Abstract
Costus speciosus (Koen) J.E. Sm. plant and its extracts are used for treatment of fever, snake bites, jaundice, and as a purgative, astringent and antibacterial agent. In the present study, an attempt has been made to evaluate in vitro antioxidant activity of different extracts of this plant by DPPH radical scavenging activity, total antioxidant capacity, nitric oxide scavenging activity, ion chelating activity, hydroxyl radical scavenging activity and its correlation with total phenolic content.
Key Words: Costus speciosus, Phenolic content, Antioxidant activity
Introduction
Costus speciosus (Koen) J.E. Sm. (Zingiberaceae) is an erect herbaceous plant up to 2 m height with long lanceolate leaves and white fragrant flowers in terminal clusters. The plant flowers during the months of July and August, the aerial parts withering away during the winter season. It has wide distribution in India, occurring throughout the sub-Himalayan tract from Himachal Pradesh to Assam, Vindhya and Satpura hills in central India and the western ghats of Maharashtra, Karnataka, Kerala (1).
Many properties of plant products are associated with the presence of phenolic compounds, which are essential for plant development and play an important role in their defense mechanisms. The inclusion of these compounds in the regular diet might be beneficial to human health by lowering incidence of diseases. Active oxygen molecules such as (O2˚-, OOH˚), hydroxyl (OH˚) and peroxyl (ROOH˚) radicals play an important role in oxidative stress related to the pathogenesis of various important diseases. In healthy individuals, the production of free radicals is balanced by antioxidative defense system (2).
Due to the increasing safety concerns about synthetic antioxidants, exploitation of cheaper and safer sources of antioxidants based on natural origin is the focus of research nowadays. Recently, there has been growing interest in oxygen containing free radicals in biologic systems and their implied roles as causative agents in the etiology of a variety of chronic disorders. Currently available synthetic antioxidants like butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT), tertiary butylated hydroquinone and gallic acid esters, have been suspected to cause or prompt negative health effects. Hence, strong restrictions have been placed on their application and there is a trend to substitute them with naturally occurring antioxidants. Moreover, these synthetic antioxidants also show low solubility and moderate antioxidant activity (3). Hence, finding natural sources of antioxidants is the focus of this study.
Antioxidant compounds in food play a key role in health promotion. Scientific evidences suggest that antioxidants reduce risk for chronic diseases including cancer and heart disease. Primary sources of naturally occurring antioxidants are whole grains, fruits and vegetables. Plant sourced food antioxidants like vitamin C, vitamin E, carotenes, phenolic acids, phytate and phytoestrogens have been recognized as having the potential to reduce disease risks. Various antioxidant activity methods have been used to monitor and compare the antioxidant activity of foods and herbal drugs. In recent years, oxygen radical absorbance capacity assays and enhanced chemiluminescence assays have been used to evaluate antioxidant activity of foods, serum and other biological fluids. Special equipment and technical skills are needed these analysis and the radical-scavenging activity of antioxidants against free radicals like the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, the superoxide anion radical (O2), the hydroxyl radical (OH), or the peroxyl radical (ROO) are measured. The variation in food antioxidant activity by different method arises from the specificity of the free radical being used as the reactant. Other methods determine the resistance of lipid or lipid emulsions to oxidation in the presence of the antioxidant being tested. The malondialdehyde (MDA) or thiobarbituric acid-reactive-substances (TBARS) assays have been extensively used since 1950 has to estimate the peroxidation of lipids in membrane and biological systems. These methods can be time-consuming since they depend on the oxidation of a substrate that is influenced by temperature, pressure, matrix etc. and may not be practical when large numbers of samples are involved (4). Antioxidant activity methods using free radicals are fast, easy and simple. Some methods are summarized here. A solid-phase spectrophotometry using immobilized tetrabenzo-[b,f,j,n][1,5,9,13]-tetraazacyclohexadecine-Cu(II) complex was proposed by Olga A. Zaporozhets (5) in order to measure the antioxidant potency of herbal products. The absorbance of the modified sorbent (ìmax) 712 nm) increases proportionally to the total antioxidant activity of the sample solution on silica gel.
Fluorometric Analysis (6) quantified the hydroxyl radical scavenging capacity and efficacy of a novel organosiliceous anionic hydride compound and silica hydride. The method measures a direct relationship between the hydroxyl radical scavenging capability of the antioxidant compound and the linear decrease in signal from a fluorescent 2-hydroxyterephthalate product created by reacting to a Fe2+-EDTA complex in the presence of a potential radical scavenger. Bioassay-guided fractionation, column separation on Diaion, Toyopearl HW 40(C), Sephadex LH-20 and MCI CHP20P, HPLC and spin trapping electron spin resonance (ESR) method (7).
Phenolic compounds are a large, heterogeneous group of secondary plant metabolites that are widespread in the plant kingdom (8). Polyphenols are the products of plant metabolism and can range from simple molecules to highly polymerized compounds. Phenolics display a vast variety of structures; here only flavonoids, tannins and phenolic acids are reviewed. Flavonoids, a subclass of polyphenols, are the most common polyphenolic compounds found in nature and are further divided into several subclasses including flavones, flavonols, isoflavones, anthocyanins, flavanols, and proanthocyanidins. Flavonoids and other plant phenolics are especially common in leaves, flowering tissues and woody parts such as the stem and bark (9).
The aim of this study was to determine in vitro antioxidant activity of the solvent extracted material of Costus speciosus and its correlation with the total phenolic content.
Experimental
Folin-Ciocalteu phenol reagent was purchased from Sigma Co. All the other chemicals and solvents used in this study were of analytical grade and obtained from HiMedia Chemicals Mumbai, India.
Extraction
C. speciosus was collected from the forest of Bahuli-gaon Tal- Igatpuri Dist-Nashik Maharashtra, India. The whole plant got certificate of authentication from Botanical Survey of India, Pune. A voucher specimen was deposited in herbarium of our laboratory. The shad dried rhizomes were pulverized and subjected to successive extraction using Soxhlet apparatus. petroleum ether (60:80), cyclohexane, benzene, ethyl acetate, chloroform, acetone, methanol and water were significantly applied for extractions used in sequence.
DPPH radical scavenging activity
DPPH scavenging activity was measured by spectrophotometric method (10). To 1 mL of various concentrations of the extract, 1 mL of DPPH solution (0.1 mM) was added. An equal amount of methanol and DPPH served as control. After 20 min of incubation in dark, the absorbance was recorded at 517 nm. The experiments were performed in triplicate and the percentage of inhibition calculated (11) as the following:
Total antioxidant capacity
Total antioxidant capacity was measured by spectrophotometric method (12). 0.1 mL of the extract (10 mg/mL) dissolved in water was mixed in eppendorf tube with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate).The tubes were capped and incubated in a thermal block at 95 °C for 90 min. After cooling to room temperature, the absorbance of aqueous solution was measured at 695 nm against blank. Ascorbic acid was used as the standard and total antioxidant capacity was expressed as the equivalents of ascorbic acid.
Scavenging of nitric oxide radical
Nitric oxide was generated from sodium nitroprusside and measured by Griess’ reaction (13). Sodium nitroprusside (5 mM) in standard phosphate buffer saline solution (0.025 M, pH 7.4) was incubated with different concentrations (2-1000 μg/mL) of the test extract dissolved in phosphate buffer saline (0.025 M, pH 7.4) and the tubes were incubated at 25°C for 5 h. Control experiments were conducted in the identical manner using the equivalent amounts of buffer. After 5 h, 0.5 mL of the sample was diluted with 0.5 mL of Griess’ reagent (1% sulphanilamide, 2 % o-phosphoric acid and 0.1 % napthyl ethylenediamine dihydrochloride). The absorbance of the chromophore formed during diazotization of nitrite with suphanilamide and its subsequent coupling with napthyl ethylene diamine was read at 546 nm. The experiments were repeated in triplicate.
Ion chelating activity
Ion chelating activity was determined by colorimetric method (14, 15).The reaction mixture containing 1 mL 0.05% o-phenathroline in methanol, 2 mL ferric chloride 200 μM and 2 mL various concentrations of the test compound was incubated at ambient temperature for 10 min and the absorbance of the sample was measured at 510 nm. The experiments were performed in triplicate.
Scavenging of hydroxyl radical
Extracts of different concentrations prepared in 2% alcohol were taken in different test tubes and evaporated on a water bath. To these, 1 mL of Iron-EDTA solution (Iron was added as 2.0 mg Fe (FeSO4) mixed with Na2EDTA as an aqueous solution in a 1:1 molar ratio), 0.5 mL of 0.018% EDTA and 1 mL of DMSO (0.85% v/v in 0.1 M phosphate buffer, pH 7.4) were added and the reaction was initiated by adding 0.5 mL of 0.22% ascorbic acid to each of the test tubes. Test tubes were tightly capped and heated on water bath at 80°-90°C for 15 min. The reaction was terminated by addition of 1 mL of ice-cold trichloroacetic acid (17.5% w/v), kept aside for 2 min and the formaldehyde formed was determined by adding 3 mL of Nash reagent (75 g of ammonium acetate, 3 mL glacial acetic acid, 2 mL acetyl acetone in to 1 L distilled water) after 10-15 min for color development (16). Intensity of yellow color formed was spectrophotometrically measured at 412 nm against reagent blank and the scavenging percentage of hydroxyl radical was calculated by comparing the results of the samples with that of the blank (17).
Estimation of phenolic content
Phenolics compounds are a large, heterogeneous group of secondary plant metabolites that are widespread in plant kingdom (18). Phenolics contain different chemical classes tannins, catechins, flavonoids, steroids, etc. The phenolic content is estimated by various methods. These are Folin Ciocalteu method (19), chromatographic response function (HPLC) (20), reversed phase HPLC (21), protein dye binding (22). Considering the available facilities, Folin- Ciocalteu method was preferred to determine the phenolic content of the extracts.
Preparation of sample
100 mg of extract was added into 40 mL ethanol and then was mixed and sonicated for about 30 min and shaked about 10 min. The volume was made up to 100 mL with HPLC grade water. Mixed well, this solution was filtered with No.1 Whatman paper. An aliquot of this solution was mixed with 0.5 mL of Folin-Ciocalteu phenol reagent. After 5 min, 1.5 mL of 20% sodium carbonate solution was added and the volume was made up to 10 mL with HPLC grade water. After 2 h, the solution was filtered with No.1 Whatman paper and the absorbance at 760 nm was recorded. The same solution without the extract solution was used as blank solution. The blank was similarly prepared without using any extract. The standard solutions were prepared and analyzed by the same manner using 20 mg of accurately weighted gallic acid. The same solution without gallic acid was used as the blank solution.
Calculation of phenolic content
Calculation of content of total phenols in percent was based on gallic acid standard.
A= Absorbance
W= Weight
Results and Discussions
The anti-oxidant activity of Petroleum ether (60:80), cyclohexane, benzene, ethyl acetate, chloroform, acetone, methanol and aqueous extracts of C. speciosus were measured by different methods including DPPH scavenging activity, total antioxidant capacity, scavenging of nitric oxide radical, ion chelating activity, scavenging of hydroxyl radical. The aim was to evaluate the activity and mechanism involved therein. The results obtained by these studies were mentioned in Table 1.
Table 1.
IC 50 μg/mL values obtained from different experiments
| Extracts | DPPH activity | Total antioxidant Capacity | Nitric oxide activity | Ion chelating activity | Hydroxyl radical activity |
|---|---|---|---|---|---|
| Petroleum Ether | 2.42 ±0.05*** | 0.17±0.20*** | 1.60±0.53*** | 2.09±0.86*** | 2.93±0.45* |
| Cyclohexane | 2.65±0.05*** | 0.71±0.1*** | 1.89±0.29*** | 2.40±0.53*** | 2.87±0.58* |
| Benzene | 15.30±0.1* | 12.58±0.75** | 12.56±0.61* | 13.41±0.68* | 13.46±1.00** |
| Ethyl acetate | 3.52±0.36*** | 2.17±0.46*** | 3.54±0.70*** | 2.89±0.65*** | 2.92±0.53* |
| Chloroform | 7.75±0.23*** | 2.62±0.75*** | 5.96±0.88*** | 4.49±0.51*** | 3.56±0.59* |
| Acetone | 6.48±0.41*** | 3.17±0.69*** | 1.86±0.96*** | 8.78±1.22** | 3.1±0.35* |
| Methanol | 14.26±0.88*** | 13.43±0.61* | 20.01±1.76*** | 12.34±1.00*** | 10.06±1.12* |
| Aqueous | 8.95±0.45*** | 12.07±0.88*** | 8.8±0.96** | 11.43±0.88*** | 8.33±0.87* |
| Standard (Ascorbic acid) | 16.14±0.20 | 15.08±0.87 | 12.54±0.8 | 6.9±0.93 | 4.63±0.67 |
Values are in the Mean ± S.D., n = 3 for each experiment, data were analyzed by one way ANOVA followed by Turkey test using Graph pad Instat software, * P > 0.05, ** P > 0.01, *** P < 0.001 as compared with standard ascorbic acid
According to DPPH assay, benzene extract showed maximum activity with IC50 = 15.30 μg/mL and no significant change when compared with standard ascorbic acid having IC50 = 16.14 μg/mL. DPPH is relatively stable free radical.The assay based on measurement of scaveinging ability of antioxidants towards stable DPPH radical. From the present result it may be postulated that C. speciosus reduces the radical when it react with hydrogen donars in antioxidant principles. DPPH radicals react with suitable reducing agents, the electrons become paired off and solution looses colour stochiometrically depending on the number of electrons taken up (23). It revealed that benzene extract have maximum antioxidant activity when compared with standard antioxidant ascorbic acid in DPPH assay.
In the Total Antioxidant Capacity experiment it was seen that Methanol and Benzene extract showed maximum activity with IC50 values 13.43 ± 0.61 and 12.58, respectively. The total antioxidant capacities of extracts were calculated based on the formation of phosphomolybdenum complex that was measured spectrophotometrically at 695 nm.
Nitric oxide radical scavenging activity was maximum to benzene extract with IC50 = 12.56 μg/mL. In the experiment of ion chelating activity benzene extract showed maximum activity with IC50 = 13.41 μg/mL as compared with ascorbic acid IC50 = 6.9 μg/mL. In the assay of hydroxyl radical activity benzene extract showed maximum activity with IC50 = 13.46 μg/mL compared with 4.63 μg/mL of ascorbic acid. Nitric oxide is free radical produced in biological cells, involved in regulation of various physiological processes. Nitric oxide is very unstable species under aerobic conditions. It reacts with oxygen to produce stable product nitrate and nitrite through intermediates NO2, N2O4 and N3O4. In the present study, nitrite produced by incubation of solution of sodium nitroprusside in standard phosphate saline buffer at 25°C was reduced by extracts of C. speciosus. This may be due to antioxidant principles in extracts which compete with oxygen to react with nitric oxides and thus inhibit generation of nitrite (24).
Ortho substituted phenolic compounds may exert prooxidant effect by interacting with iron.
O-phenthroline quantitatively forms complex with Fe2+ which get disrupted in the presence of chelating agent (25). The studied extract interfered with the formation of ferrous-o-phenanthroline complex, thereby suggesting that extract has metal chelating activity.
Hydroxy radical scavenging activities of extracts were assayed by generating hydroxyl radicals using ascorbic acid-iron-EDTA (26). The hydroxyl radical formed by the oxidation react with DMSO to yield formaldehyde. The formaldehyde production from DMSO provides a convenient method to detect hydroxyl radicals formed during oxidation of DMSO by Fe3+/ ascorbic acid system which was used to detect hydroxyl radical.
The total obtained phenolic content is shown in Table 2.
Table 2.
Total phenolic content in different extracts of C.speciosus
| Extract | % Phenolic Content |
|---|---|
| Petroleum ether (60:80) | 0.62 |
| Cyclohexane | 0.66 |
| Benzene | 4.38 |
| Ethyl acetate | 1.50 |
| Chloroform | 1.00 |
| Acetone | 0.66 |
| Methanol | 1.14 |
| Aqueous | 0.52 |
% Phenolic Content represents Gallic Acid Equivalents
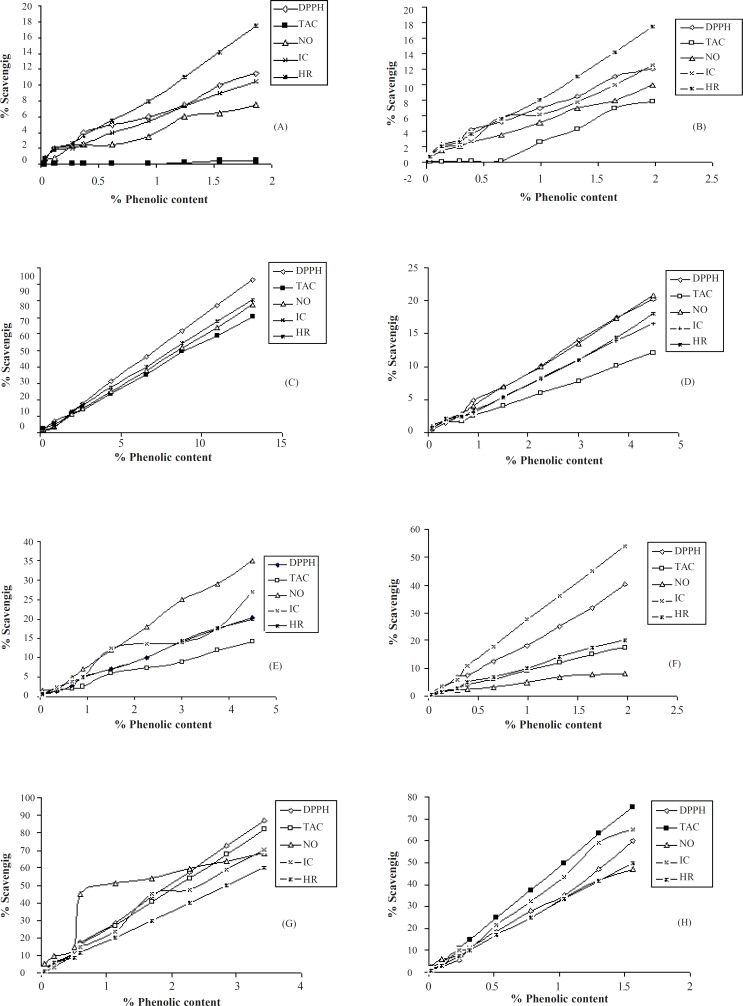
It was revealed that benzene extract had the maximum phenolic content 4.38 %The correlation coefficients for phenolic content and antioxidant activity of different extracts were studied as given in Figure 1 and it was found that benzene extract showed good correlation coefficient (r2) for all antioxidant methods. Among all the extracts analyzed, a significant phenolic content and antioxidant activity were found for benzene extract so it can be predicted that the antioxidant activity may be due to the total phenolic content in the plant. Previously it was revealed that the antioxidant activity of phenolic is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers (27). This is the first report on the antioxidant activity of Costus speciosus extracts and the activity may be due to redox properties of the phenolic content.
Figure 1.
Correlation of antioxidant activity by different methods with phenolic content in (A) Petroleum ether, (B) Cyclohexane, (C) Benzene, (D)Ethyl acetate, (E) Chloroform, (F) Acetone, (G)Methanol, (H)Aqueous Extracts
Acknowledgment
The authors are thankful to Dr. H. N. More, Principal, Bharati Vidyapeeth College of Pharmacy, Kolhapur, Maharashtra, India for providing facilities to carry out this work.
Values are in the Mean ± S.D., n = 3 for each experiment, data were analyzed by one way ANOVA followed by Turkey test using Graph pad Instat software, * P > 0.05, ** P > 0.01, *** P < 0.001 as compared with standard ascorbic acid.
References
- 1.Sarin YK, Bedi KL, Atal CK. Costus speciosus rhizome as source of diosgenin. Curr. Sci. 1974;43:569–570. [Google Scholar]
- 2.Halliwell B. Antioxidants and human diseases: a general introduction. Nutr. Rev. 1997;55:S44–S52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SM. Toxicological aspects of antioxidants used as food additives. In: Hudson BJF, editor. Food Antioxidants. London: Elsevier; 1990. pp. 253–307. [Google Scholar]
- 4.Prakash A. Medallion Laboratories Analytical Progress. Minneapolis : Plymouth Ave North; 2001. pp. 1–4. [Google Scholar]
- 5.Olga A, Olena AK, Natalia AL, Valentina NB. A new test method for the evaluation of total antioxidant activity of herbal products. J. Agric. Food Chem. 2004;52:21–25. doi: 10.1021/jf0343480. [DOI] [PubMed] [Google Scholar]
- 6.Stephanson CJ, Stephanson AM, Flanagan GP. Evaluation of hydroxyl radical-scavenging abilities of silica hydride, an antioxidant compound, by a Fe2+-EDTA-induced 2-hydroxyterephthalate fluorometric analysis. J. Med. Food. 2003;6:249–253. doi: 10.1089/10966200360716661. [DOI] [PubMed] [Google Scholar]
- 7.Hou WC, Lin RD, Lee TH, Huang YH, Hsu FL, Lee MH. The phenolic constituents and free radical scavenging activities of Gynura formosana Kiamnra. J. Sci. Food Agri. 2004;85:615–621. [Google Scholar]
- 8.Strube M, Dragstedt LO, Larsen JC. Naturally Occurring Antitumourigens. I. Plant Phenols. Copenhagen: The Nordic Council of Ministers; 1993. pp. 39–40. [Google Scholar]
- 9.Larson RA. The antioxidants of higher plants. Phytochem. 1988;27:969–978. [Google Scholar]
- 10.Sreejayan N, Rao MNA. Free radical scavenging activity by curcuminoids. Drug Res. 1996;46:169–171. [PubMed] [Google Scholar]
- 11.Prasanth Kumar V, Shasidhara S, Kumar MM, Sridhara BY. Effect of Luffa echinta on lipid peroxidation and free radical scavenging activity. J. Pharm. Pharmacol. 2000;52:891–894. doi: 10.1211/0022357001774589. [DOI] [PubMed] [Google Scholar]
- 12.Shirwaikar A, Govindrajan R, Rastogi S, Vijaykumar M, Rawat AKS, Ehlotra SM, Puspangandan P. Studies on the Antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003;26:1424. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate and 15N in biological fluids. Anal. Biochem. 1982;239:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Benzie IFF, Strain JT. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 15.Benzie IF, Szeto YT. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999;47:633–636. doi: 10.1021/jf9807768. [DOI] [PubMed] [Google Scholar]
- 16.Bhavani B, Pogozelski WK, Tullius TD. DNA strand breaking by hydroxyl radical is governed by the accessible surface area of the hydrogen atoms of the DNA backbones. Pro. National Acad. Sci. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating system. Biochem. 1991;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- 18.Peter BK, Leland JC, Sara W, James AD, Harry LB. Natural Product from Plants. New York : CRC Press; 1999. p. 102. [Google Scholar]
- 19.Mc Donalds, Prenzler PD, Autolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–74. [Google Scholar]
- 20.Martin KL, Choisnard CE, Lamien AM, Wouessidjewe D, Nacoulma OG. Experimental design optimization for screening relevant free phenolic acid from various preparation used in Burkina Faso folk medicine. Afr. J. Trad. CAM. 2006;3:115–128. [Google Scholar]
- 21.Cheryld LE, David PM. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chem. 1999;76:902–906. [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Blois MS. Antioxidant dtermination by the use of stable free radicals. Nature. 1958;26:1199–1203. [Google Scholar]
- 24.Sainani GS, Manika JS, Sainani RG. Oxidative stress: a key factor in pathogenesis of chronic diseases. Med Uptake. 1997;1:1–4. [Google Scholar]
- 25.Marcocci L, Packer L, Droy-Lefaiz MT, Sekaki A, Gardes-Albert M. Antioxidant action of Gingko biloba extract. Meth. Enzymol. 1994;234:462–465. doi: 10.1016/0076-6879(94)34117-6. [DOI] [PubMed] [Google Scholar]
- 26.Murthy KNC, Singh RP, Jayprakasha GK. Antioxidant activity of Vitis vinifera (Grapes) J. Agric. Food Chem. 2002;50:5909–5912. doi: 10.1021/jf0257042. [DOI] [PubMed] [Google Scholar]
- 27.Macheix JJ, Fleuriet A. Phenolic acids in fruits. In: Rice-Evans CA, Packer L, editors. Flavonoids in Health and Disease. New York: Marcel Dekker Inc; 1998. pp. 35–59. [Google Scholar]