Abstract
Objectives
This study sought to perform a systematic review and meta-analysis to understand the role of stress cardiac magnetic resonance imaging (CMR) in assessing cardiovascular prognosis in patients with known or suspected coronary artery disease (CAD).
Background
Although stress CMR is excellent for the diagnosis of obstructive CAD, the prognostic value of stress CMR has been less well described.
Methods
PubMed, Cochrane CENTRAL, and metaRegister of Controlled Trials were searched for stress CMR studies with >6 months of prognostic data. Primary endpoints were cardiovascular death, myocardial infarction (MI), and a composite outcome of cardiovascular death or MI during follow-up. Summary effect estimates were generated with random-effects modeling, and annualized event rates were assessed.
Results
Nineteen studies (14 vasodilator, 4 dobutamine, and 1 that used both) involved a total of 11,636 patients with a mean follow-up of 32 months. Patients had a mean age of 63 ± 12 years, 63% were male, and 26% had previous MI; mean left ventricular ejection fraction was 61 ± 12%; and late gadolinium enhancement was present in 29% and ischemia in 32%. Patients with ischemia had a higher incidence of MI (odds ratio [OR]: 7.7; p < 0.0001), cardiovascular death (OR: 7.0; p < 0.0001), and the combined endpoint (OR: 6.5; p < 0.0001) compared with those with a negative study. The combined outcome annualized events rates were 4.9% for a positive versus 0.8% for a negative stress CMR (p < 0.0001), 2.8% versus 0.3% for cardiovascular death (p < 0.0001), and 2.6% versus 0.4% for MI (p < 0.0005). The presence of late gadolinium enhancement was also significantly associated with a worse prognosis.
Conclusions
A negative stress CMR study is associated with very low risk of cardiovascular death and MI. Stress CMR has excellent prognostic characteristics and may help guide risk stratification of patients with known or suspected CAD.
Keywords: late gadolinium enhancement, myocardial perfusion, prognosis, stress cardiac MRI
Stress cardiac magnetic resonance imaging (CMR), either with vasodilator or dobutamine stress, has been shown to have excellent diagnostic accuracy for detection of significant coronary artery disease (CAD) (1–4). In addition, CMR provides valuable clinical data, including details on left ventricular function, the presence of late gadolinium enhancement (LGE), and whether there is structural or valvular heart disease. As a result, stress CMR is increasingly being used to assess chest pain in patients with known or suspected CAD. In addition, stress CMR may have a role after ST-segment elevation myocardial infarction (MI) to assess for residual ischemia due to coronary stenoses in noninfarct-related arteries (5,6). Furthermore, stress CMR can be used in patients with dilated cardiomyopathy to assess for ischemia and myocardial scar burden with LGE (7,8). Given the increasing health care costs associated with cardiovascular imaging, it is critical to validate the prognostic utility of stress CMR (9,10).
Over the past several years, multiple studies have been published regarding stress CMR assessment of prognosis. However, many of these studies are limited because they are small and single centered. Prognostic validation of stress CMR is critical because a negative stress CMR can be reassuring that the patient has a very low risk for major adverse cardiovascular events (MACE). Alternatively, patients with stress-induced wall motion abnormalities, abnormal perfusion, and/or LGE are at higher risk of MACE. In the current environment of escalating medical costs, the prognostic performance of stress CMR may also help justify its use compared with more commonly used stress modalities such as stress echocardiography and stress nuclear perfusion imaging. Given the multiple small and single-centered studies, we performed a systematic review and meta-analysis of studies reporting prognostic data from patients undergoing stress CMR to assess for myocardial ischemia in those with known or suspected CAD.
Methods
Eligibility criteria
We included any of the following: 1) study assessing for myocardial ischemia with stress CMR; 2) with ≥6 months of prognostic follow-up data, including cardiac death and/or MI; and 3) excluding populations composed of patients with cardiomyopathy or acute MI within the last 14 days.
Search strategy
To identify eligible studies for inclusion in the current systematic review and meta-analysis, 2 independent reviewers (M.J.L. and C.M.M.) systematically searched (October 2012) Cochrane CENTRAL, meta-Register of Controlled Trials, and PubMed for studies assessing prognosis in patients with known or suspected CAD after undergoing stress CMR. Key words used were “prognosis” OR “outcome” AND “stress magnetic resonance imaging” or “dobutamine magnetic resonance imaging” or “adenosine magnetic resonance imaging.” In addition, we consulted experts, reviewed citations from eligible studies, and explored “see related articles” for key publications in PubMed. The search was limited to studies published in peer-reviewed journals and thus excludes trials presented in abstract form only. We restricted the review to studies that enrolled adults only. No language restriction was applied. The current systematic review and meta-analysis was performed in accordance with guidelines of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses ) groups (11,12).
Study selection
Two investigators (M.J.L. and C.M.M.) independently and in duplicate scanned all abstracts and obtained full-text reports of articles that indicated or suggested eligibility. After obtaining full reports, the same reviewers independently assessed eligibility from the full-text articles, with divergences resolved after consensus. Study quality was evaluated by the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (13), in which the quality of the selected trials was determined on the basis of selection of the study groups (0 to 4 points), comparability of the study groups (0 to 2 points), and ascertainment of the outcome of interest (0 to 3 points).
Data collection
Data abstraction and study appraisal were performed by the same 2 aforementioned investigators. Clinical outcomes of interest were cardiovascular death, MI, or the composite outcome of cardiovascular death or MI during follow-up. Clinical outcomes data were directly abstracted when reported. Unadjusted hazard ratios were used to determine the number of events if not provided for each group, and annualized event rates (AERs) for studies were calculated by dividing the number of events by the follow-up duration.
Data analysis
Dichotomous variables are reported as proportions (percentages); continuous variables are reported as mean ± SD or median (range). Binary outcomes from individual studies were combined with a random-effects model, leading to computations of odds ratios (ORs) with 95% confidence intervals (CIs). I2 was calculated as a measure of statistical heterogeneity, with I2 values of 25%, 50%, and 75% representing mild, moderate, and severe inconsistency, respectively. Small study or publication bias was explored with funnel plots, Egger’s test (14), and Peters’ test (15). Finally, meta-regression and sensitivity analyses (including exclusion of 1 study at a time) were conducted to explore heterogeneity.
Statistical analysis was performed by using Review Manager (RevMan) 5 version 5.1.7 freeware package (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and NCSS 2007 (NCSS LLC, Kaysville, Utah), with statistical significance for hypothesis testing set at the 0.05 two-tailed level. AERs were compared by using weighted comparison of means in which we provide SD and SE of the difference of the means to provide significance by the Student’s t test (16).
Results
Results of the literature search
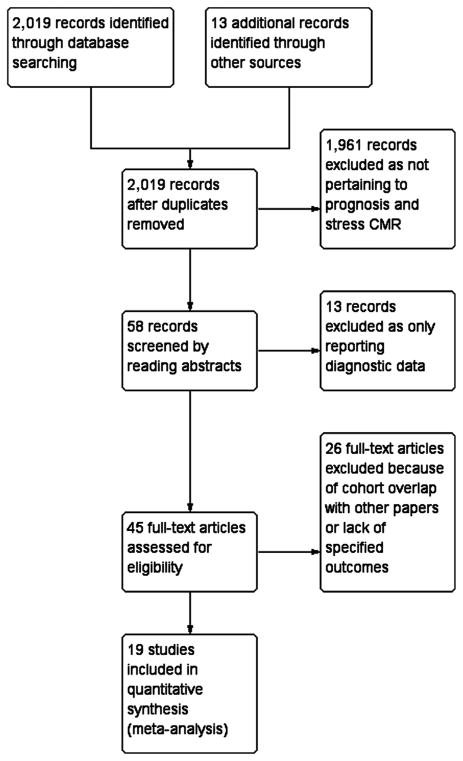
Our literature search identified 2,019 relevant abstracts of full-text articles; of these, 58 unique articles were abstracted for review. Forty-five of these articles warranted full-text review. Twenty-six articles (5–9,17–37) were excluded for various reasons, including cohort overlap with other articles or lack of our prespecified outcomes, leaving 19 articles for detailed study (38–56). The details of our flow diagram can be found in Figure 1, and study characteristics are presented in Table 1. Only 1 study included patients undergoing stress CMR at 3.0-T and 1.5-T; the rest of the studies were performed at 1.5-T.
Figure 1. Flow Diagram of the Review Process.
CMR = cardiac magnetic resonance imaging.
Table 1.
Study Characteristics
| Stress CMR Studies First Author (Ref. #) | Year Published | No. of Patients Included | Follow-Up (Months) | Stress Agent | Study Design | Quality Assessment Score | Field Strength (T) | Definition of Positive Stress CMR | Population |
|---|---|---|---|---|---|---|---|---|---|
| Bertaso et al. (38) | 2012 | 362 | 22 | Adenosine | Retrospective, single-center | 3/2/3 | 1.5 | Reversible perfusion defect | Consecutive patients referred for stress CMR |
| Bingham et al. (39) | 2011 | 908 | 31 ± 14 | Adenosine | Prospective, single-center | 4/2/3 | 1.5 | Stress perfusion defect. No resting perfusion performed | Consecutive patients referred for stress CMR |
| Bodi et al. (40) | 2012 | 1,722 | 13 ± 11 | Dipyridamole | Prospective, multicenter | 4/2/3 | 1.5 | Reversible perfusion defect in at least one segment | Patients with chest pain or to assess ischemia in intermediate coronary stenoses |
| Buckert et al. (41) | 2013 | 1,152 | 50 ± 25 | Adenosine | Prospective, single-center | 4/2/3 | 1.5 | Reversible perfusion defect extending beyond any area of LGE | Patients with stable angina referred for stress CMR |
| Coelho-Filho et al. (42) | 2011 | 405 | 30 | 92% Adenosine 8% Dipyridamole |
Prospective, single-center | 4/2/3 | 1.5 or 3.0 | Stress perfusion defect without matching segmental LGE | Consecutive patients referred for stress CMR |
| Doesch et al. (43) | 2009 | 81 | 30 ± 8 | Adenosine | Prospective, single-center | 4/2/3 | 1.5 | Reversible perfusion defect extending beyond any area of LGE | Assessment of ischemia in patients with intermediate coronary stenoses |
| Ingkanisorn et al. (44) | 2006 | 135 | 16 | Adenosine | Prospective, single-center | 4/2/3 | 1.5 | Reversible perfusion defect in >1 segment | Patients with chest pain and negative troponins referred from the ED |
| Jahnke et al. (45) | 2011 | 679 | 57 ± 26 | Adenosine and dobutamine | Retrospective, single-center | 3/2/3 | 1.5 | Reversible perfusion defect | Consecutive patients to assess chest pain or dyspnea for combined stress adenosine or dobutamine CMR |
| Krittayaphong et al. (46) | 2011 | 1,232 | 35 ± 16 | Adenosine | Retrospective, single-center | 3/2/3 | 1.5 | Reversible perfusion defect extending beyond any area of LGE | All patients age >18 years referred for stress CMR |
| Lerakis et al. (47) | 2009 | 103 | 9 (5–15) | Adenosine | Retrospective, single-center | 3/1/3 | 1.5 | Perfusion defect at rest or stress, resting wall motion abnormality, or LGE | Patients with low-risk chest pain referred from the ED |
| Lo et al. (48) | 2011 | 203 | 38 ± 19 | Adenosine | Retrospective, single-center | 3/2/3 | 1.5 | Reversible perfusion defect | Patients with known or suspected CAD to assess for ischemia |
| Lubbers et al. (49) | 2012 | 125 | 22 (11 to 43) | Adenosine | Prospective, single-center | 3/2/3 | 1.5 | Perfusion defect in >2 segments (or 1 segment at apex) | Consecutive patients referred from outpatient cardiology clinic to assess for ischemia |
| Pilz et al. (50) | 2008 | 218 | 12 | Adenosine | Prospective, single-center | 3/2/3 | 1.5 | Only negative tests included | Patients with suspected CAD but adenosine CMR negative for ischemia or LGE |
| Steel et al. (51) | 2009 | 254 | 17 (8–56) | 89% Adenosine 11% Dipyridamole |
Retrospective, single-center | 3/2/3 | 1.5 | Reversible perfusion defect in at least 1 segment. | Patients with chest pain referred for stress CMR |
| Vogel-Claussen et al. (52) | 2009 | 27 | 14 ± 5 | Adenosine | Prospective, single-center | 4/1/3 | 1.5 | Reversible perfusion defect | Patients with low-risk chest pain referred from the ED |
| Charoenpanichkit et al. (53) | 2010 | 353 | 72 ± 24 | Dobutamine | Prospective, single-center | 4/2/3 | 1.5 | New or worsening stress-induced wall motion abnormality in >1 segment | Consecutive patients referred for stress CMR |
| Gebker et al. (54) | 2011 | 1,167 | 25 ± 10 | Dobutamine | Prospective, single-center | 4/2/3 | 1.5 | New or worsening stress-induced wall motion abnormality in >1 segment, or a biphasic response | Consecutive patients to assess chest pain or dyspnea |
| Kelle et al. (55) | 2011 | 1,017 | 44 ± 24 | Dobutamine | Retrospective, single-center | 3/2/3 | 1.5 | New or worsening stress-induced wall motion abnormality in >1 segment or a biphasic response | Patients with known or suspected CAD to assess for ischemia |
| Korosoglou et al. (56) | 2010 | 1,493 | 24 ± 12 | Dobutamine | Retrospective, single-center | 3/2/3 | 1.5 | New or worsening stress induced wall motion abnormality in >1 segment | Consecutive patients with known or suspected CAD to assess for ischemia |
CAD = coronary artery disease; CMR = cardiac magnetic resonance imaging; ED = emergency department; LGE = late gadolinium enhancement.
The 19 studies with a weighted mean follow-up of 32 months (median 25 months; range 9 to 72 months) included a total of 11,636 patients with known or suspected CAD undergoing stress CMR (14 vasodilator stress [38–44,46–52]; 4 dobutamine stress [53–56]; and 1 using both vasodilator and dobutamine stress [45]) (median 362 patients; average 612 patients [range: 27 to 1,722]). Patients had a weighted mean age of 63 ± 12 years, and 63% of patients were male. The population also had a typical distribution of cardiovascular risk factors: 42% with CAD, 26% with previous MI, 66% with hypertension, 60% with hyperlipidemia, 24% with diabetes mellitus, and 25% with a history of smoking. With regard to stress CMR, the weighted mean left ventricular ejection fraction was 61 ± 12%, LGE was present in 29% of patients when reported, and 32% of patients had a positive stress CMR. Baseline patient characteristics are demonstrated in Table 2.
Table 2.
Baseline Patient Characteristics
| Stress CMR Studies First Author (Ref. #) | Age (yrs) | Male (%) | Known CAD (%) | Prior Revascularization (%) | Prior PCI (%) | Prior CABG (%) | Prior MI (%) | Hypertension (%) | Hyperlipidemia (%) | Diabetes Mellitus (%) | Smoking history (%) | LVEF (%) | LGE Present (%) | Positive Stress (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bertaso et al. (38) | 62 ± 12 | 58 | 43 | NR | NR | 0 | NR | 58 | 60 | 24 | 24 | 67 ± 12 | 21 | 25 |
| Bingham et al. (39) | 65 | 59 | 49 | NR | 33 | 15 | 35 | 64 | NR | 25 | 6 | 63 | 38 | 33 |
| Bodi et al. (40) | 64 ± 11 | 62 | NR | NR | 14 | 7 | 23 | 62 | 55 | 28 | 22 | 62 ± 13 | 28 | 41 |
| Buckert et al. (41) | 62 ± 12 | 72 | NR | NR | 41 | 12 | 36 | 63 | 57 | 21 | 24 | 64 ± 13 | 41 | 27 |
| Coelho-Filho et al. (42) | 57 ± 14 | 59 | NR | NR | 16 | 8 | 20 | 56 | 57 | 22 | 15 | 57 ± 13 | 30 | 31 |
| Doesch et al. (43) | 64 ± 10 | 83 | 100 | 35 | 27 | 7 | 30 | 72 | 58 | 28 | 40 | 56 ± 10 | NR | 56 |
| Ingkanisorn et al. (44) | 56 ± 14 | 56 | 17 | 12 | NR | NR | 7 | 42 | 53 | 10 | 30 | 65 ± 11 | 10 | 21 |
| Jahnke et al. (45) | 61 ± 10 | 69 | 54 | 48 | NR | NR | 24 | 78 | 74 | 23 | 35 | 57 ± 8 | NR | 48/41 |
| Krittayaphong et al. (46) | 65 ± 11 | 48 | 12 | 11 | NR | NR | NR | 63 | 62 | 35 | 15 | 64 ± 17 | 27 | 34 |
| Lerakis et al. (47) | 57 ± 12 | 37 | 13 | NR | NR | NR | NR | 64 | 39 | 29 | 19 | NR | 5 | 10 |
| Lo et al. (48) | 62 ± 12 | 59 | 16 | NR | 12 | 3 | 10 | 70 | 46 | 30 | 29 | 65 ± 13 | 13 | 21 |
| Lubbers et al. (49) | 61 ± 11 | 54 | NR | NR | NR | NR | 0 | 46 | 86 | 15 | 63 | NR | NR | 10 |
| Pilz et al. (50) | 63 ± 13 | 56 | 0 | 0 | 0 | 0 | 0 | 68 | 37 | 9 | 35 | 61 ± 9 | 0 | 0 |
| Steel et al. (51) | 58 ± 13 | 59 | NR | 26 | 18 | 11 | 22 | 57 | 61 | 25 | 11 | 58 ± 11 | 28 | 29 |
| Vogel-Claussen et al. (52) | 56 ± 13 | 56 | 19 | 19 | 4 | 15 | NR | 78 | 78 | 33 | 67 | NR | 15 | 19 |
| Charoenpanichkit et al. (53) | 64 ± 12 | 54 | NR | NR | NR | NR | 36 | 69 | 55 | 36 | 42 | 55 ± 13 | NR | 31 |
| Gebker et al. (54) | 63 ± 10 | 67 | 48 | NR | 40 | 18 | 31 | 74 | 65 | 23 | 31 | 57 ± 7 | NR | 40 |
| Kelle et al. (55) | 61 ± 11 | 68 | 52 | 43 | NR | NR | 25 | 73 | 70 | 17 | 44 | 57 ± 10 | NR | 30 |
| Korosoglou et al. (56) | 65 ± 13 | 74 | 55 | NR | 40 | 12 | NR | 71 | 53 | 19 | 18 | 60 ± 12 | NR | 20 |
| Median | 62 | 59 | 43 | 23 | 18 | 10 | 24 | 64 | 58 | 24 | 29 | 61 | 24 | 29 |
| Weighted mean | 63 ± 12 | 63 | 42 | 28 | 29 | 11 | 26 | 66 | 60 | 24 | 25 | 61 ± 12 | 29 | 32 |
CABG = coronary artery bypass graft surgery; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NR = not reported; PCI = percutaneous coronary intervention; other abbreviations as in Table 1.
Evidence of ischemia in stress CMR and cardiovascular outcome
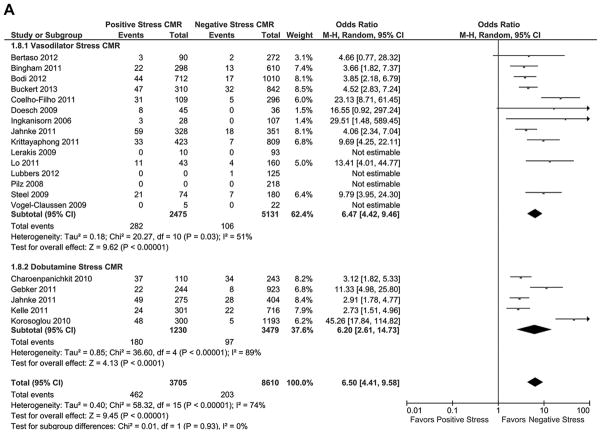
Of the 19 studies reporting the combined outcome of cardiovascular death and MI during follow-up (Fig. 2A), patients with a positive stress CMR had a greater incidence of the combined outcome compared with patients who had a negative stress CMR (OR: 6.5 [95% CI: 4.41 to 9.58]; p < 0.00001, I2 = 74%). There was no significant difference between the prognostic characteristics of vasodilator and dobutamine stress CMR. Patients with a positive stress CMR had a significantly greater AER of the combined outcome (Table 3, Fig. 3) than patients with a negative stress CMR (4.9 ± 3.1% vs. 0.8 ± 0.7%, respectively; T score = 5.69, p < 0.000002). There was no significant difference in the combined outcome AERs for patients undergoing vasodilator stress CMR versus dobutamine stress CMR in patients with a positive stress CMR (4.9 ± 3.5% vs. 4.7 ± 2.4%, respectively; T score = 0.15, p = 0.89) or in patients with a negative stress CMR (0.9 ± 0.8% vs 0.7 ± 0.7%; T score = 0.58, p = 0.57).
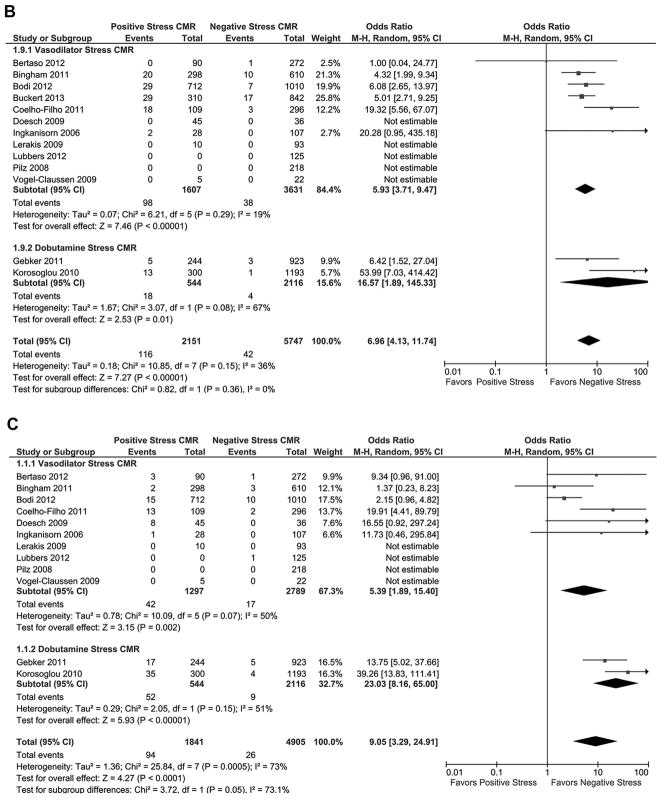
Figure 2. Individual and Pooled Risk of Cardiovascular Outcomes for Stress CMR.
Forest plots comparing clinical outcomes of patients with known or suspected coronary artery disease (CAD) with positive stress cardiac magnetic resonance imaging (CMR) and negative stress CMR. Outcomes included (A) combined cardiovascular outcomes including cardiovascular death and nonfatal myocardial infarction (MI), (B) cardiovascular death, and (C) nonfatal MI. CI = confidence interval.
Table 3.
AERs of Studies for Combined Outcome of Cardiovascular Death and MI, Cardiovascular Death, and MI Comparing Patients With Positive Stress CMR and Patients With a Negative Stress CMR
| Studies First Author (Ref. #) | Combined Event AER |
Cardiovascular Death AER
|
Nonfatal MI AER |
|||
|---|---|---|---|---|---|---|
| Positive Stress CMR | Negative Stress CMR | Positive Stress CMR | Negative Stress CMR | Positive Stress CMR | Negative Stress CMR | |
| Vasodilator stress CMR studies
| ||||||
| Bertaso et al. (38) | 1.8 | 0.4 | 0 | 0.2 | 1.8 | 0.2 |
| Bingham et al. (39) | 2.9 | 0.8 | 2.6 | 0.6 | 0.3 | 0.2 |
| Bodi et al. (40) | 5.7 | 1.6 | 3.8 | 0.6 | 1.9 | 0.9 |
| Buckert et al. (41) | 3.6 | 0.9 | 2.2 | 0.5 | 1.4 | 0.4 |
| Coelho-Filho et al. (42) | 12.2 | 0.7 | 6.7 | 0.4 | 5.5 | 0.3 |
| Doesch et al. (43) | 7.1 | 0 | 0 | 0 | 7.1 | 0 |
| Ingkanisorn et al. (44) | 8.0 | 0 | 5.4 | 0 | 2.7 | 0 |
| Jahnke et al. (45) | 3.8 | 1.1 | NR | NR | NR | NR |
| Krittayaphong et al. (46) | 2.7 | 0.3 | NR | NR | NR | NR |
| Lerakis et al. (47) | 0 | 0 | 0 | 0 | 0 | 0 |
| Lo et al. (48) | 8.1 | 0.8 | NR | NR | NR | NR |
| Lubbers et al. (49) | NR | 0.4 | NR | 0 | NR | 0.4 |
| Pilz et al. (50) | NR | 0 | NR | 0 | NR | 0 |
| Steel et al. (51) | 19.0 | 4.2 | NR | NR | NR | NR |
| Vogel-Claussen et al. (52) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Dobutamine stress CMR studies
| ||||||
| Charoenpanichkit et al. (53) | 5.6 | 2.3 | NR | NR | NR | NR |
| Gebker et al. (54) | 4.3 | 0.4 | 1.0 | 0.2 | 3.3 | 0 |
| Jahnke et al. (45) | 3.8 | 1.5 | NR | NR | NR | NR |
| Kelle et al. (55) | 2.2 | 0.8 | NR | NR | NR | NR |
| Korosoglou et al. (56) | 8.0 | 0.2 | 2.2 | 0.04 | 5.8 | 0.2 |
Values are %.
AER = annualized event rate; CMR = cardiac magnetic resonance imaging; NR = not reported; MI = myocardial infarction.
Figure 3. AERs of Cardiovascular Outcomes for Stress CMR.
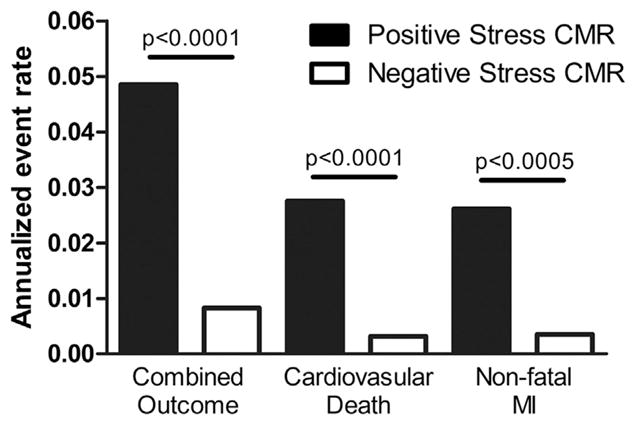
Weighted mean annualized event rates (AERs) for combined cardiovascular outcome of cardiovascular death and nonfatal MI, cardiovascular death, and non-fatal MI comparing patients with positive stress CMR (solid bars) and patients with a negative stress CMR (open bars). Abbreviations as in Figure 2.
Of the 13 studies reporting cardiovascular death during follow-up (Fig. 2B), patients with a positive stress CMR had a significantly greater risk of cardiovascular death during follow-up compared with patients who had a negative stress CMR (OR: 6.96 [95% CI: 4.13 to 11.74]; p < 0.00001, I2 = 36%). When comparing the cardiovascular death AERs (Table 3, Fig. 3), patients with a positive stress CMR had significantly greater risk of cardiovascular death during follow-up than patients with a negative stress CMR (2.8 ± 1.6% vs. 0.3 ± 0.3%, respectively; T score = 5.58, p < 0.00002).
Of the 13 studies reporting nonfatal MI during follow-up (Fig. 2C), patients with a positive stress CMR had a significantly higher incidence of MI during follow-up compared with patients who had a negative stress CMR (OR: 7.73 [95% CI: 3.28 to 18.23]; p < 0.00001, I2 = 73%). When comparing the MI AERs (Table 3, Fig. 3), patients with a positive stress CMR had a significantly higher incidence of MI during follow-up than patients with a negative stress CMR (2.6 ± 2.0% vs. 0.4 ± 0.3%, respectively; T score = 4.1, p < 0.0005).
Meta-regression analysis was performed to determine whether any clinical variables were associated with the combined cardiovascular outcome, cardiovascular death, or nonfatal MI. All variables from Table 2 were included in the meta-regression. Meta-regression analysis demonstrated that only previous MI (correlation −0.64; R2 = 0.41; p < 0.04) was associated with an increased incidence of combined cardiovascular outcomes. Among the studies reporting LGE, there was a significant correlation between previous MI and LGE (correlation 0.98; R2 = 0.96; p < 0.0001).
LGE during stress CMR and cardiovascular outcomes
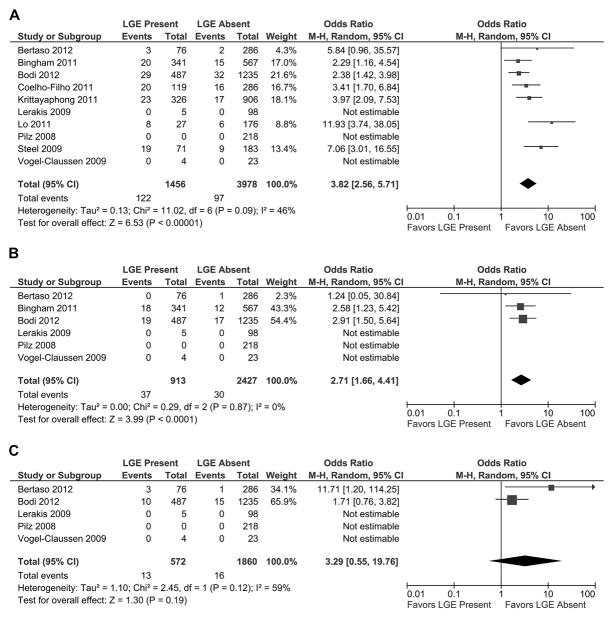
Of the 10 studies reporting the combined outcome of cardiovascular death and MI during follow-up and presence of LGE (Fig. 4A), patients with evidence of LGE had a worse outcome than patients without LGE (OR: 3.82 [95% CI: 2.56 to 5.71]; p < 0.00001, I2 = 46%). When comparing the combined outcome AERs (Table 4, Fig. 5), patients with LGE had significantly worse outcomes than patients without LGE (4.6 ± 4.0% vs. 1.4 ± 1.0%, respectively; T score = 2.45, p < 0.03). Of the 6 studies reporting cardiovascular death during follow-up and presence of LGE (Fig. 4B), patients with evidence of LGE had a higher incidence of cardiovascular death during follow-up than patients without LGE (OR: 2.71 [95% CI: 1.66 to 4.41]; p < 0.0001, I2 = 0%). When comparing cardiovascular death AERs, patients with LGE had a significantly greater risk than patients without LGE (2.4 ± 1.4% vs. 0.8 ± 0.5%, respectively; T score = 2.54, p < 0.04). Of the 5 studies reporting nonfatal MI during follow-up and presence of LGE (Fig. 4C), patients with LGE had a trend toward a higher incidence of MI during follow-up than patients without LGE (OR: 3.29 [95% CI: 0.55 to 19.76]; p = 0.19, I2 = 59%). However, when comparing MI AERs, patients with LGE had a significantly higher incidence of MI during follow-up than patients without LGE (1.9 ± 0.3% vs. 0.8 ± 0.5%, respectively; T score = 3.66, p < 0.008).
Figure 4. Individual and Pooled Risk of Cardiovascular Outcomes Based on the Presence of LGE.
Forest plots comparing clinical outcomes of patients with known or suspected CAD with late gadolinium enhancement (LGE) on CMR and without LGE on CMR. Outcomes included (A) combined cardiovascular outcomes including cardiovascular death and nonfatal MI, (B) cardiovascular death, and (C) nonfatal MI. Abbreviations as in Figure 2.
Table 4.
AERs of Studies for Combined Outcome of Cardiovascular Death and Nonfatal MI, Cardiovascular Death, and Nonfatal MI Comparing Patients With LGE on CMR and Patients Without LGE on CMR
| Stress CMR Studies First Author (Ref. #) | Combined Event AER |
Cardiovascular Death AER
|
Nonfatal MI AER |
|||
|---|---|---|---|---|---|---|
| LGE Present | LGE Absent | LGE Present | LGE Absent | LGE Present | LGE Absent | |
| Bertaso et al. (38) | 2.2 | 0.4 | 0 | 0.2 | 2.2 | 0.2 |
|
| ||||||
| Bingham et al. (39) | 2.0 | 0.9 | 1.4 | 0.7 | NR | NR |
|
| ||||||
| Bodi et al. (40) | 5.5 | 2.4 | 3.6 | 1.3 | 1.9 | 1.1 |
|
| ||||||
| Coelho-Filho et al. (42) | 6.7 | 2.2 | NR | NR | NR | NR |
|
| ||||||
| Krittayaphong et al. (46) | 2.4 | 0.6 | NR | NR | NR | NR |
|
| ||||||
| Lerakis et al. (47) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Lo et al. (48) | 9.4 | 1.1 | NR | NR | NR | NR |
|
| ||||||
| Pilz et al. (50) | 0 | NR | 0 | NR | 0 | |
|
| ||||||
| Steel et al. (51) | 18.9 | 3.5 | NR | NR | NR | NR |
|
| ||||||
| Vogel-Claussen et al. (52) | 0 | 0 | 0 | 0 | 0% | 0 |
Values are %.
AER = annualized event rate; CMR = cardiac magnetic resonance imaging; LGE = late gadolinium enhancement; NR = not reported; MI = myocardial infarction.
Figure 5. AERs of Cardiovascular Outcomes Based on the Presence of LGE.
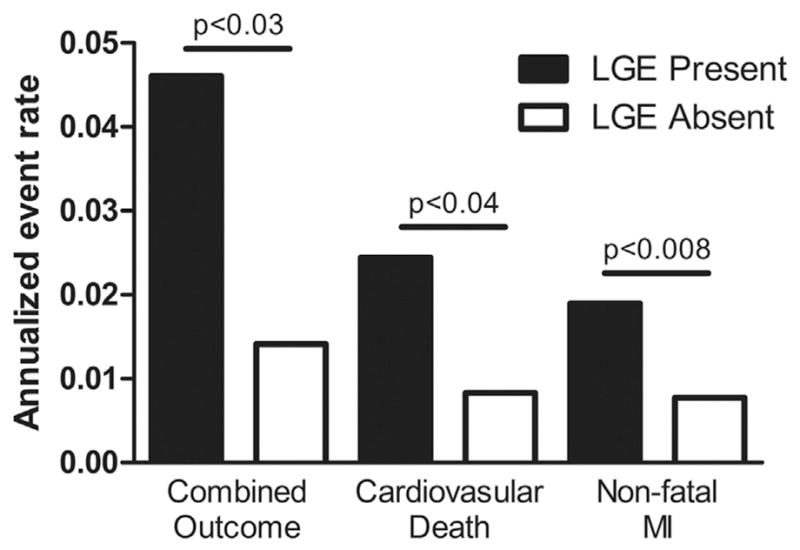
Weighted mean AERs for combined cardiovascular outcome of cardiovascular death and nonfatal MI, cardiovascular death, and nonfatal MI comparing patients with LGE on CMR (solid bars) and patients without LGE on CMR (open bars). Abbreviations as in Figures 2 to 4.
Assessment of publication bias
Funnel plots were visually inspected for all outcomes for both assessment of ischemia and LGE. There was no significant asymmetry of the funnel plots for the different outcomes though heterogeneity, with an elevated I2 value noted in some outcomes (Figs. 2A and 2C). However, Peter’s test could not rule out the presence of publication bias (R2 = 0.16; p = 0.15). Exclusion of 1 study at a time from the outcomes analysis did not affect the findings (data not shown).
Discussion
The findings of this systematic review and meta-analysis show that stress CMR provides excellent prognostic stratification of patients with known or suspected CAD. The data demonstrate that patients with a stress CMR negative for evidence of ischemia have <1% AER of either cardiovascular death or nonfatal MI, whereas patients with ischemia on stress CMR have a 5% AER of either cardiovascular death or nonfatal MI. Furthermore, there was no significant difference between vasodilator stress CMR and dobutamine stress CMR in terms of prognostic characteristics. This finding is important because vasodilator stress CMR is being used more frequently and has favorable characteristics given its ease of performance. In addition, the presence of LGE during CMR suggested an increased risk of MACE. Further studies are necessary to determine whether LGE provides incremental prognostic information in patients undergoing stress CMR. The findings of this meta-analysis in a large number of patients with a median follow-up of 25 months support the role of stress CMR for identifying patients at either low or high risk for future MACE.
Stress CMR has evolved into a powerful tool to provide comprehensive cardiac assessment. This imaging modality not only provides assessment of ischemia but can also identify the presence of LGE and valvular heart disease and can assess cardiac structure and function. Our data suggest that patients with a negative stress CMR have a prognosis comparable to those patients who have a negative stress myocardial perfusion imaging or stress echocardiogram (57–60). The combined event rate of the included studies in our meta-analysis was 5.1% during follow-up (AER 1.9%) with 2.0% for cardiovascular death (AER 0.9%) and 1.9% for nonfatal MI (AER 0.8%) in studies providing the individual outcomes. The total cardiovascular death AER was also comparable to that seen in patients undergoing coronary computed tomographic angiography in the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) trial (1.06%) (61). The robust prognostic data for stress CMR suggest that this imaging modality should be considered as an excellent alternative to stress nuclear myocardial perfusion imaging and stress echocardiography in patients who cannot exercise. This is especially true given the excellent diagnostic characteristics of stress CMR for CAD (1–4). Furthermore, research is currently underway to explore the possibility of performing exercise stress CMR to assess for myocardial ischemia (62), which may provide further valuable exercise and electrocardiography data. Large multicenter trials are currently accruing longer-term follow-up data that will provide further valuable prognostic data for stress CMR (CE-MARC [Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography for Diagnosis of Coronary Heart Disease] [4], EuroCMR Registry [63], and MR-IMPACT-II [Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease] [3]). In the current financial environment, these findings provide justification for prospective randomized trials to assess the comparative effectiveness of stress CMR compared with alternative stress-testing modalities.
Study limitations
Limitations of systematic reviews pertinent to the current study include lack of raw and uniform data from included studies, inclusion of different stress modalities, variable censoring of data for patients that underwent revascularization after stress CMR, estimation of events from hazard ratios in some studies, which assumes a linear event rate, and differences in length of follow-up (for which we attempted to adjust for by using AERs). Another limitation was the inability to assess prognosis and the degree of ischemia on stress CMR. We also included single-center, retrospective studies, as well as studies that only reported data on patients with negative stress tests. The studies in this systematic review used magnets with a field strength of 1.5-T, but the data regarding 3.0-T imaging were limited. Another limitation is the lack of information regarding the adequacy of medical therapy after stress CMR. In addition, there is the possibility of publication bias, as small studies may have been performed that did not show a significant difference in prognosis and were not published. Although the random-effects pooling method adjusts for it, another limitation of this meta-analysis was the heterogeneity observed between studies. Overall pooling can be fraught with significant heterogeneity and inconsistency. Finally, meta-regression techniques are limited because we did not have access to all the raw patient information and therefore can only assess the correlation between the variable prevalence in a study and the outcome, and the results should thus be viewed with caution and as hypothesis-generating.
Conclusions
Stress CMR seems to provide excellent prognostic risk stratification for patients with known or suspected CAD. In addition, patients with the presence of LGE on CMR are at increased risk of cardiovascular death or nonfatal MI. Stress CMR seems comparable to other stress-testing modalities for assessment of prognostic risk.
Acknowledgments
Dr. Kramer has received research equipment support from Siemens Healthcare and has served as a consultant for Synarc. Dr. Salerno has received research support from Siemens Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- AER
annualized event rate
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance imaging
- LGE
late gadolinium enhancement
- MACE
major adverse cardiovascular event(s)
- MI
myocardial infarction
- OR
odds ratio
References
- 1.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343–53. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Hamon M, Fau G, Nee G, Ehtisham J, Morello R. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. doi: 10.1186/1532-429X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwitter J, Wacker CM, Wilke N, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775–81. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodi V, Sanchis J, Nunez J, et al. Prognostic value of a comprehensive cardiac magnetic resonance assessment soon after a first ST-segment elevation myocardial infarction. J Am Coll Cardiol Img. 2009;2:835–42. doi: 10.1016/j.jcmg.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Bruder O, Breuckmann F, Jensen C, et al. Prognostic impact of contrast-enhanced CMR early after acute ST segment elevation myocardial infarction (STEMI) in a regional STEMI network: results of the “Herzinfarktverbund Essen. Herz. 2008;33:136–42. doi: 10.1007/s00059-008-3102-8. [DOI] [PubMed] [Google Scholar]
- 7.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukiji M, Nguyen P, Narayan G, et al. Peri-infarct ischemia determined by cardiovascular magnetic resonance evaluation of myocardial viability and stress perfusion predicts future cardiovascular events in patients with severe ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2006;8:773–9. doi: 10.1080/10976640600737615. [DOI] [PubMed] [Google Scholar]
- 9.Hundley WG. The use of cardiovascular magnetic resonance to identify adverse cardiac prognosis: an important step in reducing image-related heath care expenditures. J Am Coll Cardiol. 2010;56:1244–6. doi: 10.1016/j.jacc.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiribiri A, Bettencourt N, Nagel E. Cardiac magnetic resonance stress testing: results and prognosis. Curr Cardiol Rep. 2009;11:54–60. doi: 10.1007/s11886-009-0009-9. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, et al. [Accessed December 30, 2012];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 15.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Kerry SM. Statistics notes. Weighted comparison of means. BMJ. 1998;316:129. doi: 10.1136/bmj.316.7125.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50:1174–9. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischaemic cascade. Heart. 2009;95:49–55. doi: 10.1136/hrt.2007.139683. [DOI] [PubMed] [Google Scholar]
- 19.Dall’Armellina E, Morgan TM, Mandapaka S, et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. J Am Coll Cardiol. 2008;52:279–86. doi: 10.1016/j.jacc.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Waha S, Desch S, Eitel I, et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–8. doi: 10.1093/eurheartj/ehq247. [DOI] [PubMed] [Google Scholar]
- 21.de Waha S, Desch S, Eitel I, et al. Relationship and prognostic value of microvascular obstruction and infarct size in ST-elevation myocardial infarction as visualized by magnetic resonance imaging. Clin Res Cardiol. 2012;101:487–95. doi: 10.1007/s00392-012-0419-3. [DOI] [PubMed] [Google Scholar]
- 22.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Img. 2010;3:1030–6. doi: 10.1016/j.jcmg.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartlage G, Janik M, Anadiotis A, et al. Prognostic value of adenosine stress cardiovascular magnetic resonance and dobutamine stress echocardiography in patients with low-risk chest pain. Int J Cardiovasc Imaging. 2012;28:803–12. doi: 10.1007/s10554-011-9885-3. [DOI] [PubMed] [Google Scholar]
- 24.Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–57. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 25.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–33. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 26.Husser O, Bodi V, Sanchis J, et al. Head to head comparison of quantitative versus visual analysis of contrast CMR in the setting of myocardial stunning after STEMI: implications on late systolic function and patient outcome. Int J Cardiovasc Imaging. 2010;26:559–69. doi: 10.1007/s10554-010-9601-8. [DOI] [PubMed] [Google Scholar]
- 27.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 28.Johansen A, Hoilund-Carlsen PF, Vach W, Christensen HW, Moldrup M, Haghfelt T. Prognostic value of myocardial perfusion imaging in patients with known or suspected stable angina pectoris: evaluation in a setting in which myocardial perfusion imaging did not influence the choice of treatment. Clin Physiol Funct Imaging. 2006;26:288–95. doi: 10.1111/j.1475-097X.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelle S, Egnell C, Vierecke J, et al. Prognostic value of negative dobutamine-stress cardiac magnetic resonance imaging. Med Sci Monit. 2009;15:MT131–6. [PubMed] [Google Scholar]
- 30.Korosoglou G, Haars A, Michael G, et al. Quantitative evaluation of myocardial blush to assess tissue level reperfusion in patients with acute ST-elevation myocardial infarction: incremental prognostic value compared with visual assessment. Am Heart J. 2007;153:612–20. doi: 10.1016/j.ahj.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Korosoglou G, Gitsioudis G, Voss A, et al. Strain-encoded cardiac magnetic resonance during high-dose dobutamine stress testing for the estimation of cardiac outcomes: comparison to clinical parameters and conventional wall motion readings. J Am Coll Cardiol. 2011;58:1140–9. doi: 10.1016/j.jacc.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 32.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 33.Pilz G, Eierle S, Heer T, et al. Negative predictive value of normal adenosine-stress cardiac MRI in the assessment of coronary artery disease and correlation with semiquantitative perfusion analysis. J Magn Reson Imaging. 2010;32:615–21. doi: 10.1002/jmri.22289. [DOI] [PubMed] [Google Scholar]
- 34.Wallace EL, Morgan TM, Walsh TF, et al. Dobutamine cardiac magnetic resonance results predict cardiac prognosis in women with known or suspected ischemic heart disease. J Am Coll Cardiol Img. 2009;2:299–307. doi: 10.1016/j.jcmg.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh TF, Dall’Armellina E, Chughtai H, et al. Adverse effect of increased left ventricular wall thickness on five year outcomes of patients with negative dobutamine stress. J Cardiovasc Magn Reson. 2009;11:25. doi: 10.1186/1532-429X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–72. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 37.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 38.Bertaso AG, Richardson JD, Wong DT, et al. Prognostic value of adenosine stress perfusion cardiac MRI with late gadolinium enhancement in an intermediate cardiovascular risk population. Int J Cardiol. 2012 Jun 2; doi: 10.1016/j.ijcard.2012.05.051. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation. 2011;123:1509–18. doi: 10.1161/CIRCULATIONAHA.109.907659. [DOI] [PubMed] [Google Scholar]
- 40.Bodi V, Husser O, Sanchis J, et al. Prognostic implications of dipyridamole cardiac MR imaging: a prospective multicenter registry. Radiology. 2012;262:91–100. doi: 10.1148/radiol.11110134. [DOI] [PubMed] [Google Scholar]
- 41.Buckert D, Dewes P, Walcher T, Rottbauer W, Bernhardt P. Intermediate-term prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: A prospective follow-up study in a consecutive patient population. J Am Coll Cardiol Img. 2013;6:56–63. doi: 10.1016/j.jcmg.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Coelho-Filho OR, Seabra LF, Mongeon FP, et al. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. J Am Coll Cardiol Img. 2011;4:850–61. doi: 10.1016/j.jcmg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doesch C, Seeger A, Doering J, et al. Risk stratification by adenosine stress cardiac magnetic resonance in patients with coronary artery stenoses of intermediate angiographic severity. J Am Coll Cardiol Img. 2009;2:424–33. doi: 10.1016/j.jcmg.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Ingkanisorn WP, Kwong RY, Bohme NS, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–32. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 45.Jahnke C, Furundzija V, Gebker R, et al. Gender-based prognostic value of pharmacological cardiac magnetic resonance stress testing: head-to-head comparison of adenosine perfusion and dobutamine wall motion imaging. Int J Cardiovasc Imaging. 2011;28:1087–98. doi: 10.1007/s10554-011-9919-x. [DOI] [PubMed] [Google Scholar]
- 46.Krittayaphong R, Chaithiraphan V, Maneesai A, Udompanturak S. Prognostic value of combined magnetic resonance myocardial perfusion imaging and late gadolinium enhancement. Int J Cardiovasc Imaging. 2011;27:705–14. doi: 10.1007/s10554-011-9863-9. [DOI] [PubMed] [Google Scholar]
- 47.Lerakis S, McLean DS, Anadiotis AV, et al. Prognostic value of adenosine stress cardiovascular magnetic resonance in patients with low-risk chest pain. J Cardiovasc Magn Reson. 2009;11:37. doi: 10.1186/1532-429X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo KY, Leung KF, Chu CM, Loke KL, Chan CK, Yue CS. Prognostic value of adenosine stress myocardial perfusion by cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease. QJM. 2011;104:425–32. doi: 10.1093/qjmed/hcq238. [DOI] [PubMed] [Google Scholar]
- 49.Lubbers DD, Rijlaarsdam-Hermsen D, Kuijpers D, et al. Performance of adenosine “stress-only” perfusion MRI in patients without a history of myocardial infarction: a clinical outcome study. Int J Cardiovasc Imaging. 2012;28:109–15. doi: 10.1007/s10554-010-9775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilz G, Jeske A, Klos M, et al. Prognostic value of normal adenosine-stress cardiac magnetic resonance imaging. Am J Cardiol. 2008;101:1408–12. doi: 10.1016/j.amjcard.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel-Claussen J, Skrok J, Dombroski D, et al. Comprehensive adenosine stress perfusion MRI defines the etiology of chest pain in the emergency room: comparison with nuclear stress test. J Magn Reson Imaging. 2009;30:753–62. doi: 10.1002/jmri.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charoenpanichkit C, Morgan TM, Hamilton CA, et al. Left ventricular hypertrophy influences cardiac prognosis in patients undergoing dobutamine cardiac stress testing. Circ Cardiovasc Imaging. 2010;3:392–7. doi: 10.1161/CIRCIMAGING.109.912071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebker R, Jahnke C, Manka R, et al. The role of dobutamine stress cardiovascular magnetic resonance in the clinical management of patients with suspected and known coronary artery disease. J Cardiovasc Magn Reson. 2011;13:46. doi: 10.1186/1532-429X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelle S, Chiribiri A, Vierecke J, et al. Long-term prognostic value of dobutamine stress CMR. J Am Coll Cardiol Img. 2011;4:161–72. doi: 10.1016/j.jcmg.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Korosoglou G, Elhmidi Y, Steen H, et al. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1,493 consecutive patients: assessment of myocardial wall motion and perfusion. J Am Coll Cardiol. 2010;56:1225–34. doi: 10.1016/j.jacc.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11:551–61. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 58.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49:227–37. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 59.Makani H, Bangalore S, Halpern D, Makwana HG, Chaudhry FA. Cardiac outcomes with submaximal normal stress echocardiography: a meta-analysis. J Am Coll Cardiol. 2012;60:1393–401. doi: 10.1016/j.jacc.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 60.Shaw LJ, Hendel R, Borges-Neto S, et al. Prognostic value of normal exercise and adenosine (99m)Tc-tetrofosmin SPECT imaging: results from the multicenter registry of 4,728 patients. J Nucl Med. 2003;44:134–9. [PubMed] [Google Scholar]
- 61.Chow BJ, Small G, Yam Y, et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–72. doi: 10.1161/CIRCIMAGING.111.964155. [DOI] [PubMed] [Google Scholar]
- 62.Raman SV, Dickerson JA, Jekic M, et al. Real-time cine and myocardial perfusion with treadmill exercise stress cardiovascular magnetic resonance in patients referred for stress SPECT. J Cardiovasc Magn Reson. 2010;12:41. doi: 10.1186/1532-429X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner A, Bruder O, Schneider S, et al. Current variables, definitions and endpoints of the European cardiovascular magnetic resonance registry. J Cardiovasc Magn Reson. 2009;11:43. doi: 10.1186/1532-429X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]