;45(1):257–285. [Google Scholar]
- 12.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 13.Hibi N, Higashiguchi S, Hashimoto T, Yamada Y. Gene expression in tobacco low-nicotine mutants. Plant Cell. 1994;6(5):723–735. doi: 10.1105/tpc.6.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leete E, Chedekel MR. Metabolism of nicotine in Nicotiana glauca . Phytochemistry. 1974;13(9):1853–1859. doi: 10.1016/0031-9422(74)85101-0. [DOI] [Google Scholar]
- 15.Lewis RS. Identification of germplasm of possible value for confronting an unfavorable inverse genetic correlation in tobacco. Crop Sci. 2006;46(4):1764–1771. doi: 10.2135/cropsci2005.12-0519. [DOI] [Google Scholar]
- 16.Lian YY, Wang YB, Qiu J, Zhang ZF, Cao JM, Ning Y, Yue S, Niu P. Analysis on major alkaloids contents and constituent proportions of flue-cured tobacco from different tobacco production regions. Chin Tobacco Sci. 2008;29(4):6–9. (in Chinese) [Google Scholar]
- 17.Lin GH, Zhou JH, Fan QF, Yang HQ, Cao RX. Effects of topping technology on leaf yield, quality and alkaloid content of flue-cured tobacco. Chin Tobacco Sci. 2002;23(4):8–12. (in Chinese) [Google Scholar]
- 18.Miller RG. The jackknife: a review. Biometrika. 1974;61(1):1–15. doi: 10.1093/biomet/61.1.1. [DOI] [Google Scholar]
- 19.Scherling C, Ulrich K, Ewald D, Wechwerth W. A metabolic signature of the beneficial interaction of the endophyte Paenibacillus sp. isolate and in vitro-grown poplar plants revealed by metabolomics. Mol Plant Microbe Interact. 2009;22(8):1032–1037. doi: 10.1094/MPMI-22-8-1032. [DOI] [PubMed] [Google Scholar]
- 20.Shi CH, Xue JM, Yu YG, Yang XE, Zhu J. Analysis of genetic effects on nutrient quality traits in indica rice. Theor Appl Genet. 1996;92(8):1099–1102. doi: 10.1007/BF00224055. [DOI] [PubMed] [Google Scholar]
- 21.Shi HZ, Huang YJ, Liu GS, Zhao MQ, Bush LP. Alkaloid content and proportion in Chinese tobacco and cigarettes. Acta Tabacco Sin. 2001;7(2):8–12. doi: 10.3321/j.issn:1004-5708.2001.02.002. (in Chinese) [DOI] [Google Scholar]
- 22.Shi HZ, Li JP, Li ZP, Bush LP, Wang CJ, Liu GS. Study on decreasing nicotine conversion in Chinese burley hybrid through genetic improvement. Sci Agric Sin. 2007;40(1):153–160. (in Chinese) [Google Scholar]
- 23.Shi QM, Li CJ, Zhang FS. Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. J Exp Bot. 2006;57(11):2899–2907. doi: 10.1093/jxb/erl051. [DOI] [PubMed] [Google Scholar]
- 24.Shoji T, Kajikawa M, Hashimoto T. Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell. 2010;22(10):3390–3409. doi: 10.1105/tpc.110.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siminszky B, Gavilano L, Bowen SW, Dewey RE. Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. PNAS. 2005;102(41):14919–14924. doi: 10.1073/pnas.0506581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilp J, Zwickenpflug W, Richter E. Nitrosation of dietary myosmine as risk factor of human cancer. Food Chem Toxicol. 2002;40(8):1223–1228. doi: 10.1016/S0278-6915(02)00039-X. [DOI] [PubMed] [Google Scholar]
- 27.Yan XF, Chen SX. Regulation of plant glucosinolate metabolism. Planta. 2007;226(6):1343–1352. doi: 10.1007/s.00425-007-0627-7. [DOI] [PubMed] [Google Scholar]
- 28.Zador E, Jones D. The biosynthesis of a novel nicotine alkaloid in the trichomes of Nicotiana stocktonii . Plant Physiol. 1986;82(2):479–484. doi: 10.1104/pp.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Wang X, Guo J, Xia Q, Zhao G, Zhou H, Xie F. Metabolic profiling of chinese tobacco leaf of different geographical origins by GC-MS. J Agric Food Chem. 2013;61(11):2597–2605. doi: 10.1021/jf400428t. [DOI] [PubMed] [Google Scholar]
- 30.Zhao JH, Zhang JS, Wang Y, Wang RG, Wu C, Fan LJ, Ren XL. DNA methylation polymorphism in flue-cured tobacco and candidate markers for tobacco mosaic virus resistance. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(11):935–942. doi: 10.1631/jzus.B1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J. Analytic methods for seed models with genotype×environment interactions. Acta Genet Sin. 1996;23(1):56–58. (in Chinese) [PubMed] [Google Scholar]
- 32.Zhu J, Weir BS. Diallel analysis for sex-linked and maternal effects. Theor Appl Genet. 1996;92(1):1–9. doi: 10.11007/BF00222944. [DOI] [PubMed] [Google Scholar]
- 33.Zwickenpflug W, Meger M, Richter E. Occurrence of the tobacco alkaloid myosmine in nuts and nut products of Arachus hypogaea and Corylus avellana . J Agric Food Chem. 1998;46(7):2703–2706. doi: 10.1021/jf9801419. [DOI] [Google Scholar]



