Abstract
delta-Aminolevulinate in plants, algae, cyanobacteria, and several other bacteria such as Escherichia coli and Bacillus subtilis is synthesized from glutamate by means of a tRNA(Glu) mediated pathway. The enzyme glutamyl tRNA(Glu) reductase catalyzes the second step in this pathway, the reduction of tRNA bound glutamate to give glutamate 1-semialdehyde. The hemA gene from barley encoding the glutamyl tRNA(Glu) reductase was expressed in E. coli cells joined at its amino terminal end to Schistosoma japonicum glutathione S-transferase (GST). GST-glutamyl tRNA(Glu) reductase fusion protein and the reductase released from it by thrombin digestion catalyzed the reduction of glutamyl tRNA(Glu) to glutamate 1-semialdehyde. The specific activity of the fusion protein was 120 pmol.micrograms-1.min-1. The fusion protein used tRNA(Glu) from barley chloroplasts preferentially to E. coli tRNA(Glu) and its activity was inhibited by hemin. It migrated as an 82-kDa polypeptide with SDS/PAGE and eluted with an apparent molecular mass of 450 kDa from Superose 12. After removal of the GST by thrombin, the protein migrated as an approximately equal to 60-kDa polypeptide with SDS/PAGE, whereas gel filtration on Superose 12 yielded an apparent molecule mass of 250 kDa. Isolated fusion protein contained heme, which could be reduced by NADPH and oxidized by air.
Full text
PDF
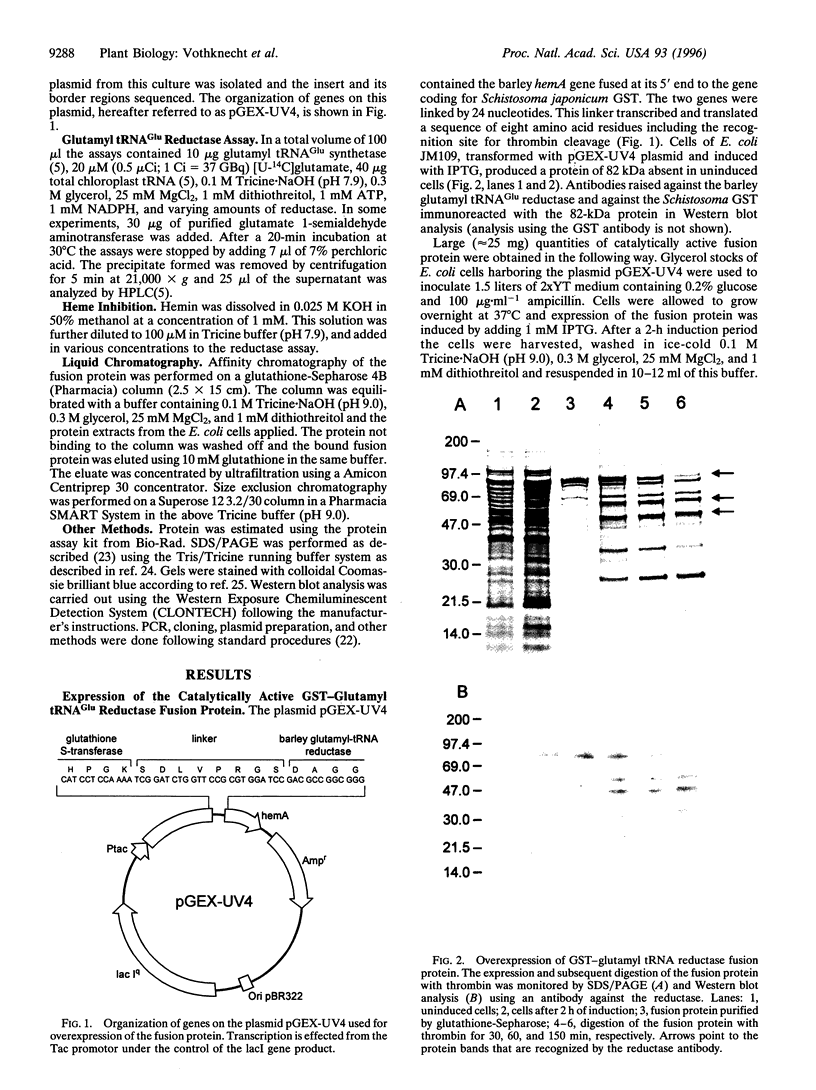
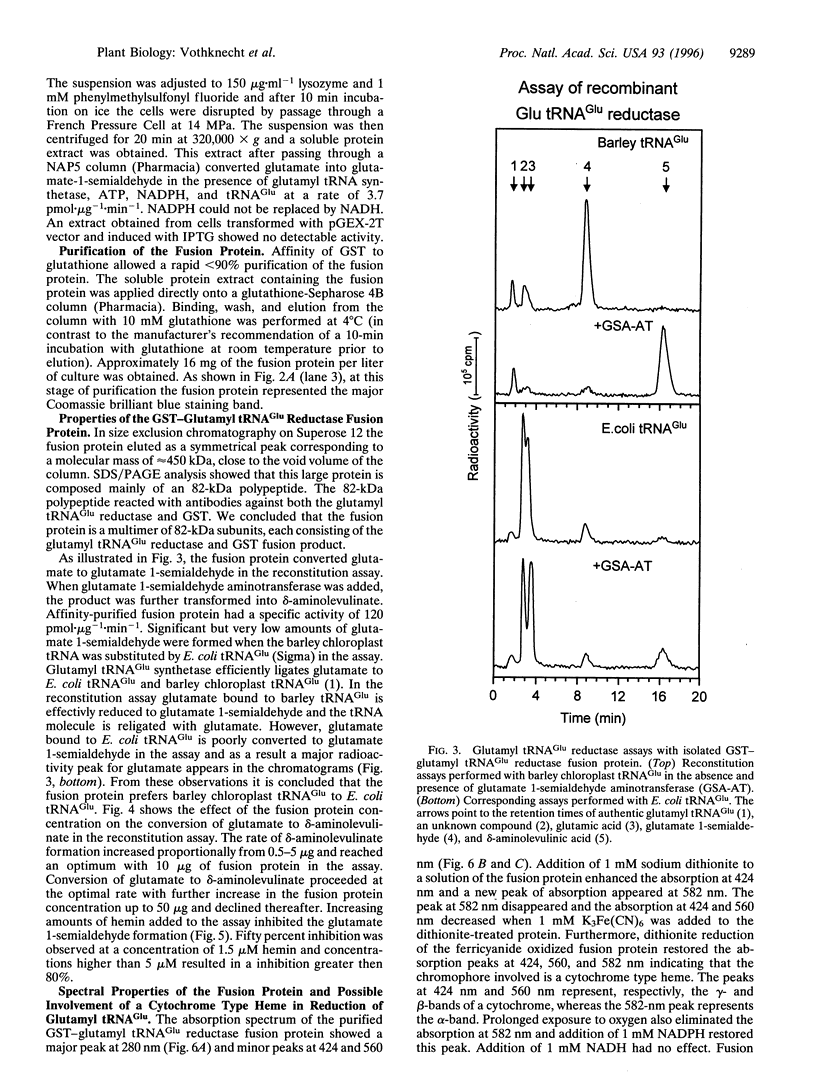
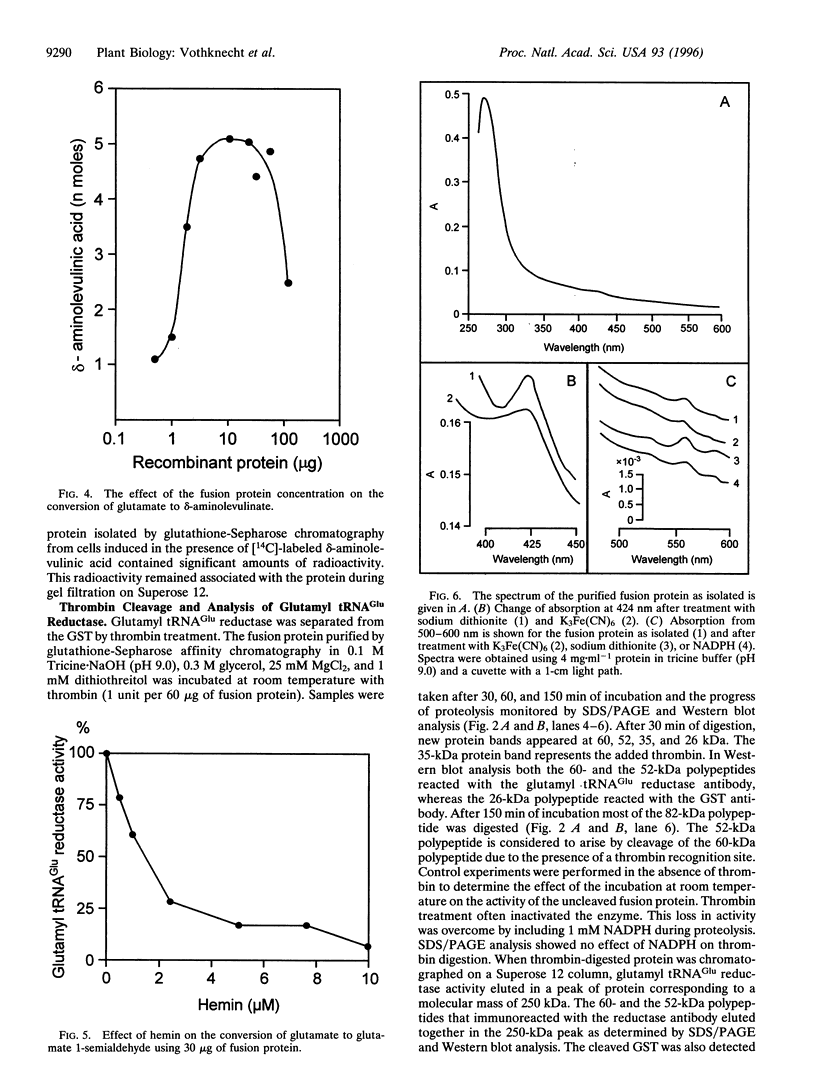

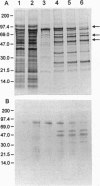
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahara N., Murakami K., Korbrisate S., Hashimoto Y., Murooka Y. Cloning and characterization of the hemA gene for synthesis of delta-aminolevulinic acid in Xanthomonas campestris pv. phaseoli. Appl Microbiol Biotechnol. 1994 Feb;40(6):846–850. doi: 10.1007/BF00173986. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Cloning and expression of a structural gene from Chlorobium vibrioforme that complements the hemA mutation in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1656–1659. doi: 10.1128/jb.172.3.1656-1659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. W., Jahn D., O'Neill G. P., Söll D. Purification of the glutamyl-tRNA reductase from Chlamydomonas reinhardtii involved in delta-aminolevulinic acid formation during chlorophyll biosynthesis. J Biol Chem. 1990 Mar 5;265(7):4058–4063. [PubMed] [Google Scholar]
- Drolet M., Péloquin L., Echelard Y., Cousineau L., Sasarman A. Isolation and nucleotide sequence of the hemA gene of Escherichia coli K12. Mol Gen Genet. 1989 Apr;216(2-3):347–352. doi: 10.1007/BF00334375. [DOI] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Fujino E., Fujino T., Karita S., Sakka K., Ohmiya K. Cloning and sequencing of some genes responsible for porphyrin biosynthesis from the anaerobic bacterium Clostridium josui. J Bacteriol. 1995 Sep;177(17):5169–5175. doi: 10.1128/jb.177.17.5169-5175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm B. Identification of a hemA gene from Synechocystis by complementation of an E. coli hemA mutant. Hereditas. 1992;117(2):195–197. doi: 10.1111/j.1601-5223.1992.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Ilag L. L., Kumar A. M., Söll D. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell. 1994 Feb;6(2):265–275. doi: 10.1105/tpc.6.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D., Michelsen U., Söll D. Two glutamyl-tRNA reductase activities in Escherichia coli. J Biol Chem. 1991 Feb 5;266(4):2542–2548. [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar D., Avissar Y. J., Wyche J. H., Beale S. I. Structure and expression of the Chlorobium vibrioforme hemA gene. Arch Microbiol. 1991;156(4):281–289. doi: 10.1007/BF00262999. [DOI] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Petricek M., Rutberg L., Schröder I., Hederstedt L. Cloning and characterization of the hemA region of the Bacillus subtilis chromosome. J Bacteriol. 1990 May;172(5):2250–2258. doi: 10.1128/jb.172.5.2250-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoppidan B., Kannangara C. G. Purification and partial characterisation of barley glutamyl-tRNA(Glu) reductase, the enzyme that directs glutamate to chlorophyll biosynthesis. Eur J Biochem. 1994 Oct 15;225(2):529–537. doi: 10.1111/j.1432-1033.1994.00529.x. [DOI] [PubMed] [Google Scholar]
- Rieble S., Beale S. I. Purification of glutamyl-tRNA reductase from Synechocystis sp. PCC 6803. J Biol Chem. 1991 May 25;266(15):9740–9745. [PubMed] [Google Scholar]
- Schröder I., Hederstedt L., Kannangara C. G., Gough P. Glutamyl-tRNA reductase activity in Bacillus subtilis is dependent on the hemA gene product. Biochem J. 1992 Feb 1;281(Pt 3):843–850. doi: 10.1042/bj2810843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkamp E., Jahn M., Jahn D., Kumar A. M., Söll D. Glutamyl-tRNA reductase from Escherichia coli and Synechocystis 6803. Gene structure and expression. J Biol Chem. 1992 Apr 25;267(12):8275–8280. [PubMed] [Google Scholar]
- Von Wettstein D., Gough S., Kannangara C. G. Chlorophyll Biosynthesis. Plant Cell. 1995 Jul;7(7):1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willows R. D., Kannangara C. G., Pontoppidan B. Nucleotides of tRNA (Glu) involved in recognition by barley chloroplast glutamyl-tRNA synthetase and glutamyl-tRNA reductase. Biochim Biophys Acta. 1995 Sep 19;1263(3):228–234. doi: 10.1016/0167-4781(95)00105-p. [DOI] [PubMed] [Google Scholar]