Abstract
Family twin and adoption studies have noted the heritability of specific biological factors that influence suicidal behaviour. Exposure to stress is one of the factors that strongly contribute to suicide attempts. The biological response to stress involves the hypothalamic-pituitary-adrenal axis (HPA). Therefore, we found it interesting to study polymorphisms of genes involved in the HPA axis (CRHR1, NR3C1, and AVPBR1). The study was performed on 597 patients, 225 of whom had a history of suicide attempts. We did not observe any significant differences in the studied polymorphisms between the group of patients with a history of suicide attempts and the control subjects. Our haplotype analysis of the AVPR1b gene revealed an association between the GCA haplotype and suicide attempts; however, this association was not significant after correcting for multiple testing. We did not observe any other association in haplotype and MDR analysis. We report here a comprehensive analysis of the HPA axis genes and a lack of association for genetic variations regarding the risk of suicide attempts in affective disorder patients. Nonetheless, the inconsistencies with the previously published results indicate the importance of the further investigation of these polymorphisms with respect to the risk of suicide attempts.
1. Introduction
According to the WHO, approximately 1 million people commit suicide each year. Therefore, suicide is considered a major public health problem. The causes of suicidal behaviour and suicide attempts are complex in that both genetic and environmental factors can play a role. Exposure to an acute or chronic stress accompanied by psychiatric conditions, including substance abuse, depressed mood, anxiety, and also psychotic features, can lead to suicide attempts.
Family twin and adoption studies have noted the heritability of these features, suggesting that there is a specific biological influence on suicidal behaviour. Genes containing different variants, interindividual differences in psychology, and related stress resilience may cause a vulnerability to suicidal behaviour [1], as well as age, sex and the history of the trauma. A majority of the genetic studies have involved serotonin-related genes because levels of serotonin and its metabolites are thought to play a role in suicidal behaviour. Meta-analyses have confirmed an association between suicidal behaviour and 5HTT, (serotonin transporter), tryptophan hydroxylase (TPH1), and brain-derived neurotropic factor (BDNF) expression, as well as the expression of tyrosine kinase receptor type 2 (NTRK2) [2, 3].
The biological response to stress involves the hypothalamic-pituitary-adrenal axis (HPA), and prolonged stress may change its function. These changes can be marked by altered HPA activity (high or low cortisol levels) or reactivity (reduced or increased feedback regulation). These HPA disturbances are also thought to be partially predictive of suicidal behaviour [4–6] and are regarded as an endophenotype of suicidal behaviour [5]. In particular, AVP receptor upregulation may be critical for sustaining corticotropic responsiveness during chronic stress or depression [7]. Jokinen et al. suggested that dexamethasone suppression test (DST) nonsuppression may be a biological suicide predictor in depressed male suicide attempters [8, 9]. Those patients may be genetically predisposed to react with high levels of HPA activity in response to a low stress and may experience a chronic state of HPA activation [10, 11]. This reaction occurs most likely via altered CRHR1 (the gene controlling activation of HPA axis) expression and functionality. Recently, Merali and colleagues found that CRHR1 mRNA expression was changed in the frontal cortices of suicide victims [12]. Hyperactivity of the HPA axis predicts a worse treatment outcome [13], and Binder et al. suggested that dysregulation of the HPA axis may even predict a nonresponse to antidepressant treatment particularly among males [14]. Both low CSF5-HIAA and DST nonsuppression contribute to a fourfold increased suicide risk in depressed subjects in a meta-analysis performed by Mann and Currier [5]. Some studies have reported no association between the DST test and individual susceptibility to suicide [15]. McGowan et al. observed epigenetic modification of the glucocorticoid receptor gene as a function of childhood abuse in the brains of suicide completers [16]. Thus, it is possible that HPA axis dysfunction is not linearly related to suicide risk [17]. Therefore, it would be interesting to study the polymorphisms of genes encoding receptors and transporters involved in the regulation of HPA axis function. Taking into account the limited number of genetic studies that have been published previously on the association between HPA axis genes and the history of suicide attempts, we aimed in the present study to investigate the possible association between NR3C1, CRHR1, and AVPR1b gene polymorphisms and suicidal behaviour in unipolar (UP) and bipolar (BP) patients.
2. Materials and Methods
2.1. Patients
We included 597 patients (367 female, 230 male), aged 18–84 (mean = 47, SD = 14) who met DSM-IV criteria for bipolar disorder (391 BPI, 104 BPII) or recurrent depression (n = 102) and were living in the Wielkopolska region of Poland. The diagnosis was established using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) [18]. Among patients with BP disorder, 197 people had a history of suicide attempt(s); among the UP patients, 28 people had a history of suicide attempt(s). The range of duration for disease in our group was 1–54 years (mean 15 years, SD = 11). Informed consent was obtained from each patient after the nature of the procedure had been fully explained to them.
2.2. Control Group
The control group consisted of 712 subjects. Control subjects were recruited from a group of healthy volunteers, blood donors and hospital staff, and students of the University of Medical Sciences in Poznan and the Clinical Neuropsychology Unit, Collegium Medicum Bydgoszcz. Only 40% of the control group were psychiatrically screened using the Polish version of the M.I.N.I. Plus scale to exclude individuals with any serious mental health problems. The mean age was 37.7, SD = 12.5. The control group was matched with age and sex. The study was approved by our local ethics committee.
2.3. Genotyping
DNA was extracted from using the salting out method [19]. SNP selection was carried out under the following criteria: functionality (in experimental functional studies), high frequency (MAF > 0.05), indication as a tag SNP in HapMap, or previously reported associations with psychiatric disorders (both positive and negative findings). The SNPs chosen included both coding regions of known functionality and noncoding regions (introns, UTRs) that could affect gene regulation. The NR3C1, CRHR1, and AVPR1b polymorphisms were genotyped using TaqMan SNP Genotyping assays (Applied Biosystems) and TaqMan Genotyping Master Mix. The list of SNPs analysed and the ID numbers of the TaqMan assays were used according to previously described findings [20]. For each reaction plate, nontemplate controls (water) and genomic control DNA samples were included. To check for genotyping accuracy control TaqMan SNP genotyping assay was performed, 15% of randomly chosen samples from both groups, and identical genotypes were identified in all repeated samples. The clinical status of the subjects was not known during genotyping. The genotyping success rates were between 96.03% and 98.97%. The total number of genotyped patients differs on the different SNPs due to genotyping errors and therefore exclusions from further studies.
2.4. Statistical Analysis
Two-tailed Pearson's chi-squared (χ 2) test and Fisher's exact test were, respectively, used to test differences in the genotypic and allelic distribution between the groups of patients and the control subjects. Two-tailed power analysis was also performed. Calculations were performed using Statistica version 9.0. Odds ratios were calculated using 2 × 2 contingency tables by using Fisher's exact test in a demo version of GraphPad InStat 3 software. Linkage disequilibrium (LD) between the CRHR1, AVPR1b, and NR3C1 polymorphisms was examined by pairwise comparisons of r 2 and D′ using Haploview version 4.1 [21]. Corrections for multiple testing were performed for multiple comparisons in the haplotype analysis and were completed for 10,000 permutations.
Higher-order gene-gene interactions among the tested SNPs were analysed using the nonparametric and genetic model-free multifactor dimensionality reduction (MDR) approach (v.2.0 beta 8.3). All interactions were tested using 10-fold cross-validation in an exhaustive search considering all possible SNP combinations. The model with the highest testing balance accuracy and cross-validation consistency of >5 out of 10 was selected as the “best model.” Statistical significance was determined using a 1000-fold permutation test (MDR permutation testing module, v.1.0 beta 2). The software is available online (http://www.epistasis.org/).
The power to detect an association for an odds ratio of 1.5 for our sample was about 80% for each SNP.
3. Results
3.1. HWE Analysis
The genotype distributions were in Hardy-Weinberg equilibrium, except the following polymorphisms: rs16940655 of CRHR1, rs10052957, and rs258813 of NR3C1, and rs28632197 of AVPBR1 in the group of the patients and the control group. We did not include those polymorphisms into the further analysis.
3.2. Association Analysis
We did not observe any significant differences in the studied polymorphisms between the group of patients with a history of suicide attempts and the control subjects or in genotype distribution (results shown in Tables 1, 2, and 3, 0 = controls, 1 = suicidal patients) or allele frequencies (data not shown). Analysis stratified by gender also did not reveal any statistically significant differences between patients with suicide attempts and the control group (data not shown). We also compared the bipolar patients with the history of suicide attempts versus bipolar patients without suicide attempts in the past, but no significance was found between the two groups (data not shown).
Table 1.
Polymorphisms in GR (NR3C1) gene (genotypes).
| rs41423247 | CC | CG | GG | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0 | 65 | 13.03 | 239 | 47.9 | 195 | 39.8 | 0.55 |
| 1 | 27 | 13.5 | 87 | 43.5 | 86 | 43 | |
|
| |||||||
| rs6195 | CC | CT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 450 | 88.93 | 56 | 11.07 | 0 | 0 | 0.28 |
| 1 | 177 | 88.5 | 22 | 11.0 | 1 | 0.5 | |
|
| |||||||
| rs6198 | CC | CT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 14 | 2.8 | 116 | 23.2 | 370 | 74 | 0.33 |
| 1 | 3 | 1.53 | 54 | 27.55 | 139 | 70.92 | |
|
| |||||||
| rs6191 | AA | AC | CC | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 136 | 27.15 | 244 | 48.7 | 121 | 24.15 | 0.72 |
| 1 | 57 | 29.38 | 88 | 45.36 | 49 | 25.26 | |
|
| |||||||
| rs6196 | AA | AG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 366 | 72.05 | 129 | 25.39 | 13 | 2.56 | 0.2 |
| 1 | 146 | 73 | 53 | 26.5 | 1 | 0.5 | |
|
| |||||||
| rs33388 | AA | AT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 138 | 27.27 | 245 | 48.42 | 123 | 24.31 | 0.84 |
| 1 | 58 | 29.15 | 92 | 46.23 | 49 | 24.62 | |
0: control. 1: suicidal patients.
Table 2.
Polymorphisms in AVPR1b gene (genotypes).
| rs28536160 | CC | CT | TT | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0 | 2 | 0.4 | 73 | 14.48 | 429 | 85.12 | |
| 1 | 0 | 0 | 22 | 11 | 178 | 89 | 0.31 |
|
| |||||||
| rs28373064 | AA | AG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 349 | 68.84 | 139 | 27.42 | 19 | 3.75 | |
| 1 | 133 | 66.5 | 60 | 30 | 7 | 3.5 | 0.78 |
|
| |||||||
| rs35369693 | CC | CG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 1 | 0.2 | 51 | 10.39 | 439 | 89.41 | 0.09 |
| 1 | 0 | 0 | 31 | 16.15 | 161 | 83.85 | |
0: control. 1: suicidal patients.
Table 3.
Polymorphisms of CRHR1 gene (genotypes).
| rs4076452 | CC | CG | GG | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0 | 11 | 2.54 | 142 | 32.79 | 280 | 64.67 | 0.48 |
| 1 | 5 | 3.09 | 45 | 27.78 | 112 | 69.14 | |
|
| |||||||
| rs12936511 | CC | CT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 394 | 91.8 | 34 | 7.93 | 1 | 0.23 | |
| 1 | 149 | 91.98 | 12 | 7.41 | 1 | 0.62 | 0.75 |
|
| |||||||
| rs4792887 | CC | CT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 332 | 77.2 | 94 | 21.86 | 4 | 0.93 | |
| 1 | 130 | 79.75 | 32 | 19.63 | 1 | 0.61 | 0.77 |
|
| |||||||
| rs242950 | CC | CT | TT | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 325 | 75.4 | 99 | 22.97 | 7 | 1.62 | 0.81 |
| 1 | 126 | 77.78 | 34 | 20.99 | 2 | 1.23 | |
|
| |||||||
| rs878886 | CC | CG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 304 | 70.2 | 118 | 27.25 | 11 | 2.5 | 0.5 |
| 1 | 106 | 65.4 | 51 | 31.48 | 5 | 3.09 | |
|
| |||||||
| rs173365 | AA | AG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 78 | 18.01 | 206 | 47.58 | 149 | 34.41 | 0.9 |
| 1 | 28 | 17.18 | 77 | 47.24 | 58 | 35.58 | |
|
| |||||||
| rs110402 | AA | AG | GG | P | |||
| n | % | n | % | n | % | ||
|
| |||||||
| 0 | 98 | 22.9 | 216 | 50.47 | 114 | 26.64 | |
| 1 | 39 | 24.07 | 87 | 53.7 | 36 | 22.22 | 0.5 |
0: control. 1: suicidal patients.
3.3. Haplotype Analysis
Linkage disequilibrium analysis of the three analysed genes revealed strong linkage between the polymorphisms. For the NR3C1 gene, we observed linkage for 5 out of 8 studied polymorphisms grouped in one haplotype block (D′ = 1.0, LOD = 39.11, r 2 = 0.162) (Figure 1).
Figure 1.
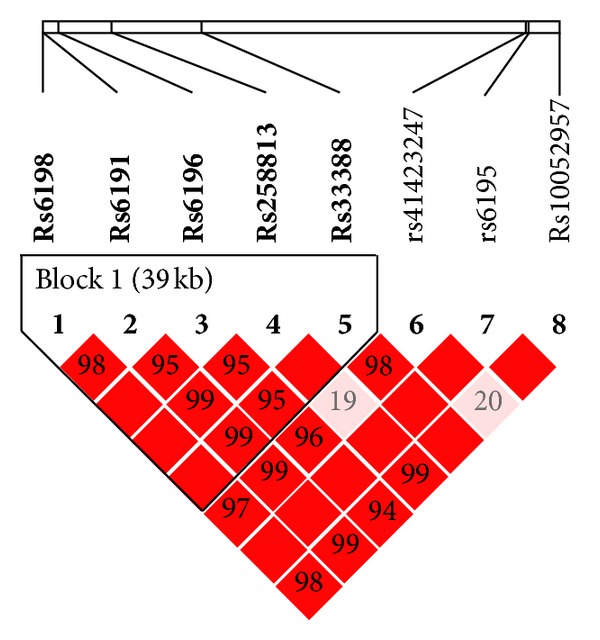
Relative positions and LD estimates between NR3C1 polymorphisms in the analyzed population. Coloured squares correspond to D′ values with numerical estimates given within the squares.
However, none of the haplotypes were significantly more frequent in the group of patients with suicide attempts compared to the control group (Table 4).
Table 4.
Comparison of haplotype frequencies for the analysed NR3C1 polymorphisms between patients with suicide attempts and the control group.
| Haplotype | Haplotype frequency | Case : control ratio | χ 2 | P |
|---|---|---|---|---|
| Block 1 | ||||
| TCAGT | 0.478 | 0.475 : 0.479 | 0.015 | 0.901 |
| TAAGA | 0.222 | 0.218 : 0.224 | 0.072 | 0.788 |
| CAAAA | 0.150 | 0.170 : 0.142 | 1.711 | 0.190 |
| TAGAA | 0.144 | 0.135 : 0.147 | 0.332 | 0.564 |
For the CRHR1 gene, we observed linkage in 6 out of 7 polymorphisms grouped in two haplotype blocks: rs4076452, rs4792887, are rs110402 in one block (D′ = 1.0, LOD = 37.66, r 2 = 0.216) and rs173365, rs242950, and rs878886 in the other block (D′ = 1.0, LOD = 54.9, r 2 = 0.285) (Figure 2).
Figure 2.
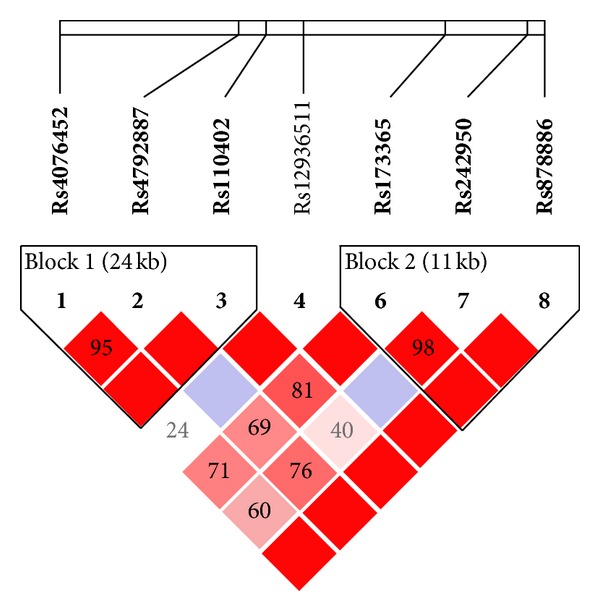
Relative positions and LD estimates between AVPR1b polymorphisms in the analyzed population. Coloured squares correspond to D′ values with numerical estimates given within the squares.
Our haplotype analysis did not reveal any significant differences in the haplotype frequencies of the two blocks between patients with suicide attempts and control subjects (Table 5).
Table 5.
Comparison of haplotype frequencies for the analysed CRHR1 polymorphisms between patients with suicide attempts and the control group.
| Haplotype | Haplotype frequency | Case : control ratio | χ 2 | P |
|---|---|---|---|---|
| Block 1 | ||||
| GCA | 0.489 | 0.510 : 0.481 | 0.755 | 0.385 |
| GCG | 0.323 | 0.321 : 0.323 | 0.0050 | 0.943 |
| CTG | 0.110 | 0.104 : 0.112 | 0.166 | 0.683 |
| CCG | 0.074 | 0.065 : 0.077 | 0.487 | 0.485 |
|
| ||||
| Block 2 | ||||
| GCC | 0.584 | 0.592 : 0.581 | 0.118 | 0.731 |
| ACG | 0.169 | 0.187 : 0.162 | 1.061 | 0.303 |
| ATC | 0.127 | 0.118 : 0.130 | 0.312 | 0.576 |
| ACC | 0.120 | 0.103 : 0.127 | 1.29 | 0.256 |
For the AVPR1b gene, we observed linkage disequilibrium for 3 out of 4 analysed polymorphisms grouped in one haplotype block (D′ = 0.98, LOD = 45.56, r 2 = 0.302) (Figure 3).
Figure 3.
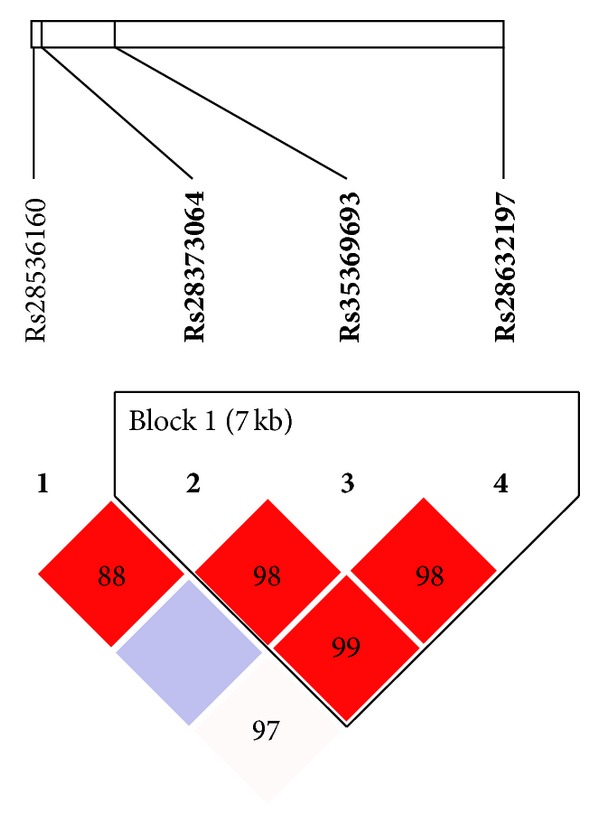
Relative positions and LD estimates between CRHR1 polymorphisms in the analyzed population. Coloured squares correspond to D′ values with numerical estimates given within the squares.
In our haplotype analysis, we observed an association between the GCA haplotype and suicide attempts; however, this association was not significant after correction for multiple testing (P value of 0.130 after 10,000 permutations) (Table 6).
Table 6.
Comparison of haplotype frequencies for the analysed AVPR1b polymorphisms between patients with suicide attempts and the control group.
| Haplotype | Haplotype frequency | Case : control ratio | χ 2 | P |
|---|---|---|---|---|
| Block 1 | ||||
| AGG | 0.823 | 0.814 : 0.826 | 0.291 | 0.589 |
| GGG | 0.076 | 0.068 : 0.079 | 0.547 | 0.459 |
| GCA | 0.060 | 0.081 : 0.052 | 4.087 | 0.043* |
| GGA | 0.041 | 0.037 : 0.042 | 0.162 | 0.687 |
*Not significant after correction for multiple test using 10.000 permutations (P = 0.130).
3.4. Gene × Gene Interaction Analysis
The results of our exhaustive MDR analysis evaluating combinations of all tested SNPs are summarised in Table 7.
Table 7.
Multilocus interaction model for the risk of suicide attempts with the NR3C1. AVPR1b and CRHR1 genes by the MDR method.
| Model | Loci combination | Testing balanced accuracy (%) | Cross-validation consistency (%) | P value |
|---|---|---|---|---|
| 2-locus | Rs258813 of NR3C1 and rs110402 of CRHR1 | 47 | 30 | 0.989 |
| 3-locus | Rs41423247 and rs258813 of NR3C1 and rs110402 of CRHR1 | 52 | 50 | 0.760 |
| 4-locus | Rs41423247 and rs258813 of NR3C1. rs28373064 of AVPR1b and rs110402 of CRHR1 | 50 | 90 | 0.589 |
The best combination of possibly interactive polymorphisms in predicting suicidal attempts was observed in the 4-locus model. However, no significance was observed for this combination (the testing balanced accuracy for this 4-locus model was 90%, cross-validation consistency was 5/10 (50%), and an empiric P value of 0.589 is based on 1000-fold permutations). Similarly, other combinations of the analysed polymorphisms also did not reach statistical significance in predicting susceptibility to an increased risk of suicide attempts.
4. Discussion
The results of our study suggested a possible association between the haplotype of the AVPR1b gene and suicide attempts, which did not reach significance after multiple testing correction. We did not find any interactions for genotypes or for the alleles of the studied polymorphisms in the group of suicidal patients. Also Dempster et al. suggested the involvement of the AVPR1b gene polymorphisms in the etiology of childhood onset mood disorders particularly in females [22]. Ben-Efraim et al. found association between the high Beck scale results in patients with suicide attempts and rs33990840 and a major 6-SNP haplotype of AVPR1b gene [23]. Previously, we reported the association between the rs28536160 polymorphism of the AVPR1b gene and rs1293651 of the CRHR1 gene and bipolar patients with psychotic features [20]. In this study, we did not find association between polymorphisms of CRHR1 gene and suicidal patients although Wasserman et al. identified a CRHR1 SNP that showed an association in the suicide attempters exposed to low-medium stress and a relationship between neurotic personality traits and suicidality. The genetic variation in the TBX19 gene (a regulator of the HPA axis) was observed by Wasserman et al. [24, 25]. In suicidal males, they found an association between the T and A alleles of SNPs rs4792287 and rs110402 and the T allele of rs12936511 of the CRHR1 gene. Polymorphisms in the CRHR1 gene have also been associated with depression and the treatment efficiency of depression [26–29]. Papiol et al. showed associations of the A allele of the CRHR1 rs110402 SNP with hte age of onset and with seasonal pattern of major depression [29]. Bradley et al. reported that the T and G alleles of the rs4792887 and rs110402 CRHR1 SNPs were linked with depression [26]. In the study performed by De Luca et al. an association between haplotype variation at the CRHR2 locus and suicidal behaviour was observed [30]. Interestingly, they also found interactions between polymorphisms of CRHR1 gene in suicidal schizophrenic patients [31]. We did not find an association between the polymorphisms of the NR3C1, CRHR1, and AVPBR1 genes and the whole group of affective disorder patients (data not published). However, in the study performed by Szczepankiewicz et al. [32], an association between NR3C1 polymorphisms and depression was found.
The main limitations of our study that might have yielded false-positive or- negative results include the following: the limited sample size of the patient group (n = 225), the fact that only 40% of the control group had been screened for psychiatric disorders, and the lack of Hardy-Weinberg equilibrium for the four analysed SNPs. However, taking into consideration the lifetime prevalence of psychiatric disorders and the power of the studied polymorphisms (>80%), it seems unlikely that these factors affected our results.
5. Conclusions
We report here a comprehensive analysis of HPA axis genes and a lack of association of genetic variation with the risk of suicide attempts in affective disorder patients. Nonetheless, the inconsistencies with the previously published associations indicate the importance of further investigation of those polymorphisms with respect to the risk of suicide attempts.
Conflict of Interests
The authors report no potential conflict of interests.
Acknowledgment
This study was supported by the Ministry of Science and Higher Education Grants nos. IP 2011053771 and 2011/01/B/NZ5/02795.
References
- 1.Wasserman D, Wasserman J, Sokolowski M. Genetics of HPA-axis, depression and suicidality. European Psychiatry. 2010;25(5):278–280. doi: 10.1016/j.eurpsy.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 2.McGuffin P, Perroud N, Uher R, et al. The genetics of affective disorder and suicide. European Psychiatry. 2010;25(5):275–277. doi: 10.1016/j.eurpsy.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Pawlak J, Dmitrzak-Węglarz M, Skibinska M, et al. Association between suicidal behavior and genes of serotonergic system in affective disorders. in press Nowiny Lekarskie, 2013.
- 4.Coryell W, Young E, Carroll B. Hyperactivity of the hypothalamic-pituitary-adrenal axis and mortality in major depressive disorder. Psychiatry Research. 2006;142(1):99–104. doi: 10.1016/j.psychres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Mann JJ, Currier D. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Archives of Suicide Research. 2007;11(1):3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- 6.Pfennig A, Kunzel HE, Kern N, et al. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biological Psychiatry. 2005;57(4):336–342. doi: 10.1016/j.biopsych.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Pompili M, Serafini G, Innamorati M, et al. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: a selective overview for the implications of suicide prevention. European Archives of Psychiatry and Clinical Neuroscience. 2010;260(8):583–600. doi: 10.1007/s00406-010-0108-z. [DOI] [PubMed] [Google Scholar]
- 8.Jokinen J, Mårtensson B, Nordström AL, Nordström P. CSF 5-HIAA and DST non-suppression-independent biomarkers in suicide attempters? Journal of Affective Disorders. 2008;105(1–3):241–245. doi: 10.1016/j.jad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Jokinen J, Nordström P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. Journal of Affective Disorders. 2009;116(1-2):117–120. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen J, Nordström A-L, Nordström P. CSF 5-HIAA and DST non-suppression: orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatry Research. 2009;165(1-2):96–102. doi: 10.1016/j.psychres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Jokinen J, Nordström P. HPA axis hyperactivity as suicide predictor in elderly mood disorder inpatients. Psychoneuroendocrinology. 2008;33(10):1387–1393. doi: 10.1016/j.psyneuen.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABAA receptor subunits in frontal cortical brain region. Journal of Neuroscience. 2004;24(6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer JP, Appelhof BC, van Rossum EFC, et al. Prediction of treatment response by HPA-axis and glucocorticoid receptor polymorphisms in major depression. Psychoneuroendocrinology. 2006;31(10):1154–1163. doi: 10.1016/j.psyneuen.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Binder EB, Künzel HE, Nickel T, et al. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34(1):99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Pitchot W, Scantamburlo G, Pinto E, et al. Vasopressin-neurophysin and DST in major depression: relationship with suicidal behavior. Journal of Psychiatric Research. 2008;42(8):684–688. doi: 10.1016/j.jpsychires.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 16.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGirr A, Diaconu G, Berlim MT, Turecki G. Personal and family history of suicidal behaviour is associated with lower peripheral cortisol in depressed outpatients. Journal of Affective Disorders. 2011;131(1–3):368–373. doi: 10.1016/j.jad.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC, USA: American Psychiatric Press; 1996. [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3, article 1215) doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leszczyńska-Rodziewicz A, Szczepankiewicz A, Dmitrzak-Wȩglarz M, Skibińska M, Hauser J. Association between functional polymorphism of the AVPR1b gene and polymorphism rs1293651 of the CRHR1 gene and bipolar disorder with psychotic features. Journal of Affective Disorders. 2012;138(3):490–493. doi: 10.1016/j.jad.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 22.Dempster EL, Burcescu I, Wigg K, et al. Evidence of an association between the vasopressin V1b receptor gene (AVPR1B) and childhood-onset mood disorders. Archives of General Psychiatry. 2007;64(10):1189–1195. doi: 10.1001/archpsyc.64.10.1189. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Efraim YJ, Wasserman D, Wasserman J, Sokolowski M. Family-based study of AVPR1B association and interaction with stressful life events on depression and anxiety in suicide attempts. Neuropsychopharmacology. 2013;38(8):1504–1511. doi: 10.1038/npp.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes, Brain and Behavior. 2008;7(1):14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman D, Wasserman J, Rozanov V, Sokolowski M. Depression in suicidal males: genetic risk variants in the CRHR1 gene. Genes, Brain and Behavior. 2009;8(1):72–79. doi: 10.1111/j.1601-183X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 26.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licinio J, O’Kirwan F, Irizarry K, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Molecular Psychiatry. 2004;9(12):1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Zhu F, Wang G, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neuroscience Letters. 2007;414(2):155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Papiol S, Arias B, Gastó C, Gutiérrez B, Catalán R, Fañanás L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. Journal of Affective Disorders. 2007;104(1–3):83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 30.De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. European Psychiatry. 2007;22(5):282–287. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 31.De Luca V, Tharmalingam S, Zai C, et al. Association of HPA axis genes with suicidal behaviour in schizophrenia. Journal of Psychopharmacology. 2010;24(5):677–682. doi: 10.1177/0269881108097817. [DOI] [PubMed] [Google Scholar]
- 32.Szczepankiewicz A, Leszczyńska-Rodziewicz A, Pawlak J, et al. Glucocorticoid receptor polymorphism is associated with major depression and predominance of depression in the course of bipolar disorder. Journal of Affective Disorders. 2011;134(1–3):138–144. doi: 10.1016/j.jad.2011.06.020. [DOI] [PubMed] [Google Scholar]


