Abstract
Background
Utilizing livers from donors after cardiac death could significantly expand the donor pool. We have previously shown that normothermic (37°C) extracorporeal liver perfusion significantly improves transplantation outcomes of ischemic rat livers. Here we investigate whether recovery of ischemic livers is possible using sub-normothermic machine perfusion at 20°C and 30°C.
Methods
Livers from male Lewis rats were divided into five groups after 1 h of warm ischemia (WI): (1) WI only, (2) 5 h of static cold storage (SCS), or 5 h of MP at (3) 20°C, (4) 30°C, and (5) 37°C. Long-term graft performance was evaluated for 28 d post-transplantation. Acute graft performance was evaluated during a 2 h normothermic sanguineous reperfusion ex vivo. Fresh livers with 5 h of SCS were positive transplant controls while fresh livers were positive reperfusion controls.
Results
Following machine perfusion (MP) (Groups 3, 4, and 5), ischemically damaged livers could be orthotopically transplanted into syngeneic recipients with 100% survival (N ≥ 4) after 4 wk. On the other hand, animals from WI only, or WI + SCS groups all died within 24 h of transplantation. Fresh livers preserved using SCS had the highest alanine aminotransferase (ALT), aspartate aminotransferase (AST), and the lowest bile production during reperfusion, while at 28 d post-transplantation, livers preserved at 20°C and 30°C had the highest total bilirubin values.
Conclusions
MP at both 20°C and 30°C eliminated temperature control in perfusion systems and recovered ischemically damaged rat livers. Postoperatively, low transaminases suggest a beneficial effect of subnormothermic perfusion, while rising total bilirubin levels suggest inadequate prevention of ischemia- or hypothermia-induced biliary damage.
Keywords: liver transplantation, reperfusion injury, sub-normothermic machine perfusion
INTRODUCTION
It is estimated that approximately 15% of deaths due to chronic liver disease and cirrhosis in the United States could be prevented by whole organ transplantation [1], however, the lack of donor organs limits the prevalence of this treatment option. A significant number of donors experience cardiac death (DCD). Organs from these donors are rarely considered for transplantation as static cold storage (SCS) [2], the current gold standard of organ preservation, is incapable of reversing the ischemic damage these organs have sustained. Encouraging results in animal models have shown that both normothermic (37°C) extracorporeal machine perfusion (37MP) [3–5], and hypothermic (4°C) machine perfusion (4MP) that aims to augment SCS, can improve that state of ischemically damaged organs [6, 7]. However, 4MP has been shown to compromise graft integrity with cold-induced damage [8], and 37MP requires a labor-intensive and complex heated perfusion system, hindering its translation into the clinical setting. Further, it is unknown whether normothermic conditions are necessary or, in fact, optimal.
Reducing perfusion temperature into the subnormothermic range may enable a less complex system to be used by reducing the need for strict temperature control as well as lowering oxygen demand during perfusion. Therefore, we investigated whether MP-based recovery of warm ischemic (WI) livers was also possible at both 20°C and 30°C and evaluated survival in a rat model of orthotopic liver transplantation, as well short-term graft function in an ex vivo perfusion setup. After orthotopic liver transplantation into syngeneic recipients, long-term graft performance was evaluated over the span of 28 d. Short-term graft function was determined using a 2 h ex vivo reperfusion model of 50% saline and 50% whole blood post-MP recovery. Early graft evaluation during reperfusion revealed comparable performance by WI+MP livers to fresh livers, while long-term post-transplantation evaluation showed 100% survival in WI+MP livers. It is conceivable, therefore, that MP may be reduced in complexity without compromising function, thereby bringing it a step closer to translation into standard clinical practice.
METHODS
Groups
Experiments were performed using male Lewis rats weighing 250–300 g (Charles River Labs, Wilmington, MA, USA). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Sub-committee on Research Animal Care, Committee on Research, Massachusetts General Hospital. Animals were divided into five groups based on how they were treated after 1 h of WI: (1) WI only; (2) 5 h of SCS; 5 h of machine perfusion at (3) 20°C, (4) 30°C, and (5) 37°C. All groups were subsequently transplanted and monitored for 28 d, or subjected to a 2 h normothermic sanguineous reperfusion in order to assess early graft function [9]. Each group comprised n ≥ 4 livers. Fresh livers with 6 h of SCS were positive transplant controls, while fresh livers were positive reperfusion controls.
Isolation of Donor Livers
All animals were anesthetized with isoflurane using a Tech 4 vaporizer (Surgivet, Waukesha, WI, USA). The donor liver isolation procedure used was a modification of Delriviere’s technique [10] and is described in detail elsewhere [11]. Briefly, a transverse abdominal incision was made to expose the portal vein (PV), the common bile duct (CBD), and the inferior vena cava (IVC). The CBD was cannulated (12 cm for bile capture during perfusion or 0.3 cm for transplantation, 22 G polyethylene stent; Surflo, Terumo, Somerset, NJ, USA). The IVC and PV were skeletonized and the right phrenic vein emptying into the supra-hepatic vena cava (SHVC) was ligated. Heparin was administered (1 u/g). The hepatic artery (HA) was ligated, then the IVC followed by the PV were clamped and the clock started for ischemic duration. The diaphragm was opened, the SHVC was transected, and the liver was removed and weighed.
Warm ischemia was induced by placing livers in a temperature-controlled chamber filled with saline and maintained at 34 ± 0.1°C for 1 h during which time they were cuffed. Ex vivo ischemia ensured a constant temperature [12] and enabled a severe model of warm ischemia [13]. After 1 h of warm ischemia, livers to be machine-perfused were flushed with saline and then connected to the perfusion system, while SCS livers were flushed with 10 mL ice cold University of Wisconsin (UW) solution and stored fully-immersed for 5 h in a bowl of ice cold UW. Fresh livers for reperfusion were flushed through the PV with 10 mL of saline upon clamping the vein in situ and were then placed in a bowl of room temperature saline to be cuffed at the PV and IVC; average warm ischemic time prior to reperfusion was 10 ± 2 min. Fresh+SCS livers for transplantation were flushed through the PV with 10mL of ice cold UW upon clamping the vein in situ, and then stored for 6 h fully immersed in a bowl of ice cold UW upon completion of the cuffing procedure.
Machine Perfusion
Details of the perfusion system can be found elsewhere [11]. Briefly, the perfusion system consisted of a primary liver perfusion circuit and a secondary dialysis circuit. This design enabled a small blood supply to provide a high hematocrit in the primary circuit while retaining the benefit of a large reservoir of nutrients and waste dilution via the secondary circuit. The primary circuit included perfusate that recirculated via a peristaltic pump through a jacketed perfusion chamber, a membrane oxygenator, a heat exchanger, and bubble trap. The oxygenator was gassed with a mixture of 74% N2/21% O2/5% CO2 and 100% O2 to maintain a constant pH. Inflow was maintained at approximately 2 mL/min/g liver, and 10–14 cm H2O portal pressure. A fraction of the perfusate was diverted to the secondary circuit via continuous peristaltic flow through a hollow fiber dialyzer with a 2200 cm2 membrane area and a 30 kDa nominal molecular weight cut-off (Spectrum Labs, Rancho Dominguez, CA, USA) at an average rate of 3 mL/min/g wet liver weight. The secondary circuit dialyzed the perfusate by counter-current exposure to 450 mL of dialysate. The volumes of perfusate and dialysate were kept constant by varying the flow of dialysate through the dialyzer in the secondary circuit. Perfusate temperature was maintained at 37, 30, or 20°C via a water-jacketed apparatus.
Perfusate comprised Williams Medium E (Sigma Chemical), autologous erythrocytes (16%–18% hct) and plasma (25% vol/vol). To this were added: insulin (2 u/L humulin; Eli Lilly, Indianapolis, IN, USA), penicillin (40,000 u/L)/streptomycin (40,000 μg/L; Gibco and Invitrogen, Carlsbad, CA, USA), L-glutamine (0.292 g/L; Gibco), hydrocortisone (10 mg/L Solu Cortef; Pfizer, New York, NY, USA), and heparin (1000 u/L APP). The total perfusate volume used for one liver perfusion was 55–60 mL. Dialysate was identical to the perfusate but without erythrocytes or plasma.
Analysis of Perfusate Levels of Metabolites and Liver Enzymes
During MP and reperfusion, perfusate samples were collected every hour and analyzed using a Piccolo blood chemistry analyzer (Abaxis, Union City, CA, USA) to determine alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Bile was collected and weighed every hour. For analysis of the hepatic oxygen uptake rate (OUR), samples were taken from the in- and outflow (PV and IVC) of the liver and analyzed immediately using a blood gas analyzer (Rapidlab 865, Siemens, DE). The total concentration of oxygen in the samples and OUR were determined as described previously [11] using the Fick principle. All livers in the reperfusion groups were biopsied for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), hematoxylin and eosin staining. For postoperative levels of ALT, AST, and total bilirubin (TBIL), 200 μL of blood was collected on postoperative d 1, 3, 5, and 7 from tail vein puncture under isoflurane anesthesia. The samples were immediately analyzed using a Piccolo blood chemistry analyzer.
Transplantation
The cuff technique developed by Kamada and Calne [15] was modified by Delriviere [10, 14] was implemented and is described in detail elsewhere [11]. All recipient surgery was carried out by the same microsurgeon (HT). The anhepatic phase of the procedure was typically 13–15 min and did not exceed 17 min. Animals were hydrated with 8 mL/kg of warm (37°C) lactated Ringer’s solution with 5% dextrose and 2 mL/kg of NaHCO3 7% wt/vol (Abbott, North Chicago, IL, USA) by penile vein injection.
The animals were put singly in clean cages, allowed to recover from anesthesia under an infrared lamp for half an hour, and subsequently returned to regular housing. During the first 12 h post-transplantation animals were checked every 2 h and subsequently every 8 h for 1 wk, and daily afterwards.
Diluted Whole Blood Reperfusion
For an approximation of the graft response in the very early phase (0–2 h) after transplantation, we employed an extracorporeal diluted whole-blood reperfusion model. This method was considered preferable to manipulating animals for sampling immediately after transplantation, which could further stress the animals, affect survival, and introduce measurement artifacts. Fresh heparinized blood was collected on the day of experiment from retired male Lewis breeders and diluted 1:1 with saline for a total perfusion volume of 55–60 mL. The primary circuit was identical to the machine perfusion system with the exception of the secondary circuit comprising the dialyzer, which was disconnected. Reperfusion temperature was set at 37°C. Livers were reperfused for 120 min and inflow (PV) and outflow (IVC) sampling was conducted routinely.
Histology
Liver tissue slices were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Apoptosis was evaluated through TUNEL staining (Roche, Indianapolis, IN).
Statistical Analysis
Data presented are means ± SEM. Statistical analysis was performed using 2-way (analysis of variance) ANOVA at α = 0.05, with Tukey-Kramer correction for multiple comparisons.
RESULTS
Survival after Transplantation
Transplantation of all livers was successful and the animals recovered from anesthesia rapidly. One hundred percent of the animals that received MP livers survived beyond 1 mo in good health and without any external signs of jaundice (Table 1). Within 12 h of transplantation, all recipients of SCS-preserved livers died. Autopsy revealed non-homogeneously perfused livers and serous fluid in the abdomen. All recipients of directly transplanted ischemic livers died later, within 24 h of transplantation. All controls that received freshly isolated livers preserved for 6 h by SCS recovered rapidly from surgery and survived beyond 1 mo.
TABLE 1.
Survival of Recipient Rats after Transplantation
| Transplantation | Survival (d) |
|---|---|
| Fresh+SCS | >28 |
| WI | <1 |
| WI+SCS | <0.5 |
| WI+20MP | >28 |
| WI+30MP | >28 |
| WI+37MP | >28 |
Integrity and Function of Liver during Perfusion
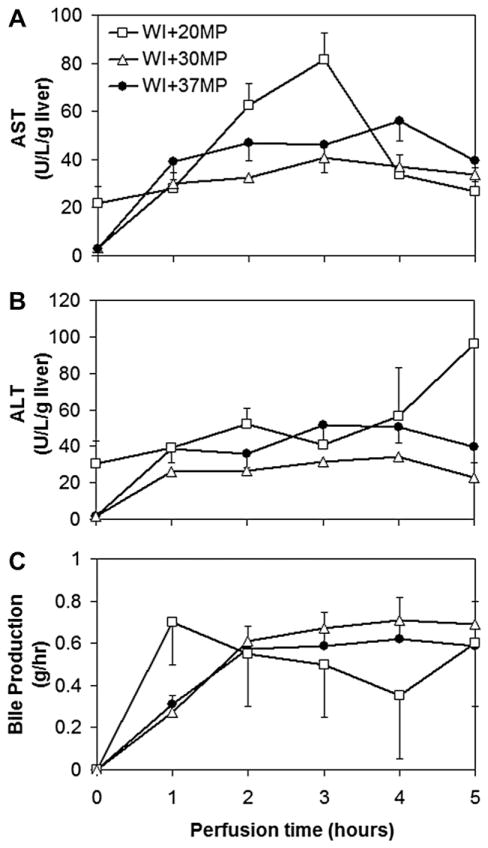
ALT and AST activities, measured hourly during perfusion and used to indicate presence of hepatocellular damage, are displayed in Fig. 1A and B. 37MP and 30MP livers have no significant differences between trends of ALT and AST release (P > 0.05), both of which illustrate a gradual increase and peak at t = 3–4 h, followed by a gradual decline. By contrast, 20 MP livers demonstrated an increasing trend in AST release such that there was a significant difference between groups at t = 4 h (P = 0.015), prior to a decrease in release such that AST levels were comparable amongst all groups at t = 6 h. ALT released by 20MP livers did not differ significantly from 30MP or 37MP on an hourly basis (P= 0.053), but overall the release was statistically higher (P = 0.016).
FIG. 1.
Function and integrity of warm ischemic livers during normothermic perfusion. (A) Asparate aminotransferase (AST) and (B) alanine aminotransferase (ALT) levels in perfusate samples collected hourly from the primary circuit, and (C) bile synthesis.
Rate of bile secretion, describing liver function, was observed to have a similar trend between 30MP and 37MP livers, increasing sharply for the first 2 h of perfusion before stabilizing at 0.6 ± 0.02 g/h and 0.7 ± 0.02 g/h, respectively (P = 0.02) for the remainder of the experiment. Though not significantly different from the other groups, 20MP livers exhibited a very different trend by increasing bile production to 0.7 ± 0.2 g/h within the first hour of perfusion, and then subsequently declining linearly over time to 0.4 ± 0.3 g/h at t = 5 h, before finally increasing to the same secretion rate of 0.6 ± 0.3 g/h as 37MP livers at t = 6 h.
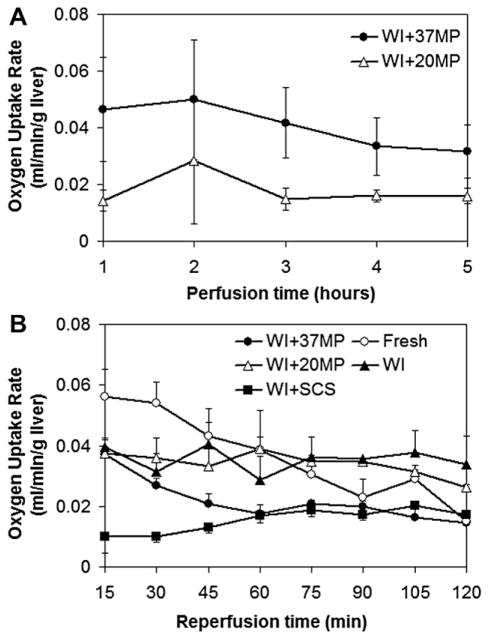
The metabolic state of the liver was assessed through its oxygen uptake rate, which was generally stable during perfusion (Fig. 2A). Average oxygen uptake rate (OUR) of 20MP livers was 43% lower compared with 37MP livers (Table 2, P = 0.02).
FIG. 2.
Oxygen uptake rate measured during (A) perfusion and (B) reperfusion.
TABLE 2.
Average Oxygen Supply and Uptake Rate of Livers After Preservation Followed by Perfusion and Reperfusion
| ODR (mL/min/g liver) | OER (mL/min/g liver) | OUR (mL/min/g liver) | |
|---|---|---|---|
| Perfusion | |||
| WI+20MP | 0.119 ± 0.018 | 0.101 ± 0.014 | 0.018 ± 0.011 |
| WI+30MP | ND | ND | ND |
| WI+37MP | 0.141 ± 0.018* | 0.098 ± 0.02 | 0.042 ± 0.017* |
| Reperfusion | |||
| Fresh | 0.112 ± 0.012 | 0.076 ± 0.024 | 0.036 ± 0.02 |
| WI | 0.098 ± 0.013** | 0.062 ± 0.008** | 0.036 ± 0.015 |
| WI+SCS | 0.106 ± 0.01 | 0.091 ± 0.008*** | 0.015 ± 0.006** |
| WI+20MP | 0.117 ± 0.009 | 0.082 ± 0.012 | 0.034 ± 0.005 |
| WI+30MP | ND | ND | ND |
| WI+37MP | 0.094 ± 0.01** | 0.073 ± 0.013 | 0.022 ± 0.004** |
ODR = oxygen delivery rate; OER = oxygen exit rate; OUR = oxygen uptake rate.
Significantly different from WI+20MP in perfusion (P < 0.05).
Significantly lower and higher than other groups in reperfusion, respectively (P < 0.05).
Short-Term Graft Performance Post-machine Perfusion
To evaluate graft function immediately post-perfusion we employed a reperfusion system using diluted whole-blood. OUR was highest in fresh livers at the onset of reperfusion at 0.07 ± 0.009 mLO2/min/g liver, while WI+SCS livers had the lowest OUR at 0.013 ± 0.005 mLO2/min/g liver (Fig. 2B). At the end of 2 h of reperfusion, all livers occupied a narrow range of OUR values of 0.015–0.04 mLO2/min/g liver. Fresh livers showed a linear decline in OUR throughout reperfusion while WI+SCS livers demonstrated a gradual increase in uptake. MP livers and WI livers both commenced at the same OUR of 0.039 ± 0.015 mLO2/min/g liver and over the course of the first hour achieved a steady value. Table 2 reveals that the lowest oxygen exit rate (OER) was 0.062 ± 0.008 mLO2/min/g liver, which illustrates usefully that though there were some oxygen delivery rates (ODR)s that were significantly lower than others, none of the livers were oxygen deprived. Curiously, the average OUR during reperfusion was similar between fresh, WI+20MP, and WI livers, while it was significantly lower in WI+37MP livers (approximately half the value observed in perfusion), and lowest in WI+SCS livers.
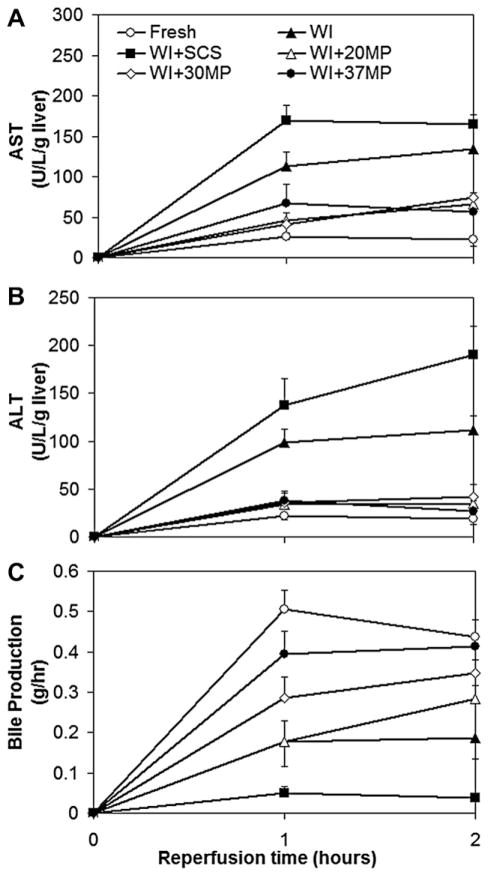
Figure 3 shows ALT and AST secretion for MP groups during reperfusion were similar to fresh livers and significantly lower than WI-only livers at all time points (P = 0.004 and P = 0.03, respectively), while WI+SCS groups were higher than WI-only livers (P = 0.16 and P = 0.056, respectively). The rate of ALT and AST release was decreased during the second hour of reperfusion relative to the first in all groups. ALT and AST values for MP livers during reperfusion were generally consistent with the values observed during machine perfusion.
FIG. 3.
Reperfusion results: (A) alanine aminotransferase (ALT), (B) aspartate aminotransferase (AST), and (C) bile synthesis.
Bile production during reperfusion was highest in fresh livers and decreased as a function of decreasing temperature, though the differences between fresh and MP groups were not significant. WI livers produced bile at a rate comparable to 20MP in the first hour but then reached a plateau in the second hour, making significantly less than fresh livers (P = 0.014). WI+SCS livers produced significantly less bile throughout reperfusion compared to fresh or MP livers (P = 0.001). MP livers produced less bile per hour during reperfusion than during machine perfusion.
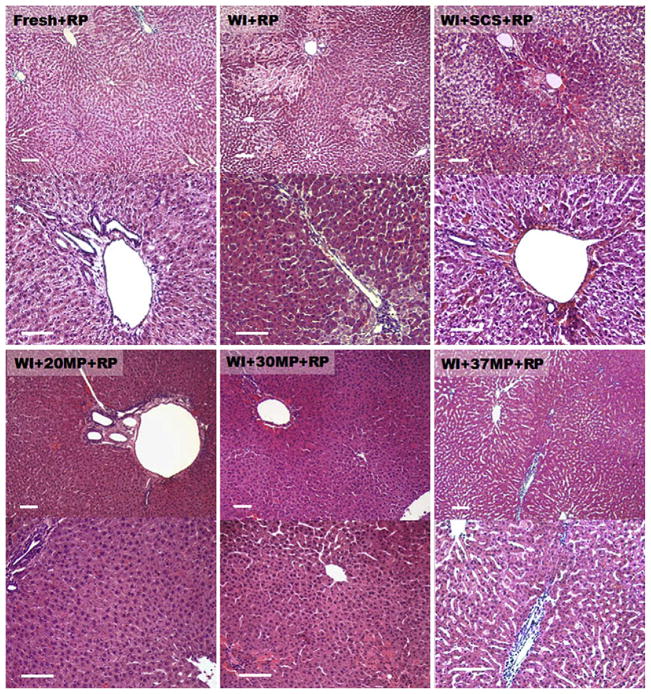
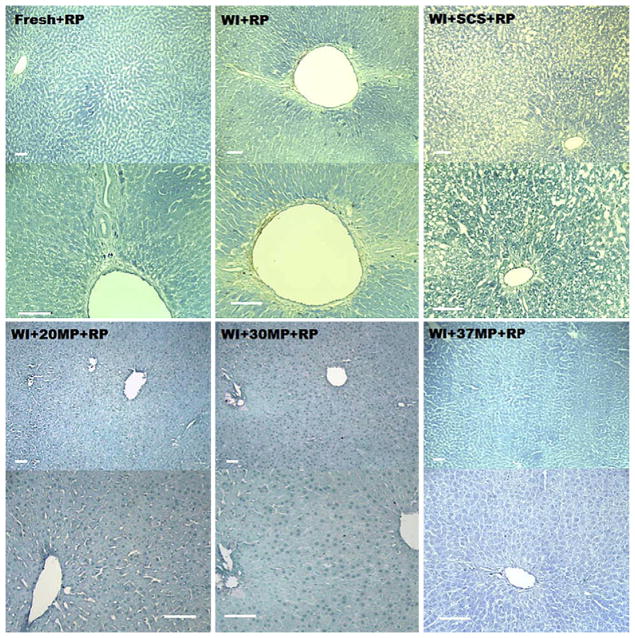
Hand E histology of livers biopsied immediately after reperfusion (Fig. 4) show that MP livers retained a homogeneous architecture that appears comparable to fresh livers. Edema appeared more prevalent in 37MP livers, and seemed to decrease with temperature, showing minimal to none at 20°C. WI livers showed focal areas of necrosis, which was exacerbated in WI+SCS livers. Figure 5 illustrates that apoptosis was negligible in all reperfused livers.
FIG. 4.
H and E staining of livers after preservation and reperfusion at 10× (top row) and 20× (bottom row) magnification. (A) Group I: fresh livers. (B) Group II: warm ischemic livers. (C) Group III: warm ischemic livers after 5 h of static cold storage in UW solution). (D) Group IV: warm ischemic livers after 20°C MP. (E) Warm ischemic livers after 30°C MP. (F) Warm ischemic livers after 37°C MP.
FIG. 5.
TUNEL of livers after preservation and reperfusion at 10× (top row) and 20× (bottom row) magnification. (A) Group I: fresh livers. (B) Group II: warm ischemic livers. (C) Group III: warm ischemic livers after 5 h of static cold storage (SCS) in University of Wisconsin (UW) solution). (D) Group IV: warm ischemic livers after 20°C MP. (E) Warm ischemic livers after 30°CMP. (F) Warm ischemic livers after 37°C MP.
Postoperative Liver Enzymes and Bilirubin
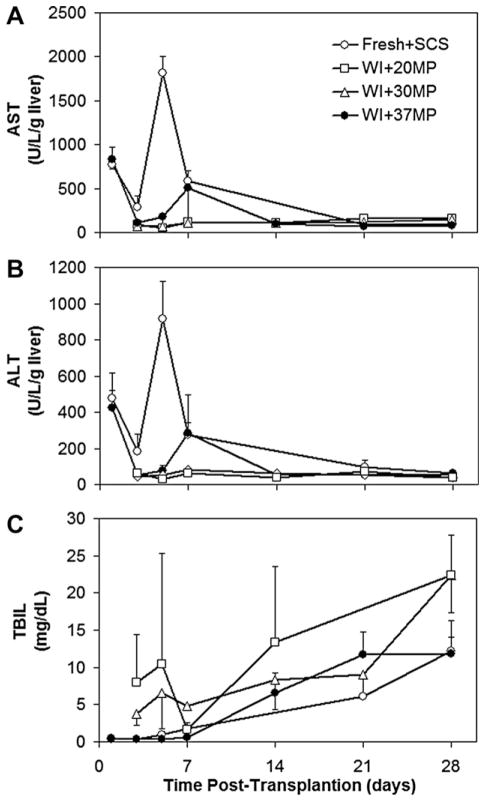
Serologic analysis was performed on the transplant recipients over the course of 28 d. Both ALT and AST levels (Fig. 6A and B) were elevated on d 1 postoperatively for all groups compared with perfusion and reperfusion values. Fresh+SCS livers displayed a spike in both enzyme levels on d 5; a much less pronounced spike was also evident in 37MP-resuscitated ischemic livers on d 7. By contrast, 30MP and 20MP livers consistently displayed the lowest levels throughout the observation period.
FIG. 6.
Values of (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), (C) total bilirubin (TBIL) measured after transplantation of healthy cold-stored livers compared with machine perfused warm ischemic livers at 20, 30, or 37°C.
Postoperative TBIL levels increased in all groups, though more so in 20MP and 30MP livers, such that recipients of 30MP livers had significantly elevated levels compared with recipients of Fresh+SCS livers at 28 d (P < 0.01). No other differences were statistically significant.
DISCUSSION
Machine perfusion of donor livers is gaining recognition as an organ preservation technique capable of both expanding the current donor pool and producing grafts superior to conventional static cold storage methods [16–20]. Recovery of damaged donor organs such as those which are ischemic [5, 21–24] or steatotic has been demonstrated in animal models [25, 26], enabling clinical application of machine perfusion at 4°C [27, 28]. To our knowledge, this study is the first to demonstrate successful transplantation of ischemic livers recovered with subnormothermic machine perfusion.
Room-temperature perfusion has already proven advantageous through retention of hepatic and biliary structural integrity [29, 30], and improved protection of marginal livers [25]. Here we demonstrate that ischemic rat livers recovered by machine perfusion at 20 and 30°C result in 100% transplantable grafts, measured 28 d postoperatively. Clinically, the recipients looked as well as those that had received livers recovered at 37°C or fresh livers stored using SCS. Interestingly, evidence that SCS further exacerbated warm ischemic damage [31, 32] was seen both in the rapidity with which recipients of these control livers died (within 12 h of transplantation, compared with WI-only recipients that died within 24 h of surgery), and the extent of ALT and AST release during reperfusion, which was highest in WI+SCS livers.
Perfusion of warm ischemic livers at 20 and 30°C did not incur further cellular damage as very little ALT and AST release occurred, which stabilized during the first hour of perfusion. Further, organ function appeared intact with stable oxygen uptake rate and bile production within the first few hours of perfusion. WI+20MP livers consumed significantly less oxygen than WI+37MP, as would be expected with a temperature-induced decline in metabolic rate [29]. From OER values in Table 2, it can be seen that neither WI+20MP livers nor WI+37MP livers used all the available oxygen delivered to them; therefore they were not oxygen limited. Perfusate without erythrocytes, oxygenated with 95%O2 and 5%CO2, at the physiologic flow rates employed in these experiments of 1.8 ± 1.2 mL/min/g liver (in vivo: 1.7–2.3 mL/min/g liver [33]) would deliver approximately 0.022 mL O2/min/g liver (using a modified Fick’s formula), which is higher than WI livers perfused at 20°C require. Future perfusions at 20°C may therefore be further reduced in complexity by removing the need for oxygen carriers.
Reperfusion data provided valuable insight as to the time frame and likely sources of warm ischemic organ failure post-transplantation. Reperfusion of WI livers demonstrated a metabolic rate of activity through OUR that was comparable to fresh or perfusion-recovered livers. This is not surprising from the perspective that these organs are still viable, as successful transplantation after machine perfusion demonstrates. However, reperfusion with (diluted) whole blood at 37°C caused a significant release of ALT and AST, and a greater reduction in the production of bile than in WI+37MP livers. Similarly, histologic analysis of liver tissue after 2 h of reperfusion showed evidence of necrosis in WI and WI+SCS groups, not seen in any of the other groups; none of the groups showed any signs of apoptosis within this time frame. It is likely, therefore, that machine perfusion is capable of ameliorating ischemia reperfusion injury [34–36] initiated immediately post-transplantation by factors present within the recipient’s blood supply.
Serologic analysis of recipients post-transplantation over the course of 28 d revealed that ALT and AST levels were lowest in 20 and 30°C perfused livers. Though all grafts showed an increasing trend in total bilirubin, likely exacerbated by the non-arterialized orthotopic liver transplantation method used [37], WI+20MP and WI+30MP livers were highest (but statistically insignificant), possibly suggestive of temperature-associated damage to the biliary epithelium. Recent work by Brockmann et al. [38] has demonstrated a more suitable porcine DCD-liver model of MP resuscitation that includes re-arterialization of the liver at transplantation, which will enable better evaluation of biliary epithelium recovery during MP.
In conclusion, we demonstrated that transplantable grafts can be recovered from warm ischemic rat livers by machine perfusion at both 20 and 30°C. Subnormothermic perfusion may remove the need for stringent temperature control, and may also eliminate dependence on oxygen carriers in perfusate, providing critical simplifications for the successful translation of this technology into clinical practice.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01DK59766, R00DK080942) and the Shriners Hospitals for Children.
References
- 1.Miniño AM, Heron MP, Murphy SL, et al. Deaths: Final data for 2004. Natl Vital Stat Rep. 2007;55:1. [PubMed] [Google Scholar]
- 2.Reddy S, Zilvetti M, Brockmann J, et al. Liver transplantation from non-heart-beating donors: Current status and future prospects. Liver Transplantation. 2004;10:1223. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 3.St Peter SD, Imber CJ, Lopez I, et al. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 4.Schön MR, Puhl G, Frank J, Neuhaus P. Hemodialysis improves results of pig-liver perfusion after warm ischemic-injury. Transplantation Proc. 1993;25:3239. [PubMed] [Google Scholar]
- 5.Schon MR, Otto K, Stephen W, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CY, Zhang JX, Jones JW, Jr, et al. Functional recovery of preserved livers following warm ischemia: Improvement by machine perfusion preservation. Transplantation. 2002;74:944. doi: 10.1097/00007890-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bessems M, Doorschodt BM, Kolkert JLP, et al. Preservation of steatotic livers: A comparison between cold storage and machine perfusion preservation. Liver Transplantation. 2007;13:497. doi: 10.1002/lt.21039. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Lee CY, Clemens MG, et al. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77:1676. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 9.Uygun K, Tolboom H, Izamis M, et al. Diluted blood reperfusion as a model for transplantation of ischemic rat livers: Alanine aminotransferase is a direct indicator of viability. Transplantation Proc. 2010;42:2463. doi: 10.1016/j.transproceed.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delriviere L, Gibbs P, Kobayashi E, et al. Detailed modified technique for safer harvesting and preparation of liver graft in the rat. Microsurgery. 1996;17:690. doi: 10.1002/(SICI)1098-2752(1996)17:12<690::AID-MICR6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Tolboom H, Pouw R, Uygun K, et al. A model for normothermic preservation of the rat liver. Tissue Eng. 2007;13:2143. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]
- 12.Heijnen BH, van Veen SQ, Straatsburg IH, et al. Pronounced effect of minor changes in body temperature on ischemia and reperfusion injury in rat liver. J Appl Physiol. 2001;91:265. doi: 10.1152/jappl.2001.91.1.265. [DOI] [PubMed] [Google Scholar]
- 13.Dutkowski P, Furrer K, Tian Y, et al. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244:968. doi: 10.1097/01.sla.0000247056.85590.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delriviere L, Gibbs P, Kobayashi E, et al. Technical details for safer venous and biliary anastomoses for liver transplantation in the rat. Microsurgery. 1998;18:12. doi: 10.1002/(sici)1098-2752(1998)18:1<12::aid-micr4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47. [PubMed] [Google Scholar]
- 16.Vogel T, Brockmann JG, Friend PJ. Ex vivo normothermic liver perfusion: An update. Curr Opin Organ Transplant. 2010;15:167. doi: 10.1097/MOT.0b013e328337349d. [DOI] [PubMed] [Google Scholar]
- 17.Monbaliu D, Brassil J. Machine perfusion of the liver: Past, present and future. Curr Opin Organ Transplant. 2010;15:160. doi: 10.1097/MOT.0b013e328337342b. [DOI] [PubMed] [Google Scholar]
- 18.Vekemans K, Liu Q, Pirenne J, et al. Artificial circulation of the liver: Machine perfusion as a preservation method in liver transplantation. Anat Rec. 2008;291:735. doi: 10.1002/ar.20662. [DOI] [PubMed] [Google Scholar]
- 19.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front Biosci. 2008;13:221. doi: 10.2741/2672. [DOI] [PubMed] [Google Scholar]
- 21.Lee CY, Jain S, Duncan HM, et al. Survival transplantation of preserved non-heart-beating donor rat livers: Preservation by hypothermic machine perfusion. Transplantation. 2003;76:1432. doi: 10.1097/01.TP.0000088674.23805.0F. [DOI] [PubMed] [Google Scholar]
- 22.Tolboom H, Pouw R, Izamis M, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009;87:170. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg. 2009;250:1. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 24.de Rougemont O, Breitenstein S, Leskoek B, et al. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250:674. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 25.Vairetti M, Ferrigno A, Carlucci F, et al. Subnormothermic machine perfusion protects steatotic livers against preservation injury: A potential for donor pool increase? Liver Transplantation. 2009;15:20. doi: 10.1002/lt.21581. [DOI] [PubMed] [Google Scholar]
- 26.Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab Eng. 2009;22:274. doi: 10.1016/j.ymben.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarrera JV, Henry SD, Chen SW, et al. Hypothermic machine preservation attenuates ischemia/reperfusion markers after liver transplantation: Preliminary results. J Surg Res. 2010 doi: 10.1016/j.jss.2010.01.038. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Guarrera JV, Estevez J, Boykin R, et al. Hypothermic machine perfusion of liver grafts for transplantation: Technical development in human discard and miniature swine models. Transplantation Proc. 2005;37:323. doi: 10.1016/j.transproceed.2004.12.094. [DOI] [PubMed] [Google Scholar]
- 29.Fujita S, Hamamoto I, Nakamura K, et al. Isolated perfusion of rat livers: Effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nippon Geka Hokan. 1993;62:58. [PubMed] [Google Scholar]
- 30.Vairetti M, Ferrigno A, Rizzo V, et al. Correlation between the liver temperature employed during machine perfusion and reperfusion damage: Role of Ca2+ Liver Transplantation. 2008;14:494. doi: 10.1002/lt.21421. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Lee CY, Clemens MG, et al. Prolonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Tranplantation. 2004;77:1676. doi: 10.1097/01.tp.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 32.Olschewski P, Gass P, Aiyakhagorn V, et al. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337. doi: 10.1016/j.cryobiol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Izamis ML, Uygun K, Berthiaume F, et al. In situ metabolic flux analysis to quantify the liver metabolic response to experimental burn injury. Biotechnol Bioeng. 2010 doi: 10.1002/bit.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: A brief review. Mol Med. 2007;14:337. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bulletin. 2004;70:71. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:1083. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 37.Imamura H, Rocheleau B, Cote J, et al. Long-term consequence of rat orthotopic liver transplantation with and without hepatic arterial reconstruction: A clinical, pathological, and hemodynamic study. Hepatology. 1997;26:198. doi: 10.1002/hep.510260126. [DOI] [PubMed] [Google Scholar]
- 38.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg. 2009;250:1. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]