Abstract
In this pilot study, we prospectively compared the response of bone metastasis assessed by our MD Anderson (MDA) bone tumor response criteria (computed tomography [CT], plain radiography [XR], and skeletal scintigraphy [SS]) with the response assessed by the World Health Organization (WHO) criteria (XR and SS). Both MDA and WHO criteria predicted progression-free survival (PFS) of patients at 6 months but not at an earlier time point.
Background
In our previous study, new MD Anderson (MDA) bone tumor response criteria (based on computed tomography [CT], plain radiography [XR], and skeletal scintigraphy [SS]) predicted progression-free survival (PFS) better than did World Health Organization (WHO) bone tumor response criteria (plain radiography [XR] and SS) among patients with breast cancer and bone-only metastases. In this pilot study, we tested whether MDA criteria could reveal bone metastasis response earlier than WHO criteria in patients with newly diagnosed breast cancer with osseous and measurable nonosseous metastases.
Methods
We prospectively analyzed bone metastasis response using each imaging modality and set of bone response criteria to distinguish progressive disease (PD) from non-PD and their association with PFS and overall survival (OS). We also compared the response of osseous metastases assessed by both criteria with the response of nonosseous measurable lesions.
Results
The median follow-up period was 26.7 months (range, 6.1–53.3 months) in 29 patients. PFS rates differed at 6 months based on the classification of PD or non-PD using either set of criteria (MDA, P = .002; WHO, P = .014), but these rates, as well as OS, did not differ at 3 months. Response in osseous metastases by either set of criteria did not correlate with the response in nonosseous metastases.
Conclusion
MDA and WHO criteria predicted PFS of patients with osseous metastases at 6 months but not at an earlier time point. We plan a well-powered study to determine the role of MDA criteria in predicting bone tumor response by incorporating 18-fluorodeoxyglucose (18F) positron emission tomography (FDG-PET)/CT to see if findings using this modality are earlier than those with WHO criteria.
Keywords: one metastasis, Bone tumor response criteria, Breast cancer, Computed tomography, Plain radiography, Skeletal scintigraphy
Introduction
During the course of breast cancer, 30% to 85% of patients are diagnosed with bone metastases.1–3 The median survival duration after diagnosis of bone metastasis is 25.2 to 72 months.4–7 Serious skeletal-related events caused by bone metastasis—including fractures, spinal cord compression, and hypercalcemia—impair a patient’s quality of life.8–10 An accurate assessment of the disease condition and elimination of skeletal complications improve a patient’s quality of life.2,8,11,12 In clinical practice, the presence of bone metastases and their response to treatment are assessed by imaging studies that evaluate the secondary effect of tumor on bone. The findings from plain radiography (XR) and computed tomography (CT) depend on the density and penetration of ionizing radiation and primarily image the cortex. Skeletal scintigraphy (SS) detects areas of new hydroxyapatite deposition. However, there is no consensus as to the best use of imaging modality for evaluating bone metastases and the response to treatment of bone metastases. This is because bone metastases can be present as osteolytic (with increased bone resorption), osteoblastic (with increased bone formation), or mixed lesions with both osteolytic and osteoblastic imaging changes.13 Several systems of assessment have been proposed.
The International Union Against Cancer (UICC) criteria, which is based on XR, and the World Health Organization (WHO) criteria, which is based on XR and SS, have been considered the standard means for assessment of bone metastasis response since the 1970s. However, because they evaluate changes in bone structure rather than directly imaging the tumor, these 2 modalities can take as long as 6 months to reflect response to therapy. The WHO criteria may have higher sensitivity than the UICC criteria because of the addition of SS to XR. However, SS also may yield false-positive findings such as the “flare phenomenon.”14,15 We previously reported a retrospective study of the efficacy of the MD Anderson (MDA) criteria, which adds CT or magnetic resonance imaging (MRI) (providing cortical and medullary bone anatomy to the response assessment) to XR and SS for assessing the response of bone metastases1,16 (Table 1). In that study, we compared the MDA criteria (based on CT, XR, and SS) with the WHO criteria (based on XR and SS) in patients with breast cancer and bone-only metastases (no measurable nonosseous disease). Patients were classified as tumor responders (those with complete response [CR]17 or partial response [PR]) or nonresponders (those with stable disease [SD]18 or progressive disease [PD]) using both sets of criteria. We demonstrated that there were significant differences in PFS between responders and nonresponders using the MDA criteria, but not the WHO criteria, at 2 to 6 months (P = .025).16 Therefore we hypothesized that the MDA criteria could reveal bone metastasis response earlier than the WHO criteria in patients with newly diagnosed breast cancer with osseous and measurable nonosseous metastases.
Table 1.
The UICC, WHO, and MDA Criteria for Detection of Bone Response
| Response Type | UICCa | WHOb | Revised Criteria for Assessment of Bone Response (MDA) |
|---|---|---|---|
| Target Diagnostic Imaging | XR | XR, SS | XR, SS, CT, MRI |
| Complete Response | Disappearance of all known disease Lytic lesions should have radiologic evidence of calcification |
Complete disappearance of all lesions on XR or scan for at least 4 wk | Complete fill-in or sclerosis of lytic lesion on XR and CT Disappearance of hot spots or tumor signal on SS, CT, or MRI Normalization of osteoblastic lesion on XR and CT |
| Partial Response | At least 50% decrease in size of measurable lesions Objective improvement in evaluable or unmeasurable lesions No new lesions or progressive lesions |
Partial decrease in size of lytic lesions, recalcification of lytic lesions, or decreased density of blastic lesions for at least 4 wk | Sclerotic rim around initially lytic lesion or sclerosis of lesions previously undetected on XR or CT Partial fill-in or sclerosis of lytic lesion on XR or CT Regression of measurable lesion on XR, CT, or MRI Regression of lesion on SS (exclude rapid regressionc Decrease in blastic lesion on XR or CT |
| No Change or Stable Disease | Unchanged or between 25% increase and 50% decrease in size of measurable lesionsd | As a result of the slow response of bone lesions, the classification of “no change” should not be applied until at least 8 wk have passed from start of therapy | No change in measurable lesion on XR, CT, or MRI No change in blastic lesion on XR, CT, or MRI No new lesion on XR, SS, CT, or MRI |
| Progressive Disease | Mixed; some lesions persist while others progress or new lesions appear Failure; some or all lesions progress and/or new lesions appear; no lesions regress |
Increase in size of existing lesions or appearance of new lesions | Increase in size of any existing measurable lesions on XR, CT, or MRI New lesion on XR, SS (excluding flare phenomena), CT, or MRI Increase in activity on SS (excluding flare phenomena) or blastic/lytic lesion on XR or CT |
Abbreviations: CT = computed tomography; MDA = MD Anderson; MRI = magnetic resonance imaging; SS = skeletal scintigraphy; XR = plain radiography; UICC = Union Against Cancer; WHO = World Health Organization.
Criteria are based on plain radiography; the duration of response is to be measured from the start of therapy until either new lesions appear or any 1 existing lesion increases by 25% or more beyond its smallest recorded size.
Occurrence of bone compression or fracture and its healing should not be used as the sole indicator for evaluation of therapy.
Rapid osteolytic progression may show decreased osteoblastic activity, resulting in regression of “hot spots” on SS. XR or CT may be helpful in detecting progressive osteolysis and thus help identify progressive disease (PD) in this situation.
If lesions that cannot be measured but are otherwise evaluable represent the bulk of disease and these lesions clearly do not respond even though measurable lesions have improved, the response is considered to be “no change” rather than an “objective regression.”
Reproduced with permission from Hamaoka T, et al. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer 2010; 102:651-7.
To test this hypothesis, in this pilot study we prospectively compared the MDA and WHO criteria by stratifying patients with breast cancer with osseous and nonosseous metastases with respect to PFS and OS. We also compared the response of osseous metastases assessed by the MDA or WHO criteria with the response of nonosseous measurable lesions using Response Evaluation Criteria in Solid Tumors (RECIST) 1.019 to determine whether bone response aligns with response in other metastatic sites.
Patients and Methods
Patient Eligibility
Patients who were newly diagnosed with bone (osseous) and measurable nonosseous metastases of breast cancer and who were treated at MD Anderson Cancer Center from September 2004 to January 2009 were enrolled in this prospective clinical study. This study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center. All patients gave informed consent.
The bone metastasis was confirmed by imaging and/or bone biopsy results. Patients were eligible if they initiated systemic treatment, including chemotherapy and/or hormone therapy, for the newly diagnosed metastatic disease. Patients who had a history of radiation therapy for bone disease, a history or presence of brain/ leptomeningeal metastases, or a history of other malignancies (except cured nonmelanoma skin cancer or cured cervical carcinoma in situ) were not eligible for this study.
Assessment of Tumor Response
Diagnostic imaging was performed before the initiation of systemic therapy and at 3, 6, and 12 months after the initiation of therapy. All images (XR, SS, and CT) were reviewed for this study independently by 2 musculoskeletal radiologists who were blinded to patient identities and outcomes. Changes in the imaging characteristics of the tumors were interpreted in accordance with the guidelines provided by the respective sets of response criteria. A response was assigned for each imaging modality. In addition, response was assessed based on the WHO and MDA criteria (Table 1). CT was assessed for osseous metastases at 3 and 6 months and for nonosseous metastases at 12 months after the initiation of therapy. MRI was excluded from the analysis because a limited number of MRI scans were available; it was not standard at our institution to assess bone tumor response with MRI at the time.
Statistical Analysis
We assessed the images and differentiated between PD and non-PD (progression-free status: CR, PR, and SD); SD was grouped with CR and PR because 1 purpose of response assessment is for treatment decision making, and patients with SD usually do not change therapy. We then analyzed whether the use of a particular imaging modality or set of bone response criteria would distinguish PD from non-PD in terms of PFS or OS by using Kaplan-Meier analyses. The survival distributions of patients with PD and patients with non-PD were compared using the log-rank test. All time-to-event intervals were calculated from the date of imaging (3 and 6 months from registration) to the date of the event, including progression for PFS and death for OS, or of the last follow-up if no event occurred. For overall survival (OS) from date of diagnosis of bone metastases, the analyses were conditioned on the patients who were alive at the time of last follow-up. For PFS from the date of diagnosis of bone metastases, the analyses were conditioned on the patients who were alive and progression free by RECIST criteria at the time of last follow-up. Analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC) and S-plus, version 8.0 (Tibco Software, Palo Alto, CA) at the end of the study.
To assess which imaging modality or set of criteria most accurately reflected true bone tumor response, we assessed agreement between the response for osseous metastases assigned on the basis of imaging results (SS alone, CT alone, WHO criteria, and MDA criteria) and clinical response for measurable nonosseous metastasis (RECIST 1.0) using McNemar’s test and Cohen’s kappa coefficient test.
Results
Patient Characteristics
Thirty-seven patients were initially enrolled in this study. Eight patients were excluded from the analysis because they declined to undergo imaging during the follow-up period. The clinical characteristics of the remaining 29 patients are shown in Table 2. The median age at diagnosis of the 29 patients was 53 years (range, 30–91 years). Chemotherapy was administered in 20 patients and endocrine therapy in 9 patients. The median follow-up period was 26.7 months (range, 6.1–53.3 months). Eight patients died within 12 months of initiation of therapy. Twenty-one of the 29 patients (72.4%) had stage IV disease at diagnosis.
Table 2.
Patient Characteristics
| Characteristic | No. |
|---|---|
| Number of Patients | 29 |
| Age, Median (range) | 53 y (30–91 y) |
| Primary Disease Stage at Diagnosis | |
| I | 1 |
| II | 5 |
| III | 0 |
| IV | 21 |
| Unknown | 2 |
| Histopathologic Findings at Diagnosis | |
| T status | |
| 1 | |
| 2 | 11 |
| 3 | 6 |
| 4 | 7 |
| Unknown | 3 |
| N status | |
| Positive | 26 |
| Negative | 2 |
| Unknown | 1 |
| Tumor grade | |
| 1 | 1 |
| 2 | 13 |
| 3 | 15 |
| Estrogen receptor status | |
| Positive | 24 |
| Negative | 5 |
| Progesterone receptor status | |
| Positive | 13 |
| Negative | 16 |
| HER2/neu status | |
| Positive | 8 |
| Negative | 21 |
| Type of surgery | |
| Mastectomy | 5 |
| Breast-conserving therapy | 3 |
| No surgery | 21 |
| Bone metastatic site | |
| Spine | 26 |
| Pelvis | 18 |
| Rib | 10 |
| Type of treatment | |
| Standard chemotherapy | 20 |
| Hormone therapy | 9 |
| Availability of images | |
| At 3 mo after treatment initiation | |
| XR images | 24 |
| SS images | 18 |
| CT images | 24 |
| At 6 mo after treatment initiation | |
| XR images | 26 |
| SS images | 23 |
| CT images | 26 |
Computed Tomography Vs. Skeletal Scintigraphy
Stratification of patients as having PD or non-PD at 3 months using CT or SS alone did not correspond to a significant difference between groups in PFS (CT alone, P = .24; SS alone, P = .994) or OS (CT alone, P = .736; SS alone, P = .276). At 6 months, CT alone did distinguish PFS (P = .007), but SS alone did not (P = .174). Neither modality distinguished OS at 6 months (CT alone, P = .373; SS alone, P = .672). Response for osseous metastases by CT or SS did not correlate with the imaging assessment of response for nonosseous metastases (at 3 months: kappa coefficients, 0 and 0.129, McNemar’s test, 0.754 and 0.039, respectively; at 6 months: kappa coefficients, −0.139 and −0.187, McNemar’s test, 0.388 and 0.035, respectively) (Table 3).
Table 3.
Agreement Between the Bone Tumor Responsea and Clinical Response for Measurable Nonosseous Metastasisb
| Bone Tumor Response Criteria |
Time of Assessment After Initiation of Therapy |
Relative to Clinical Response by RECIST for Nonosseous Metastases |
|
|---|---|---|---|
| McNemar’s Test | Kappa Coefficient | ||
| MDA | 0.999 | 0.129 | |
| WHO | 3 mo | 0.3877 | 0 |
| MDA | 0.2266 | −0.075 | |
| WHO | 6 mo | 0.3018 | −0.127 |
| CT | 0.7539 | 0 | |
| SS | 3 mo | 0.0391 | 0.129 |
| CT | 0.3877 | −0.139 | |
| SS | 6 mo | 0.0352 | −0.187 |
Abbreviations: CT = computed tomography; MDA = MD Anderson; SS = skeletal scintigraphy; WHO = World Health Organization.
Assigned on the basis of imaging results.
Determined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.
MDA Criteria vs. Who Criteria
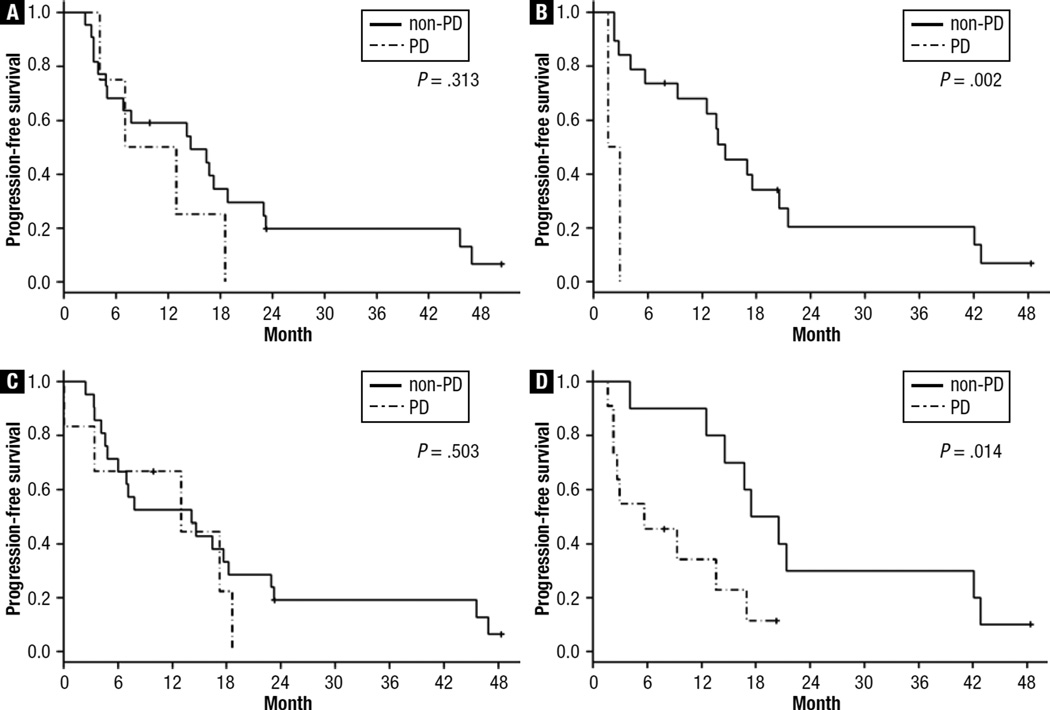
Diagnosis of the 29 patients at 3 and 6 months by MDA and WHO criteria is shown in Figure 1. Using the MDA and WHO criteria, PFS did not differ between patients classified as having PD and those classified as having non-PD at 3 months (MDA, P = .313; WHO, P = .503) but did differ in PFS at 6 months (MDA, P = .002; WHO, P = .014) (Figure 2). Neither response assessment system distinguished patients with PD from those with non-PD in terms of OS.
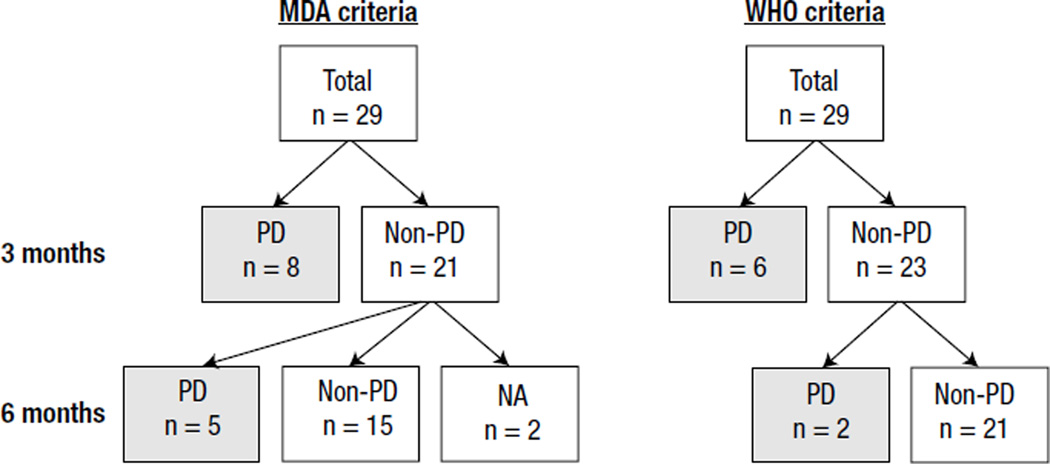
Figure 1.
Diagnosis of the 29 Patients at 3 and 6 Months by MD Anderson (MDA) and World Health Organization (WHO) Criteria. Patients Classified as Having Non–Progressive Disease (PD) (n = 21) at 3 Months had PD (n = 5) at 6 Months by the MDA Criteria. Patients Classified as Having Non-PD (n = 23) at 3 Months had PD (n = 2) at 6 Months by the WHO Criteria
Abbreviation: NA = not accessed.
Figure 2.
Kaplan-Meier Functions of Disease-Free Survival in (A) Patients Classified as Having Progressive Disease (PD) (n = 4) and Those Classified as Having Non-PD (n = 22) at 3 Months by the MDA Criteria, (B) Patients Classified as Having PD (n = 2) and Those Classified as Having Non-PD (n = 19) at 6 Months by the MDA Criteria, (C) Patients Classified as Having PD (n = 6) and Those Classified as Having Non-PD (n = 21) at 3 Months by the WHO Criteria, and (D) Patients Classified as Having PD (n = 11) and Those Classified as Having Non-PD (n = 10) at 6 Months by the WHO Criteria
Response for osseous metastases by MDA or WHO criteria did not correlate with the imaging assessment of response for nonosseous metastases (at 3 months: kappa coefficients, 0.129 and 0, McNemar’s test, 0.999 and 0.388, respectively; at 6 months: kappa coefficients, −0.075 and −0.127, McNemar’s test, 0.227 and 0.302, respectively) (Table 3).
Discussion
In this pilot study of patients with breast cancer and osseous and nonosseous metastases, we demonstrated that PFS did differ significantly between patients classified as having PD and those classified as having non-PD using both the MDA and WHO criteria at 6 months from initiation of therapy, although the number of events was small. However, contrary to our hypothesis, the MDA criteria did not enable earlier prediction of response and prognosis compared with the WHO criteria.
We expected the MDA criteria to predict response earlier than the WHO criteria based on the results of our retrospective study because the MDA criteria include CT, allowing evaluation of greater anatomic detail. There are some possibilities why the current study did not show an advantage of the MDA criteria for predicting PFS compared with the WHO criteria. First, although our previous retrospective study showed the superiority of the MDA criteria among patients with breast cancer and bone-only metastases, this study was conducted for patients with both bone and nonosseous metastases. Therefore, the MDA criteria may be applicable only for patients with bone-only metastasis. Second, the current study compared the MDA criteria with the WHO criteria at 3 and 6 months, whereas the previous study compared them in terms of the response within 2 to 6 months. Third, the current study lacks the power to differentiate the response for bone disease at 3 months because of the accrual time to recruit patients. The lack of significant differences with respect to OS in the current study is most likely related to the limited number of patients. We also did not assess the prognostic role of each modality and criteria at 12 months because only a small number of images were available. There was difficulty in successfully recruiting patients with metastatic breast cancer to this prospective study because of their poor prognosis and the necessity of taking fixed radiologic imaging (ie, with or without CT) at stated periods.
We also tested for the correlation between osseous metastasis response by the MDA or WHO criteria and clinical response for nonosseous metastases using RECIST criteria. There is no gold standard for assessing bone tumor response using imaging modalities or sets of criteria. Therefore we hypothesized that bone response parallels the response for nonosseous metastases based on RECIST. However in this study, osseous metastasis response as measured by each imaging modality or by the MDA or WHO criteria did not correlate with the imaging assessment of clinical response for nonosseous metastases. Possible explanations for the poor correlation between osseous metastasis response by the WHO criteria and clinical response include high false-positive rates on imaging studies caused by conditions such as inflammation, fracture,20–24 the flare phenomenon,14,15 or falsenegative findings such as “cold spots” on bone scans, which show rapid progression of disease as a reduction in tracer uptake.22,25,26 The low correlation between bone tumor response by the MDA criteria, which reflect anatomic changes of bone seen by CT, and clinical response of nonosseous metastases strongly indicates that there was a difference in biological behavior between osseous and nonosseous metastases. Further understanding of the different mechanisms of response to treatment of osseous and nonosseous metastases is needed.
With fluorine-18 (18F) fluorodeoxyglucose (FDG)-PET/CT, in which PET findings are fused with CT findings, determination of glucose metabolism is added to anatomic information.27,28 Because osteolytic lesions can be detected by PET and sclerotic lesions by CT, FDG-PET/CT has a high potential for more accurate assessment of bone metastases than does either PET or CT alone.27,28 Du et al reported that FDG-PET/CT immediately reflected the activity of bone metastases.29 Morris et al also demonstrated that FDG-PET/CT might be superior to SS in detecting bone metastases in patients with suspected metastatic breast cancer.30 Therefore addition of PET-CT to the MDA criteria may enable assessment of bone metastases earlier than the WHO criteria.
Conclusion
MDA and WHO criteria predicted PFS of patients with osseous metastases at 6 months but not at an earlier time point in this study. However, this study was underpowered to confirm the value of the MDA criteria. We plan a well-powered study to test the role of these criteria by incorporating FDG-PET/CT findings to predict bone tumor response earlier.
Clinical Practice Points.
The presence of bone metastases and their response to treatment are assessed by imaging studies.
WHO criteria, which have been considered the standard means for assessment of bone metastasis response, are based on XR and SS. MDA criteria are based on CT, XR, and SS.
We prospectively compared the MDA and WHO criteria by stratifying patients with breast cancer and osseous and nonosseous metastases with respect to PFS and OS.
We also compared the response of osseous metastases assessed by the MDA or WHO criteria with the response of nonosseous metastases to determine whether bone response aligns with response in other metastatic sites.
In the current study, both criteria predicted PFS of patients at 6 months but not at an earlier time point.
Response in osseous metastases by either criterion did not correlate with the response in nonosseous metastases.
In clinical practice, we still need to wait about 6 months for bone tumor response before we can predict the PFS regardless of imaging type.
Incorporating FDG-PET/CT findings into the MDA criteria to predict bone tumor response earlier needs a well-powered clinical study.
Acknowledgments
We thank Sunita Patterson of the Department of Scientific Publications at MD Anderson Cancer Center for expert editorial assistance.
This work was supported in part by an Institutional Research Grant from the University of Tex as MD Anderson Cancer Center (to T.H. and N.T.U.), by the National Institutes of Health through MD Anderson’s Cancer Center Support grant CA016672, and by a gift from Pfizer (to N.T.U.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have stated that they have no conflicts of interest.
References
- 1.Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen OS. Palliative radiotherapy of bone metastases: there is now evidence for the use of single fractions. Radiother Oncol. 1999;52:95–96. doi: 10.1016/s0167-8140(99)00109-7. [DOI] [PubMed] [Google Scholar]
- 4.Briasoulis E, Karavasilis V, Kostadima L, et al. Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer. 2004;101:1524–1528. doi: 10.1002/cncr.20545. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domchek SM, Younger J, Finkelstein DM, et al. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89:363–368. doi: 10.1002/1097-0142(20000715)89:2<363::aid-cncr22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Koenders PG, Beex LV, Kloppenborg PW, et al. Human breast cancer: survival from first metastasis. Breast Cancer Study Group. Breast Cancer Res Treat. 1992;21:173–180. doi: 10.1007/BF01975000. [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 9.Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol. 2001;19:3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 10.Rubens RD. Bone metastases—the clinical problem. Eur J Cancer. 1998;34:210–213. doi: 10.1016/s0959-8049(97)10128-9. [DOI] [PubMed] [Google Scholar]
- 11.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RE. Management of bone metastases. Oncologist. 2000;5:463–470. doi: 10.1634/theoncologist.5-6-463. [DOI] [PubMed] [Google Scholar]
- 13.Theriault RL, Hortobagyi GN. Bone metastasis in breast cancer. Anticancer Drugs. 1992;3:455–462. doi: 10.1097/00001813-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE. Assessment of response to treatment. London: Springer Verlag; 1991. [Google Scholar]
- 15.Hortobagyi GN. Bone metastases in patients with breast cancer. Semin Oncol. 1991;18:11–15. [PubMed] [Google Scholar]
- 16.Hamaoka T, Costelloe CM, Madewell JE, et al. Tumour response interpretation with new tumour response criteria vs the world Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102:651–657. doi: 10.1038/sj.bjc.6605546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. doi: 10.1056/NEJM200507073530120. discussion:199-202. [DOI] [PubMed] [Google Scholar]
- 18.Adami S, Isaia G, Luisetto G, et al. Fracture incidence and characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res. 2006;21:1565–1570. doi: 10.1359/jbmr.060715. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Citrin DL, Bessent RG, Greig WR. A comparison of the sensitivity and accuracy of the 99TCm-phosphate bone scan and skeletal radiograph in the diagnosis of bone metastases. Clin Radiol. 1977;28:107–117. doi: 10.1016/s0009-9260(77)80137-2. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RE, Rubens RD, Fogelman I. Reappraisal of the baseline bone scan in breast cancer. J Nucl Med. 1988;29:1045–1049. [PubMed] [Google Scholar]
- 22.Galasko CS, Doyle FH. The detection of skeletal metastases from mammary cancer. A regional comparison between radiology and scintigraphy. Clin Radiol. 1972;23:295–297. doi: 10.1016/s0009-9260(72)80051-5. [DOI] [PubMed] [Google Scholar]
- 23.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64. [PubMed] [Google Scholar]
- 24.Tubiana-Hulin M. Incidence, prevalence and distribution of bone metastases. Bone. 1991;(12 suppl 1):S9–S10. doi: 10.1016/8756-3282(91)90059-r. [DOI] [PubMed] [Google Scholar]
- 25.Condon BR, Buchanan R, Garvie NW, et al. Assessment of progression of secondary bone lesions following cancer of the breast or prostate using serial radionuclide imaging. Br J Radiol. 1981;54:18–23. doi: 10.1259/0007-1285-54-637-18. [DOI] [PubMed] [Google Scholar]
- 26.Cook GJ, Fogelman I. The role of positron emission tomography in the management of bone metastases. Cancer. 2000;88:2927–2933. doi: 10.1002/1097-0142(20000615)88:12+<2927::aid-cncr8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Cook GJ, Houston S, Rubens R, et al. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 28.Shie P, Cardarelli R, Brandon D, et al. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33:97–101. doi: 10.1097/RLU.0b013e31815f23b7. [DOI] [PubMed] [Google Scholar]
- 29.Du Y, Cullum I, Illidge TM, et al. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–3447. doi: 10.1200/JCO.2007.11.2854. [DOI] [PubMed] [Google Scholar]
- 30.Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–3159. doi: 10.1200/JCO.2009.27.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]