Abstract
Objective
The pleiotropic effects of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) independent of cholesterol-lowering effects are thought to be mediated through inhibition of the Rho/Rho-kinase pathway. However, we have previously demonstrated that the pleiotropic effects of regular-dose statins are mediated mainly through inhibition of the Rac1 signaling pathway rather than the Rho/Rho-kinase pathway, although the molecular mechanisms of the selective inhibition of the Rac1 signaling pathway by regular-dose statins remain to be elucidated. In this study, we tested our hypothesis that small GTP-binding protein GDP dissociation stimulator (SmgGDS) plays a crucial role in the molecular mechanisms of the Rac1 signaling pathway inhibition by statins in endothelial cells.
Approach and Results
In cultured human umbilical venous endothelial cells, statins concentration-dependently increased SmgGDS expression and decreased nuclear Rac1. Statins also enhanced SmgGDS expression in mouse aorta. In control mice, the protective effects of statins against angiotensin II–induced medial thickening of coronary arteries and fibrosis were noted, whereas in SmgGDS-deficient mice, the protective effects of statins were absent. When SmgGDS was knocked down by its small interfering RNA in human umbilical venous endothelial cells, statins were no longer able to induce Rac1 degradation or inhibit angiotensin II–induced production of reactive oxygen species. Finally, in normal healthy volunteers, statins significantly increased SmgGDS expression with a significant negative correlation between SmgGDS expression and oxidative stress markers, whereas no correlation was noted with total or low-density lipoprotein-cholesterol.
Conclusions
These results indicate that statins exert their pleiotropic effects through SmgGDS upregulation with a resultant Rac1 degradation and reduced oxidative stress in animals and humans.
Keywords: Rac, reactive oxygen species, Rho, small GTP-binding protein GDP dissociation stimulator, statins
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) are potent cholesterol-lowering drugs widely used in clinical practice for primary and secondary prevention of coronary artery disease.1,2 Furthermore, the beneficial cardiovascular effects of statins, beyond their lipid-lowering action (the so-called pleiotropic effects), have attracted much attention.3,4 The pleiotropic effects of statins could be mediated by reduced synthesis of the isoprenoids that are responsible for the post-translational modulation of intracellular proteins.1 Because membrane localization and GTPase activity of small GTP-binding proteins (eg, Rho, Rac, and Ras) are dependent on isoprenylation, the pleiotropic effects of statins have been considered to be mediated by inhibition of those small GTP-binding proteins.5,6 However, the specific cellular GTPase target protein(s) of statins still remains to be elucidated.
We have recently demonstrated that regular-dose statins (atorvastatin and pravastatin, 20 mg/d for 1 week) significantly inhibit Rac1, but not RhoA/Rho-kinase, activity in circulating leukocytes in normal healthy volunteers.7 Rac1 plays a crucial role in generating reactive oxygen species (ROS) and is an important mediator of cardiovascular hypertrophy.8 In mouse models of angiotensin II (AngII)– and pressure overload–induced cardiovascular hypertrophy, it was reported that simvastatin inhibits Rac1-mediated ROS production in the heart and vascular smooth muscle.9,10 These findings are further supported by the analysis of failing human heart tissues, where increased ROS generation is associated with increased Rac1 activity, both of which are attenuated by statins.11 More interestingly, it has been reported that the protective effects of regular-dose pitavastatin are noted in endothelial nitric oxide synthase–deficient (eNOS−/−) mice.12 Thus, we considered that the pleiotropic effects of regular-dose statins are mediated mainly through inhibition of the Rac1 pathway rather than the other pathway.7 However, the molecular mechanisms of the selective inhibition of the Rac1 signaling pathway by regular-dose statins remain to be elucidated.
To elucidate the molecular mechanisms of the preferential inhibition of the Rac1 signaling pathway by regular-dose statins, we hypothesized that the small GTP-binding protein GDP dissociation stimulator (SmgGDS) plays an important role. SmgGDS is the only guanine nucleotide exchange factor of the armadillo family of proteins.13-15 Purified SmgGDS interacts with the small GTPase C-terminal polybasic region.16 Importantly, the polybasic region of Rac1, but not that of RhoA, has a functional nuclear localization signal sequence15 and Rac1 with a functional nuclear localization signal accompanies SmgGDS into the nucleus and is degraded by the proteasome system.17 In the present study, we thus tested our hypothesis that SmgGDS plays a crucial role in the molecular mechanisms of the pleiotropic effects of statins in animals and humans.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Transfection of Human Umbilical Venous Endothelial Cells With Small Interfering RNA
Multiple small interfering RNA (siRNA) duplexes for SmgGDS were purchased from Qiagen. Multiple siRNA duplexes were transfected and tested for maximal and specific suppression of their target protein as previously described.18 A functional nontargeting siRNA that was bioinformatically designed by Qiagen was used as a control. Human umbilical venous endothelial cells (HUVECs) were transfected with HiPerFect Transfection Reagent (Qiagen) with either 10 nmol/L mock siRNA or 10 nmol/L siRNA specific for SmgGDS. After 72 hours post-transfection, the cells were analyzed by Western blot or ROS analysis.
ROS Analysis
Intracellular ROS production in HUVECs was measured as previously described.19 We treated HUVECs with AngII (1 μmol/L, Wako) for 3 hours at 37°C in 5% CO2, washed them with PBS, and loaded them with 2,7-dichlorofluorescein diacetate (5 μmol/L, Cayman) for 30 minutes at 37°C to reveal the presence of ROS as green fluorescence (488 nm) by fluorescence microscopy (BIOREVO, Keyence). The relative fluorescence intensity was measured by BZ-II analyzer (Keyence) software.
Analysis of Medial Thickening and Perivascular Fibrosis of Mouse Coronary Arteries
We performed all mouse experiments in accordance with experimental protocols approved by the Animal Care and Use Committee of the Tohoku University Graduate School of Medicine. We used the AngII-infused cardiovascular hypertrophy model12 to assess the effect of SmgGDS deficiency on the pleiotropic effects of regular-dose statins. We infused AngII (2.0 mg/kg per day) or saline for 2 weeks in 10-week-old male SmgGDS+/+ littermate control mice and SmgGDS+/− mice. We dissolved AngII in sterile saline and infused it via an osmotic mini-pump (Alzet model 2002, Alze Corp). We anesthetized the animals with isofluorane and placed the pump into the subcutaneous space of isofluorane-anesthetized mice through a small incision in the back that was closed by suturing. Both the AngII-infused and saline-infused mice were administrated either statin or placebo treatment by gavage every day for 2 weeks. All incision sites healed rapidly without any infection. Systolic blood pressure was measured using a noninvasive tail-cuff system (MK-2000, Muromachi) at 2 weeks after pump implantation. Plasma lipids (triglycerides, total cholesterol, and low-density lipoprotein (LDL)-and high-density lipoprotein-cholesterol) were analyzed with high-performance liquid chromatography system by Skylight Biotech. Histological studies were performed for the determination of medial thickness and fibrosis of coronary arteries, as previously reported.20,21
Human Study
This protocol was approved by the Human Research Committee of the Tohoku University Graduate School of Medicine, and 20 normal healthy human volunteers participated after written consent was obtained (Table II in the online-only Data Supplement). The volunteers received pravastatin (20 mg/d) or atorvastatin (20 mg/d) orally for 2 weeks in a randomized crossover manner with a 2-week washout interval. The 2-week interval is enough to restore lipid profiles to prestatin treatment levels.22 Leukocytes were isolated according to the method previously described with slight modification.23
Statistical Analysis
Comparisons of parameters between 2 groups were performed with the unpaired Student t test. Statistical analysis was performed by 1-way ANOVA followed by Dunnett test for multiple comparisons. Statistical significance was evaluated with JMP 8 (SAS Institute). A P<0.05 was considered to be statistically significant.
Results
Statins Upregulate SmgGDS in HUVECs and Mouse Aorta
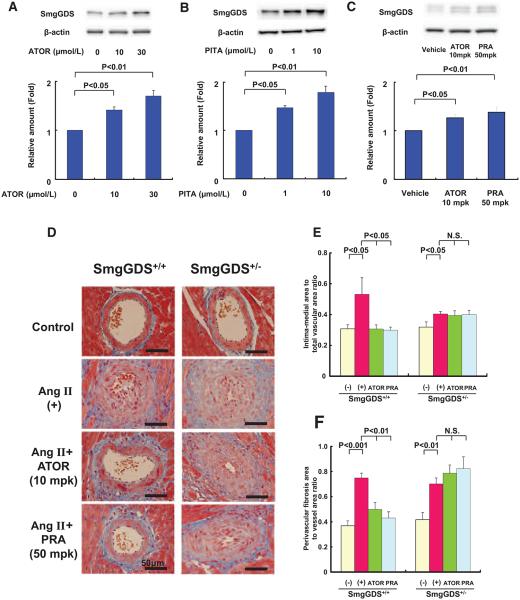
To examine whether SmgGDS levels are actually increased by statins, we first examined the effects of 2 statins (atorvastatin, 10 and 30 μmol/L, and pitavastatin, 1 and 10 μmol/L) on the SmgGDS expression in cultured HUVECs after 24 hours treatment. These 2 statins increased SmgGDS expression in HUVECs in a concentration-dependent manner (Figure 1A and 1B). Similarly, these 2 statins also increased SmgGDS expression in human coronary arterial endothelial cells (Figure IA and IB in the online-only Data Supplement). Furthermore, to explore whether the upregulation of SmgGDS noted in vitro exist in vivo, we examined the effect of statins on SmgGDS expression in mouse aorta treated with 2 statins (atorvastatin, 10 mg/kg per day and pravastatin, 50 mg/kg per day) for 1 week. These 2 statins increased SmgGDS expression in mouse aorta (Figure 1C). This is the novel finding that statins exert class effects to increase SmgGDS expression levels in vitro and in vivo.
Figure 1.
Statins upregulate small GTP-binding protein GDP dissociation stimulator (SmgGDS) and the effects of statins are absent in SmgGDS+/− mice. A and B, Expression of SmgGDS protein after incubating human umbilical venous endothelial cells with atorvastatin (ATOR) or pitavastatin (PITA) for 24 hours (n=3 each). C, Expression of SmgGDS protein in the aorta administered with vehicle, ATOR, or PITA in mice for 1 week (n=10 each). D, Representative Masson’s trichrome staining of the coronary artery in control (−), angiotensin II (AngII)-infused mice (no inhibitor, AngII[+]), and AngII-infused mice treated with either ATOR (AngII+ATOR, 10 mg/kg per day) or pravastatin (PRA) (AngII+PRA, 50 mg/kg per day). Medial thickening and perivascular fibrosis of the coronary arteries in SmgGDS+/+ (left) and SmgGDS+/− (right ) mice are shown. E and F, Quantitative analysis of cardiovascular intima-medial area and perivascular fibrosis area in SmgGDS+/+ and SmgGDS+/− mice treated with vehicle or statins at 2 weeks after AngII infusion (n=10). Results are expressed as mean±SEM. N.S. indicates not significant.
No Cardioprotective Effects of Statins in SmgGDS-Deficient Mice
Next, we examined the effects of statins on AngII-induced medial thickening and perivascular fibrosis of coronary arteries in mice in vivo. It has been reported that statins attenuate AngII-induced cardiovascular hypertrophy in wild-type mice without changes in blood pressure or plasma cholesterol levels.9,12 Thus, this effect of statins is highly likely to depend on their pleiotropic effects. AngII was administered to heterozygous SmgGDS-deficient mice (SmgGDS+/−) and littermate (SmgGDS+/+) mice with an infusion pump for 2 weeks. Systolic blood pressure was elevated in both SmgGDS+/− and littermate mice, and plasma total and LDL-cholesterol levels tended to increase (Table I in the online-only Data Supplement). There was no significant difference in blood pressure or plasma lipid profiles between the vehicle and the statin groups (Table I in the online-only Data Supplement). Analysis of microscopic examination of coronary artery sections revealed that AngII caused accelerated medial thickening (Figure 1D and 1E) and perivascular fibrosis of coronary arteries (Figure 1D and 1F). In littermate mice, the protective effects of statins against AngII-induced medial thickening of coronary arteries and fibrosis were noted, whereas in SmgGDS+/− mice, the protective effects of statins were absent (Figure 1D–1F).
Statins Upregulate SmgGDS in HUVECs Through the Phosphoinositide-3-Kinase/Akt/Glycogen Synthase Kinase-3β Pathway
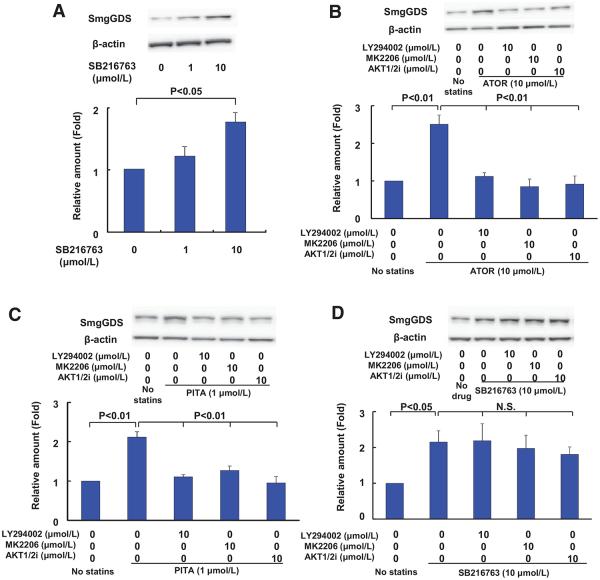
Next, we analyzed the mechanisms by which statins increase SmgGDS expression in HUVECs. It has been reported that statins increase β-catenin that is a member of the armadillo protein family like SmgGDS.24 This is achieved through statin-mediated inhibition of glycogen synthase kinase-3β (GSK-3β) and thereby inhibition of β-catenin phosphorylation.25,26 Because phosphorylated β-catenin is more prone to ubiquitination and degradation, inhibition of β-catenin phosphorylation increases the stability of β-catenin. It was previously shown that the selective GSK-3β inhibitor, SB216763, also increases the β-catenin expression level.26 Thus, we examined the possible enhancing effect of SB216763 on the SmgGDS expression in HUVECs. As we expected, SB216763 increased SmgGDS expression in a concentration-dependent manner (Figure 2A). Similar results were obtained with lithium, another GSK-3β inhibitor (data not shown). This increase in SmgGDS when GSK-3β is inactivated by SB216763 or lithium is consistent with the presence of several putative GSK-3β phosphorylation sites in SmgGDS identified by the eukaryotic linear motif program (http://elm.eu.org). This increase in SmgGDS by statins was reversed by either phosphoinositide-3-kinase (PI3K) inhibitor (LY294002), Akt inhibitor (MK2206), or AKT 1/2 inhibitor (Figure 2B and 2C). Interestingly, this increase in SmgGDS in response to SB216763 was not reversed by LY294002, MK2206, or AKT 1/2 inhibitor (Figure 2D).
Figure 2.
Statins upregulate small GTP-binding protein GDP dissociation stimulator (SmgGDS) in human umbilical venous endothelial cells (HUVECs) through the phosphoinositide-3-kinase/Akt/glycogen synthase kinase (GSK)-3β pathway. A, Expression of SmgGDS protein after incubating HUVECs with GSK-3β inhibitor SB216763 for 24 hours (n=3). B and C, The effect of LY294002 (10 μmol/L), MK2206 (10 μmol/L), and AKT 1/2 inhibitor [AKT1/2i], 10 μmol/L on statin-induced SmgGDS upregulation in HUVECs (n=3). D, The effect of LY294002 (10 μmol/L), MK2206 (10 μmol/L), and AKT1/2i (10 μmol/L) on SB216763-induced SmgGDS upregulation in HUVECs (n=3). Results are expressed as mean±SEM. N.S. indicates not significant.
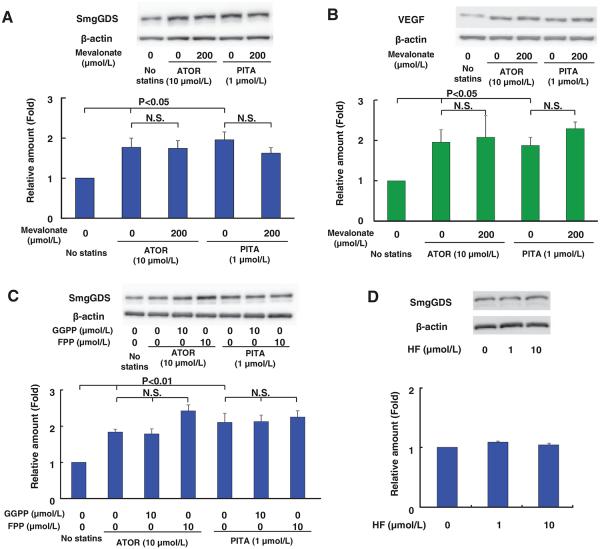
Next, we examined whether mevalonate and isoprenoids (farnesyl pyrophosphate and geranylgeranyl pyrophosphate), both of which are important products of the cholesterol biosynthesis pathway,27 mediate the statin-induced SmgGDS upregulation. Because it is known that the reduced mevalonate biosynthesis is one of the mechanisms of activation of the PI3K/Akt pathway by statins,28 we hypothesized that statins activate PI3K/Akt pathway through mevalonate, which in turn inhibits GSK-3β. Importantly, mevalonate did not inhibit the statin-induced SmgGDS upregulation (Figure 3A). This may be explained by activation of Akt via an alternative pathway. It has been shown that Akt is activated by vascular endothelial growth factor (VEGF) and that Akt activation is not reversed by mevalonate.28 Furthermore, it has been reported that statins increase mRNA and protein expression levels of VEGF in HUVECs.29 We observed that statins upregulated VEGF, which was not reversed by mevalonate (Figure 3B). However, in the present study, because of several technical reasons, including the nonspecific effects of VEGF receptor inhibitors and the presence of VEGF in culture medium, we did not further examine the possible role of VEGF in the effects of statins. In addition, because farnesyl pyrophosphate and geranylgeranyl pyrophosphate control the post-translational modification of intracellular proteins and membrane localization and activity of small GTPase,30 they could play a central role in the pleiotropic effects of statins.31,32 Splice variants of SmgGDS also could control small GTPase prenylation and membrane localization.33 However, farnesyl pyrophosphate or geranylgeranyl pyrophosphate did not inhibit the upregulation of SmgGDS by statins (Figure 3C). We also demonstrated the increase in endothelial nitric oxide synthase expression as a positive control of drug treatments (Figure II and III in the online-only Data Supplement). Thus, the enhancing effects of statins on SmgGDS expression are not mediated through reduction in these isoprenoids. Next, because it has been reported that guanine nucleotide exchange factor function is regulated by Rho-kinase,34 we examined whether the enhancing effects of SmgGDS by statins are mediated by the inhibitory effect of statins on the Rho-kinase pathway. However, a specific Rho-kinase inhibitor, hydroxyfasudil, did not increase SmgGDS expression (Figure 3D). Thus, the enhancing effects of statins on SmgGDS expression are not mediated through inhibition of the Rho-kinase pathway. Taken together, these results indicate that statins increase SmgGDS expression through inhibition of GSK-3β via the PI3K/Akt pathway.
Figure 3.
Small GTP-binding protein GDP dissociation stimulator (SmgGDS) upregulation by statins in human umbilical venous endothelial cell (HUVECs) is not reversed by inhibition of mevalonate pathway. A, The effect of mevalonate (200 μmol/L) on statin-induced SmgGDS upregulation in HUVECs (n=3). B, The effect of mevalonate (200 μmol/L) on statin-induced vascular endothelial growth factor (VEGF) upregulation in HUVECs (n=3). C, The effect of farnesyl pyrophosphate (FPP, 10 μmol/L) and geranylgeranyl pyrophosphate (GGPP, 10 μmol/L) on statin-induced SmgGDS upregulation in HUVECs (n=3). D, Expression of SmgGDS protein after incubating HUVECs with Rho-kinase inhibitor hydroxyfasudil (HF) for 24 hours (n=3). Results are expressed as mean±SEM. N.S. indicates not significant.
Statins Reduce ROS Production via SmgGDS
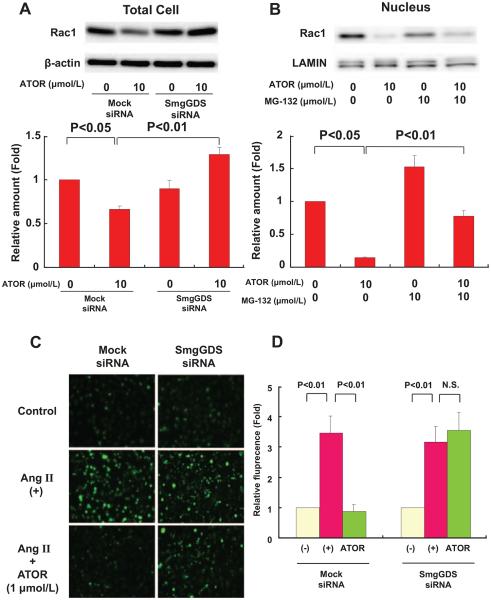
To explore the effect of statins on Rac1 expression, we examined Rac1 protein expression levels in HUVECs after 24 hours treatment with statins. Atorvastatin significantly decreased total Rac1 level (Figure 4A), which was also the case with pitavastatin (Figure IVA in the online-only Data Supplement). Next, we examined the mechanism by which statins decrease Rac1 level. It was previously reported that Rac1 is degraded by the nuclear proteasome.17 Thus, we cotreated HUVECs with statins and a proteasome inhibitor, MG-132.17 As we expected, although atorvastatin significantly decreased nuclear Rac1 expression level, MG-132 inhibited the decrease in Rac1 expression by atorvastatin in the nucleus (Figure 4B). This was also the case with pitavastatin (Figure IVB in the online-only Data Supplement). RhoA and K-Ras were not detected in nuclear fraction (Figure VB in the online-only Data Supplement). We also measured cytosolic Rac1 expression after treatment with both MG-132 and statins, and confirmed that MG-132 did not increase cytosolic Rac1 expression (Figure VC in the online-only Data Supplement). Thus, statins preferentially enhance degradation of Rac1 through degradation by proteasomes in the nucleus.
Figure 4.
Small GTP-binding protein GDP dissociation stimulator (SmgGDS) small interfering RNA (siRNA) abolishes Rac1 degradation and anti-oxidant effects of atorvastatin in human umbilical venous endothelial cells (HUVECs). A, Western blot analysis of total cell lysate of HUVECs transfected with mock or SmgGDS siRNA for 72 hours. Atorvastatin was added during the last 24 hours (n=3). B, The effect of proteasome inhibitor (MG-132) on atorvastatin-induced Rac1 reduction in the nuclear fraction (n=3). C, Representative dichlorofluorescein staining of HUVECs after preincubation with atorvastatin (1 μmol/L) for 24 hours followed by 3 hours incubation with 1 μmol/L angiotensin II (AngII). D, Densitometric analysis (n=8). Results are expressed as mean±SEM. N.S. indicates not significant.
We further examined the effect of statins on SmgGDS expression by using its siRNA in HUVECs. Densitometric analysis showed that SmgGDS expression levels were reduced by ≈85% after SmgGDS siRNA transfection, relative to mock siRNA-transfected cells (Figure VA in the online-only Data Supplement). In mock siRNA-transfected cells, atorvastatin decreased Rac1 expression in whole cells, whereas in SmgGDS siRNA–transfected cells, atorvastatin was no longer able to induce Rac1 degradation (Figure 4A). This was also the case with pitavastatin (Figure IVA in the online-only Data Supplement). Thus, we were able to further confirm that statins enhance Rac1 degradation via SmgGDS. Furthermore, we examined the possible role of Rac1 degradation in mediating the pleiotropic effects of statins by evaluating the antioxidant effects of statins. It has been reported that statins decrease AngII-induced production of ROS.10,35 In mock siRNA–transfected cells, atorvastatin (1 μmol/L) decreased AngII-induced production of ROS, whereas in SmgGDS siRNA-transfected cells, atorvastatin was no longer able to inhibit AngII-induced production of ROS (Figure 4C and 4D). This was also the case with pitavastatin (0.1 μmol/L; Figure IVC and IVD in the online-only Data Supplement).
Statins Upregulate SmgGDS in Humans
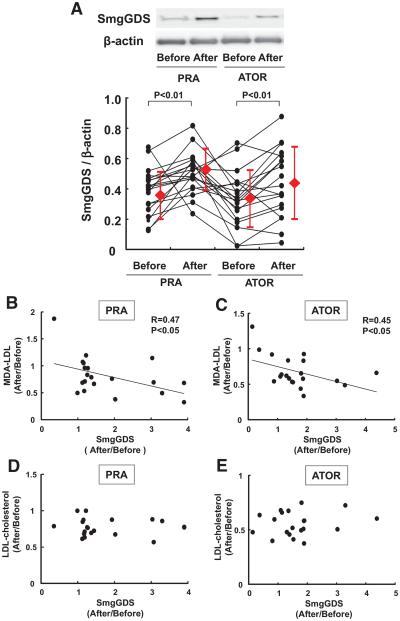
Finally, we examined the effects of statins on SmgGDS expression in normal healthy volunteers, who received pravastatin and atorvastatin (20 mg/d each) orally for 2 weeks in a crossover manner with a 2-week washout period (Figure VI in the online-only Data Supplement). Both statins significantly reduced the serum levels of total cholesterol and LDL-cholesterol even in the normolipidemic volunteers, indicating the efficacy of the statin therapy (Table III in the online-only Data Supplement). SmgGDS expression in circulating polymorphonuclear leukocytes was significantly increased after the treatment with pravastatin or atorvastatin (Figure 5A). Importantly, both pravastatin (a hydrophilic stain) and atorvastatin (a lipophilic statin) exerted a comparable enhancing effect on SmgGDS expression in humans, indicating the class effect of statins to enhance SmgGDS level in humans. Furthermore, malondialdehyde-modified–LDL-cholesterol, one of the oxidative stress markers, was significantly decreased with the statin treatment (Table III in the online-only Data Supplement). Importantly, a significant negative correlation was noted between the extent of SmgGDS level and that of malondialdehyde–LDL-cholesterol level, whereas no such correlation was noted with total or LDL-cholesterol (Figure 5B–5E).
Figure 5.
Statins upregulate small GTP-binding protein GDP dissociation stimulator (SmgGDS) in humans. A, Protein levels of SmgGDS in circulating human polymorphonuclear leukocytes. Crossover oral treatment with pravastatin (PRA, 20 mg/d for 2 weeks) or atorvastatin (ATOR, 20 mg/d for 2 weeks) significantly increased the SmgGDS level (n=20). B and C, Significant correlation between changes in SmgGDS and those in malondialdehyde (MDA)–low-density lipoprotein (LDL)-cholesterol. D and E, No correlation between changes in SmgGDS and those in LDL-cholesterol (n=20). Results are expressed as mean±SD.
Discussion
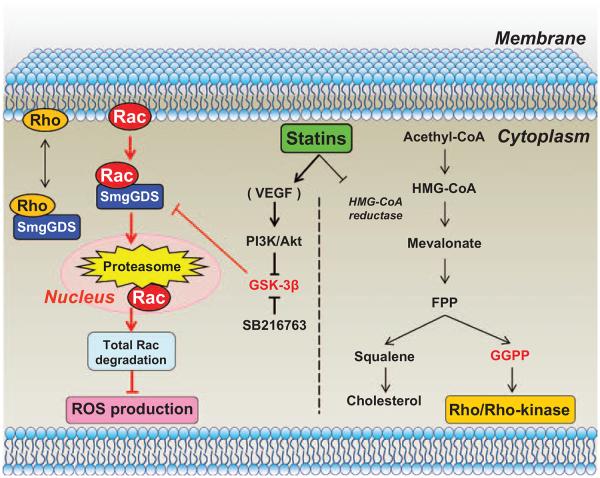
To the best our knowledge, the present study provides the first direct evidence that SmgGDS plays a crucial role in the molecular mechanisms of the pleiotropic effects of statins through enhancement of intranuclear Rac1 degradation in animals and humans (Figure 6). Multiple processes may be involved in the effects of statins on SmgGDS. First, statins increase SmgGDS expression. Second, SmgGDS binds and transports Rac1 to the nucleus. Third, Rac1 is degraded by the nuclear proteasome system. Fourth, total Rac1 degradation causes reduced ROS production. Finally, this reduced ROS production exerts cardiovascular protective effects independent of cholesterol levels, that is, the pleiotropic effects of statins (Figure 6). In addition, we also were able to demonstrate that SmgGDS does not transport RhoA to the nucleus, although it is able to bind with RhoA.15 The present findings provide a new therapeutic consideration, highlighting the importance of assessing both quantitative changes and cellular localization of small G proteins, rather than considering their activities alone.
Figure 6.
Novel mechanisms of the pleiotropic effects of statins. The present study demonstrates that regular-dose statins enhance small GTP-binding protein GDP dissociation stimulator (SmgGDS) expression through glycogen synthase kinase (GSK)-3β inhibition via phosphoinositide-3-kinase (PI3K)/Akt pathway, and that SmgGDS transports Rac1 to the nucleus, where Rac1 is degraded by the nuclear proteasome with resultant reduced reactive oxygen species (ROS) production (left). In contrast, high-dose statins exert inhibitory effects on the Rho/ Rho-kinase pathway as previously demonstrated (right). FPP indicates farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; and VEGF, vascular endothelial growth factor.
In the present study, we used 3 statins (atorvastatin, pitavastatin, and pravastatin) to examine whether the increase in SmgGDS expression is the class effects of statins. In the present in vitro study, we used atorvastatin and pitavastatin, but not pravastatin, because pravastatin is a hydrophilic statin that needs organic anion transporter to take into cells. Furthermore, we used higher concentration statins than their plasma levels in humans. We were unable to detect SmgGDS upregulation at lower concentrations of statins by Western blotting. We consider that this is because of technical limitations of the in vitro study. Instead, we were able to demonstrate that the clinical doses of statins increase SmgGDS expression in circulating leukocytes in humans. In the present in vivo study, we used atorvastatin and pravastatin to address whether lipophilic and hydrophilic statins exert similar class effects. We have previously demonstrated the pharmacokinetic profiles of atorvastatin and pravastatin in rats, confirming that the plasma concentrations of the statins in rats were equivalent to their maximum concentrations (Cmax) in humans.7 Previous reports showed that both the Rho/Rho-kinase and Rac1 pathways are involved in the pathogenesis of cardiovascular diseases.9,36,37 In the present study, we tested our hypothesis that the pleiotropic effects of regular-dose statins are mediated through inhibition of the Rac1 signaling pathway but not that of the Rho/Rho-kinase signaling pathway. High-dose statins certainly suppress the Rho/Rho-kinase signaling pathway.38 In cultured endothelial cells, we noted that statins enhanced SmgGDS expression independent of mevalonate or Rho/Rho-kinase pathway, resulting in enhanced Rac1 degradation and reduced production of ROS. However, the role of Rac1 in the nucleus and the reason for its intranuclear degradation remain to be clarified. It has been reported that Rac1 activation is indispensable for nuclear localization of β-catenin during canonical Wnt signaling.39 Rac1 interacts with β-catenin and Dickkopt 1 genes to control limb outgrowth in mice.39 Because β-catenin is a member of the armadillo protein family like SmgGDS, it is conceivable that nuclear translocation of Rac1 is necessary during fetal development. In SmgGDS+/− mice, we noted that the cardiovascular protective effects of statins were absent. Interestingly, the protective effects of statins against cardiovascular hypertrophy and diastolic dysfunction are noted even in eNOS−/− mice.12 Moreover, statin treatment ameliorated the high mortality rate in AngII-treated eNOS−/− mice associated with suppression of oxidative stress.12 It has been suggested that endothelial nitric oxide synthase activation through the Akt pathway is also important in the pleiotropic effects of statins.28 Alternatively, statins inhibit the transforming growth factor (TGF)-β1/Smad signaling pathway through suppression of oxidative stress. TGF-β1 is one of the most important profibrotic molecules, and TGF-β1 overexpression in mice results in cardiac hypertrophy and fibrosis.40 On the contrary, in TGF-β1–deficient mice, AngII-induced cardiac hypertrophy is absent.41 These effects of statins are considered to be mediated by their pleiotropic effects. The present results suggest that statins exert their cardiovascular protective effects through endothelial nitric oxide synthase–independent pathway because the Rac1 signaling pathway is upstream of the ROS/TGF-β/Smad signaling pathway.
In the present study, we used SmgGDS+/− mice because SmgGDS−/− mice die of heart failure shortly after birth, as a result of enhanced cardiomyocyte apoptosis triggered by cardiovascular overload but not developmental heart defects.42 In contrast, SmgGDS+/− mice are viable with no abnormal phenotypes, and there was no difference in AngII-induced increase in heart weight or blood pressure between SmgGDS+/− and littermate mice. The reason why AngII-induced cardiovascular phenotype did not become more serious in SmgGDS+/− mice is unclear. However, the reduction in SmgGDS expression level in SmgGDS+/− mice may not be enough to worsen cardiovascular phenotype. Thus, SmgGDS+/− mice are suitable to examine the role of SmgGDS in the pleiotropic effects of statins. However, the SmgGDS deficiency in these mice is not restricted to the cardiovascular tissues. Thus, tissue-specific SmgGDS−/− mice are needed for better understanding of the roles of SmgGDS. In addition, it has been reported that AngII infusion induces cardiac hypertrophy in both wild-type and β-catenin–mutant mice.43 In contrast, mice with stabilized β-catenin show decreased cardiac cross-sectional area at baseline and an abrogated hypertrophy response to AngII infusion.43 It is conceivable that β-catenin works in a similar way to SmgGDS in this model, and that similar result could be obtained with β-catenin–mutant mice. Currently, we are developing SmgGDS-overexpressing mice, which we expect would display phenotypes similar to Rac1-deficient mice and β-catenin–stabilized mice.
In the present clinical protocol with normal healthy volunteers, we were able to demonstrate that regular-dose statins significantly increased SmgGDS expression with a significant negative correlation between SmgGDS expression and oxidative stress markers, whereas no such correlation was noted with total or LDL-cholesterol. The present results are consistent with a previous report that statins inhibit ROS production through inhibition of Rac1 activity in the failing human heart.11 It has recently been shown that Rac1 also contributes to the pathogenesis of atrial fibrillation through activation of superoxide production, which is inhibited by statins through inhibition of Rac1 activation.44 In this study, we examined the SmgGDS expression level in polymorphonuclear leukocytes, because it is difficult to obtain blood vessels from human subjects. However, the present method is widely accepted as a method to evaluate the small GTP-binding proteins activity in cardiovascular system.7,38 Moreover, because statins significantly reduced the serum levels of total cholesterol and LDL-cholesterol even in the normolipidemic volunteers, a consistent finding with previous reports,7,45 we cannot completely rule out the possible involvement of their cholesterol-lowering effect in the present results in humans. Furthermore, the present findings in healthy volunteers remain to be confirmed in patients with cardiovascular disease (eg, coronary artery disease, hypertension, and heart failure).
Based on our previous findings, the doses of statins required to inhibit Rho-kinase are 3-fold higher than those required to inhibit Rac1 signaling.7 Therefore, we consider that >60 mg/d atorvastatin is needed to partially inhibit Rho/Rho-kinase pathway.7 In addition, combination therapy with atorvastatin and fasudil, a selective Rho-kinase inhibitor,46 should be more effective than fasudil alone for ameliorating AngII-induced cardiovascular hypertrophy in vivo.7 Indeed, to avoid adverse effects of statins (eg, rhabdomyolysis and diabetes mellitus), we recommend that combination therapy with a statin and a Rho-kinase inhibitor be used rather than monotherapy with a high-dose statin (Figure 6). Further understanding of the molecular mechanisms of the pleiotropic effects of statins will allow us to select appropriate therapies for patients. Excessive increase in SmgGDS expression may enhance its guanine nucleotide exchange factor function and, in turn, may cause the adverse effects of high-dose statins, which are still poorly understood. Activation of the guanine nucleotide exchange factor function of SmgGDS may explain the adverse effects by high-dose statins. Our present finding of the novel mechanisms of actions of statins through enhancement of Rac1 degradation, in addition to inhibition of mevalonate and Rho/Rho-kinase pathways, may have significant impacts on the current use of statins and the development of new cardiovascular drugs.
Supplementary Material
Significance.
Statins are widely used in billions of patients for primary and secondary prevention of cardiovascular diseases. There are 2 known mechanisms of statins, including the inhibition of cholesterol synthesis in the liver and that of geranylgeranyl pyrophosphate pathway with resultant inhibition of small G proteins. We have previously demonstrated that the cholesterol-independent, pleiotropic effects of regular doses of statins are mediated mainly through inhibition of the Rac1 pathway rather than the Rho/Rho-kinase pathway, although the molecular mechanisms of the selective inhibition of the Rac1 pathway remain to be elucidated. In this study, we were able to demonstrate the novel and the third mechanism of statins, by which they upregulate small GTP-binding protein dissociation stimulator with resultant selective degradation of Rac1 in animals and humans. Our findings may have significant impacts on our understanding of the cardiovascular effects of statins and the development of new class of cardiovascular drugs.
Acknowledgments
We thank A. Saito, C. Miyamoto, and T. Hiroi for excellent technical assistance and Asahi-Kasei Pharma, Pfizer, Kowa Pharmaceutical, and Daiichi Sankyo, Co, Tokyo, Japan, for providing hydroxyfasudil, atorvastatin, pitavastatin, and pravastatin, respectively.
Sources of Funding
This work was supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas (Signaling Functions of Reactive Oxygen Species), the Grant-in-Aid for Tohoku University Global COE for Conquest of Signal Transduction Diseases with Network Medicine, and the Grants-in-Aid for Scientific Research; all of which are from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.300922/-/DC1.
Disclosures
None.
References
- 1.Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Levine GN, Keaney JF, Jr, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med. 1995;332:512–521. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- 3.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 4.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 7.Rashid M, Tawara S, Fukumoto Y, Seto M, Yano K, Shimokawa H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ J. 2009;73:361–370. doi: 10.1253/circj.cj-08-0817. [DOI] [PubMed] [Google Scholar]
- 8.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wassmann S, Laufs U, Bäumer AT, Müller K, Konkol C, Sauer H, Böhm M, Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 11.Maack C, Kartes T, Kilter H, Schäfers HJ, Nickenig G, Böhm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 12.Yagi S, Aihara K, Ikeda Y, Sumitomo Y, Yoshida S, Ise T, Iwase T, Ishikawa K, Azuma H, Akaike M, Matsumoto T. Pitavastatin, an HMG-CoA reductase inhibitor, exerts eNOS-independent protective actions against angiotensin II induced cardiovascular remodeling and renal insufficiency. Circ Res. 2008;102:68–76. doi: 10.1161/CIRCRESAHA.107.163493. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Kaibuchi K, Mizuno T, Hiroyoshi M, Shirataki H, Takai Y. Purification and characterization from bovine brain cytosol of proteins that regulate the GDP/GTP exchange reaction of smg p21s, ras p21-like GTP-binding proteins. J Biol Chem. 1990;265:16626–16634. [PubMed] [Google Scholar]
- 14.Mizuno T, Kaibuchi K, Yamamoto T, Kawamura M, Sakoda T, Fujioka H, Matsuura Y, Takai Y. A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc Natl Acad Sci U S A. 1991;88:6442–6446. doi: 10.1073/pnas.88.15.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams CL. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell Signal. 2003;15:1071–1080. doi: 10.1016/s0898-6568(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamel B, Monaghan-Benson E, Rojas RJ, Temple BR, Marston DJ, Burridge K, Sondek J. SmgGDS is a guanine nucleotide exchange factor that specifically activates RhoA and RhoC. J Biol Chem. 2011;286:12141–12148. doi: 10.1074/jbc.M110.191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanning CC, Daddona JL, Ruiz-Velasco R, Shafer SH, Williams CL. The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J Biol Chem. 2004;279:44197–44210. doi: 10.1074/jbc.M404977200. [DOI] [PubMed] [Google Scholar]
- 18.Thill R, Campbell WB, Williams CL. Identification and characterization of the unique guanine nucleotide exchange factor, SmgGDS, in vascular smooth muscle cells. J Cell Biochem. 2008;104:1760–1770. doi: 10.1002/jcb.21740. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda Y, Aihara K, Sato T, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 21.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 22.Stroes ES, Koomans HA, de Bruin TW, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication. Lancet. 1995;346:467–471. doi: 10.1016/s0140-6736(95)91322-x. [DOI] [PubMed] [Google Scholar]
- 23.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergmann MW, Rechner C, Freund C, Baurand A, El Jamali A, Dietz R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J Mol Cell Cardiol. 2004;37:681–690. doi: 10.1016/j.yjmcc.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 26.Coluccia AM, Vacca A, Duñach M, Mologni L, Redaelli S, Bustos V, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes β-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;412:211–216. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 28.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frick M, Dulak J, Cisowski J, Józkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229–236. doi: 10.1016/s0021-9150(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 30.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 31.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q, Liao JK. Pleiotropic effects of statins. Circ. J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg TJ, Gastonguay AJ, Lorimer EL, Kuhnmuench JR, Li R, Fields AP, Williams CL. Splice variants of SmgGDS control small GTPase prenylation and membrane localization. J Biol Chem. 2010;285:35255–35266. doi: 10.1074/jbc.M110.129916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takefuji M, Mori K, Morita Y, Arimura N, Nishimura T, Nakayama M, Hoshino M, Iwamatsu A, Murohara T, Kaibuchi K, Amano M. Rho-kinase modulates the function of STEF, a Rac GEF, through its phosphorylation. Biochem Biophys Res Commun. 2007;355:788–794. doi: 10.1016/j.bbrc.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Custodis F, Eberl M, Kilter H, Böhm M, Laufs U. Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc Res. 2006;71:342–351. doi: 10.1016/j.cardiores.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Laufs U, Kilter H, Konkol C, Wassmann S, Böhm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 37.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 38.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 41.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takakura A, Miyoshi J, Ishizaki H, Tanaka M, Togawa A, Nishizawa Y, Yoshida H, Nishikawa Si, Takai Y. Involvement of a small GTP-binding protein (G protein) regulator, small G protein GDP dissociation stimulator, in antiapoptotic cell survival signaling. Mol Biol Cell. 2000;11:1875–1886. doi: 10.1091/mbc.11.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 44.Adam O, Frost G, Custodis F, Sussman MA, Schäfers HJ, Böhm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, Sakaino N, Kitagawa A, MUSASHI-AMI Investigators Usefulness of hydrophilic vs lipophilic statins after acute myocardial infarction: sub-analysis of MUSASHI-AMI. Circ J. 2007;71:1348–1353. doi: 10.1253/circj.71.1348. [DOI] [PubMed] [Google Scholar]
- 46.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.