Summary
Human papillomavirus–related oropharyngeal squamous cell carcinoma has a unique biology and improved prognosis. A new focus is to identify prognostic biomarkers specifically in this human papillomavirus–positive cohort. We analyzed cyclin D1 immunostaining on a tissue microarray of patients with known clinical follow-up and p16 and human papillomavirus status (by E6/E7 RNA in situ hybridization). Cyclin D1 staining was read visually and digitally. Cutoffs of 5%, 10%, and 30% were separately analyzed as was linear intensity data derived from the image analysis. For the 202 tumors, cyclin D1 expression was > 10% in 25.7% (visual) and 35.5% (digital) of the cases. It was > 30% in 15.8% (visual) and 16.5% (digital) of the cases. High cyclin D1 by both methods, cutoffs, and expression intensity was associated with poorer overall, disease-free, and disease-specific survival in univariate analysis. However, low cyclin D1 expression was also tightly associated with human papillomavirus RNA (P < 1.0 × 10−18 for all cutoffs) and p16 positivity (P < 1.0 × 10−14 for all cutoffs). In multivariate analysis using the digital 30% cutoff (the strongest cyclin D1 assessment method), only T stage, p16 status, smoking, and treatment approach associated with survival. Intensity of cyclin D1 expression did, however, significantly substratify the human papillomavirus RNA–positive patients into prognostic subgroups independent of other variables. In summary, cyclin D1 overexpression correlates strongly with patient survival in oropharyngeal squamous cell carcinoma, but its relationship with human papillomavirus status is very tight, and the complex nature of this correlation likely limits any clinical application for cyclin D1 assessment.
Keywords: Human papillomavirus, Oropharyngeal squamous cell carcinoma, p16, Cyclin D1, Prognosis
1. Introduction
Human papillomavirus (HPV)–related oropharyngeal squamous cell carcinoma (OSCC) has a unique biology and improved prognosis [1]. Although most patients respond well to therapy, approximately 10% to 15% of patients develop progressive disease, predominantly distant metastases [2,3]. T stage [4,5] and smoking status [1,6] are well established as strong prognostic factors in these cancers, but the current focus is to identify more robust prognostic biomarkers to better risk stratify the more and less aggressive HPV-positive OSCC cases for determining appropriate therapy.
Cyclin D1 functions as a cell cycle regulator. It regulates progression of the cell cycle at the G1-S checkpoint. When expressed, it forms complexes with cyclin-dependent kinase 4 or 6 in the mid-G1 phase that are regulated by inhibitors such as p16-INK4a [7]. As a result of either gene amplification [8] or rearrangement [9], overexpression of cyclin D1 can contribute significantly to tumor growth in head and neck squamous cell carcinoma (SCC) [10,11].
Both cyclin D1 and HPV are associated with tumorigenesis at different points within the cell cycle. HPV oncoprotein E7 binds to retinoblastoma protein (Rb), causing its inactivation. This results in loss of transcriptional repression of p16-INK4a, which is then markedly overexpressed. As mentioned, p16 normally regulates the cyclin D1/cyclin-dependent kinase complexes. As such, cyclin D1 and HPV E7-induced signaling effects appear to overlap significantly, with HPV-related tumors generally having low levels of cyclin D1 expression [12]. The relationship between cyclin D1 expression and prognosis in OSCC appears to be complex [13-15]. Although many studies have assessed cyclin D1 amplification and/or overexpression in head and neck SCC grouping multiple anatomical subsites or focusing on laryngeal SCC [8,9,11,16], only a few have examined it in just OSCC, with fewer still controlling for HPV. Most of these studies show cyclin D1 overexpression to be a marked adverse prognosticator [15] without controlling for HPV status. Others have shown that cyclin D1 overexpression is highly correlated with HPV status, and 2 studies, in particular, have shown overexpression of cyclin D1 to retain significant prognostic significance in OSCC independent of tumor HPV (or p16) status [17,18]. Notably, the methods used and the staining extent cutoffs used to classify tumors as positive or negative have varied widely among these studies.
This study aimed to more clearly elucidate the relationship between cyclin D1 expression, tumor HPV status, and prognosis in OSCC using a tissue microarray cohort of OSCC patients with robust clinical follow-up and known HPV status by ultrasensitive RNA in situ hybridization (ISH).
2. Materials and methods
With approval from the Human Research Protection Office, new primary cases of oropharyngeal SCC from 1997 to 2008 were identified from Radiation Oncology and Otolaryngology Head and Neck Surgery databases. Clinical follow-up information was obtained from a clinician database (W. L. T.) and electronic medical records, including survival data, smoking, and other clinical variables. All cases were reviewed by 1 study pathologist (J. S. L.) and confirmed as SCC [19-21] (Fig. 1). Other pathologic features were obtained by pathology report review. All patients were treated either with primary surgery with or without postoperative radiation and chemotherapy or were treated definitively with radiation therapy with or without chemotherapy. All radiation therapy was intensity modulated and was administered by a single radiation oncologist (W. L. T.). The paraffin blocks were obtained from department files, and a tissue microarray constructed. According to the amount of available biopsied or resected tumor tissue, duplicate 2 mm (or if only limited amounts of tumor tissue present, 0.6 mm) punches were taken. Because most of the cases (75%) were treated with primary surgery, most cases on the array had the larger (2 mm) punches. For staining evaluation, cases where there was less than 10% surface area consisting of tumor (averaged across the 2 microarray punches) were excluded.
Fig. 1.
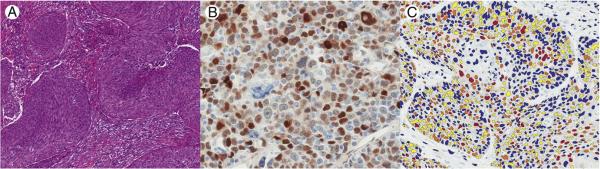
A, Nonkeratinizing SCC with well-circumscribed nests of tumor cells that have round to oval nuclei, inconspicuous nucleoli, and high mitotic activity (hematoxylin and eosin, magnification ×100). There is a conspicuous absence of stromal reaction. These findings are typical for HPV-related OSCC. B, Immunohistochemistry for cyclin D1 in OSCC with variably intense nuclear brown staining in tumor cells (hematoxylin and eosin, magnification ×400). C, Aperio Image Analysis Toolbox results showing variable nuclear colors corresponding to negative (blue), low (yellow), moderate (orange), and high (red) intensity nuclear staining for cyclin D1 (magnification ×200).
2.1. RNA ISH for high-risk HPV
ISH for high-risk HPV E6/E7 messenger RNA (mRNA) was performed by hand using the RNAscope HPV kit (Advanced Cell Diagnostics, Inc, Hayward, CA) [5,22,23] as previously described. The array (and control) slides were previously read by 1 study pathologist (J. S. L.) and classified simply as positive or negative. Positive cases had granular cytoplasmic and/or nuclear brown staining that was above any signal present on the DapB-negative control slide.
2.2. Immunohistochemistry for p16
Immunohistochemistry was performed for p16 on 4-μm whole tumor formalin-fixed, paraffin-embedded sections using a monoclonal antibody to p16 (clone E6H4, monoclonal, 1:1 dilution; Roche/MTM/Ventana Laboratories, Tucson, AZ) on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc, Tucson, AZ) according to standard protocols with appropriate controls including normal tonsil (negative) and a known positive OSCC case. Antigen retrieval, standard on the machine, used the Ventana CC1, EDTA-Tris, pH 8.0 solution. Staining was read by 1 study pathologist (J. S. L.). Staining was considered positive only if there was nuclear and cytoplasmic staining (of any intensity) and was graded in a quartile manner as follows: 0, no staining; 1+, 1% to 25% staining; 2+, 26% to 50%; 3+, 51% to 75%; and 4+, 76% to 100%. Cases were then classified binarily as positive with extensive staining (>50%; 3+ or 4+) and negative with no or only partial staining (negative or <50%; 1+ or 2+), as it is well established that only extensive expression correlates with the presence of transcriptionally active HPV [24,25].
2.3. Immunohistochemistry for cyclin D1
Immunohistochemistry was performed for cyclin D1 (clone SP4-4; monoclonal; prediluted; Ventana Medical Systems, Inc) using a Ventana Benchmark automated immunostainer. Antigen retrieval was performed with a pH 8.0 EDTA-Tris buffer, and antibody was incubated at 37°C for 16 minutes.
Cyclin D1 staining was assessed visually for percentage of positive cells by 2 study pathologists (J. B. S. and J. S. L.) and also by digital image analysis using Aperio’s Image Analysis Toolbox, Membrane Algorithm, version 9 (Aperio, Inc, Vista, CA) (Fig. 1). The standard (“out of the box”) algorithm settings were used. For the visual analysis, any tumor cell with cyclin D1 nuclear staining was considered as positive, regardless of intensity, and results were reported from 0% to 100% in 5% increments. Several binary cutoffs were modeled based on the literature and the staining data. Furthermore, the intensity of staining was taken into account. The image analysis software also provides a cell intensity value as a continuous variable. This intensity score was analyzed as a continuous variable and also as a binary variable divided into equal groups both above (“high”) and below (“low”) the median intensity value. The image analysis software also provides intensity scores of 1+ to 3+ for individual nuclei, with 1+ being weakly positive to 3+ being strongly positive. Using this, we performed additional analyses of staining with 10% and 30% cutoffs, but only counting the 2+ and 3+ cells as positive.
2.4. Statistical analysis
Associations between cyclin D1 and the other clinical and pathologic variables were determined by Fisher exact tests or χ2 tests, as proper. A Spearman rank-based correlation coefficient was calculated between the visual and digital scoring of cyclin D1. Overall, disease-free, and disease-specific survival were defined as the time interval between the start of treatment (either the date of surgical resection or start of radiation and/or chemotherapy) and the date of death due to any cause, the date of death or the date of first tumor recurrence, or the date of death in patients with known recurrent tumor present at death, respectively. The Kaplan-Meier product limit method was used to estimate empirical survival probabilities as illustrated by curves. Log-rank tests were applied to examine survival differences, indicating the significance of a categorical variable being prognostic for a survival end point. Multivariate Cox proportional hazard models were also performed to investigate the independent prognostic ability of variables after accounting for the other clinical and pathologic variables. These were indicated by hazard ratios, associated 95% confidence intervals, and Wald test P values. All tests were 2 sided with the level for significance set at 0.05. All analyses were performed in statistical software R 2.14.1 (http://cran.r-project.org).
3. Results
Demographic and clinical data for the overall cohort of 202 patients are summarized in Table 1. The patients had typical characteristics for a contemporary OSCC cohort, with most being white, male, and current or former smokers. Approximately 80% of patients were p16 positive and HPV positive by RNA ISH, and most had nonkeratinizing histology. Almost 90% of patients presented with nodal metastases, and 81.1% of patients were treated with primary surgery with or without postoperative radiation. Approximately two-thirds of patients’ tumors were T1 or T2.
Table 1.
Clinical and pathologic features
| Characteristics: N = 202 | n (%) a |
|---|---|
| Age (y), mean ± SD | 56.8 ± 9.4 |
| Sex | |
| Female | 24 (11.9) |
| Male | 178 (88.1) |
| Race | |
| White | 178 (88.1) |
| Other | 24 (11.9) |
| Smokingb | |
| No | 57 (29.2) |
| Yes | 138 (70.8) |
| Tumor histology | |
| Keratinizing | 46 (23.2) |
| Nonkeratinizing with maturation | 43 (21.7) |
| Nonkeratinizing | 109 (55.1) |
| HPV DNA by ISH | |
| Negative | 67 (37.0) |
| Positive | 114 (63.0) |
| HPV RNA by ISH | |
| Negative | 39 (20.6) |
| Positive | 150 (79.4) |
| p16 immunohistochemistryc | |
| Negative | 47 (23.4) |
| Positive | 154 (76.6) |
| T stage | |
| T1/T2 | 130 (65.7) |
| T3/T4 | 68 (34.3) |
| N Stage | |
| N0 | 24 (12.0) |
| N1/2/3 | 176 (88.0) |
| Overall stage | |
| I/II | 14 (7.0) |
| III/IV | 186 (93.0) |
| Treatment | |
| Definitive radiation | 37 (18.9) |
| Surgery ± radiation | 159 (81.1) |
| Chemotherapy | |
| No | 88 (50.9) |
| Yes | 85 (49.1) |
| Resection margin status | |
| Negative | 136 (84.0) |
| Positive | 26 (16.0) |
| Distant metastasis | |
| Negative | 183 (90.6) |
| Positive | 19 (9.4) |
| Death (any cause) | |
| Negative | 138 (68.3) |
| Positive | 64 (31.7) |
| Death (with recurrent cancer) | |
| Negative | 171 (85.5) |
| Positive | 29 (14.5) |
Only age and sex data are available for the full 202 patients. For the other major variables, the overall numbers are less than 202 patients due to lack of complete information availability.
Nonsmokers versus any lifetime smoking.
Negative, no staining or staining of less than 50% of tumor cells; positive, staining of more than 50% of tumor cells.
The 2 methods (visual and digital) of assessing cyclin D1 staining distribution strongly correlated with each other (using the ≥10% cutoff; Spearman rank correlation coefficient, 0.77). By visual analysis, 52 patients (25.7%) had 10% or more of the tumor cells positive for cyclin D1, and by digital analysis, 71 patients (35.5%) had 10% or more positive. By visual analysis, 32 patients (15.8%) had 30% or more of the tumor cells positive for cyclin D1, and by digital analysis, 33 patients (16.5%) had 30% or more positive.
Digital image analysis also provided intensity as a continuous variable between 170 and 227 (arbitrary units) and as categorical 0 to 3+ cell intensity classifiers. Of the 171 informative tumors, 23 (13.5%) had 10% or greater of the tumor cells with nuclear intensity of 2+ or 3+ (positive) for cyclin D1. In other words, this group consisted of tumors that not only had a high proportion of cyclin D1–positive cells, but also high intensity of expression.
The intensity and distribution, as expected, were highly related to each other. Intensity correlated strongly with the digital image analysis (and visual) distribution scores in continuous scale (Spearman correlation, 0.87 and 0.64, respectively) and also with the binary scales (P < .0001 both for the 10% and 30% cutoffs). Cyclin D1 expression, using all 3 different binary distribution cutoffs, and whether by visual or digital image analysis, strongly correlated with tumor HPV RNA and p16 status (Table 2). Most patients with HPV RNA–positive and/or p16-positive tumors had low cyclin D1 expression. All associations were statistically significant by all cutoffs with very low P values. Cyclin D1 intensity also strongly correlated with HPV RNA and p16 status.
Table 2.
Cyclin D1 overexpression by HPV or p16 status, assessed by digital analysis
| Cyclin D1 expression a |
HPV status (%) |
p16 status b (%) |
||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| 10% cutoff | ||||
| Negative | 2 (1.6) | 122 (98.4) | 7 (5.5) | 121 (94.5) |
| Positive | 36 (57.1) | 27 (42.9) | 39 (54.9) | 32 (45.1) |
| 30% cutoff | ||||
| Negative | 12 (7.7) | 144 (92.3) | 16 (9.6) | 150 (90.4) |
| Positive | 26 (83.9) | 5 (16.1) | 30 (90.9) | 3 (9.1) |
| 10% and 2+ or 3+ cutoff | ||||
| Negative | 12 (8.5) | 129 (91.5) | 15 (10.1) | 133 (89.9) |
| Positive | 26 (83.9) | 5 (16.1) | 30 (90.9) | 3 (9.1) |
| Binary intensity cutoff c | ||||
| Negative (“low”) | 2 (2.3) | 84 (97.7) | 7 (7.9) | 82 (92.1) |
| Positive (“high”) | 35 (41.2) | 50 (58.8) | 37 (46.3) | 53 (53.7) |
All differences were statistically significant (P < .0001).
Negative, no staining or staining of less than 50% of tumor cells; positive, staining of more than 50% of tumor cells.
Binary “low” and “high” values were the digital image analysis intensity values divided at the median.
Among the other variables, low cyclin D1 expression by all 3 distribution cutoffs statistically significantly correlated with white ethnicity, lower smoking rates, positive nodal metastases, higher overall tumor stage (I/II versus III/IV), and treatment approach. These are also all features that typify patients with HPV-related oropharyngeal SCC.
3.1. Survival analysis
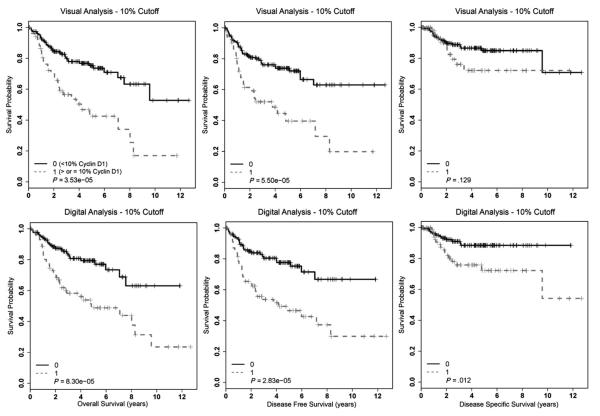
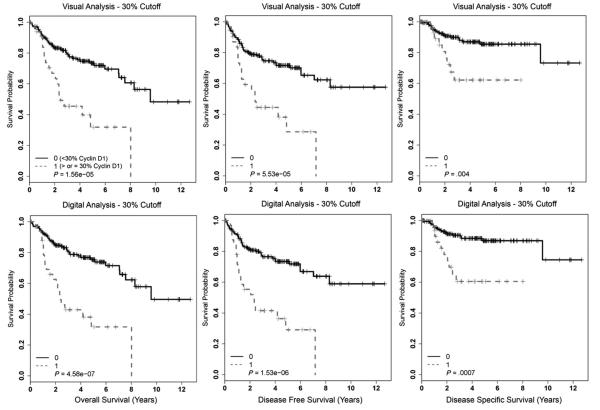
Cyclin D1 overexpression by several different methods and cutoffs correlated with statistically significantly worse survival in univariate analysis (Table 3). Kaplan-Meier survival curves using the 10% cutoff are presented in Fig. 2 and, for the 30% cutoff, are presented in Fig. 3. Cyclin D1 overexpression by both methods and cutoffs statistically significantly correlated with worse overall, disease-free, and disease-specific survival. In digital analysis considering only the 2+ and 3+ intensity nuclei with a 10% cutoff, tumors with 10% or greater of cells positive had statistically significantly worse overall (P = 7.91 × 10−7), disease-free (P = 6.22 × 10−6), and disease-specific (P = .008) survival. Although strongly correlating with poorer survival, the magnitude of these associations with patient survival is essentially equivalent to the other methods and cutoffs.
Table 3.
Univariate survival analysis by respective distribution, intensity, or combined intensity and distribution cutoff among the entire cohort and then among just the HPV RNA ISH–positive cohort
| Cyclin D1 variable | Overall survival | Disease-free survival | Disease-specific survival |
|---|---|---|---|
| Whole patient cohort | |||
| Image analysis 5% distribution cutoff | P = .17 | P = .10 | P = .55 |
| Visual 10% distribution cutoff | P < .0001 | P < .0001 | P = .13 |
| Image analysis 10% distribution cutoff | P < .0001 | P < .0001 | P = .012 |
| Visual 30% distribution cutoff | P < .0001 | P < .0001 | P = .004 |
| Image analysis 30% distribution cutoff | P < .0001 | P < .0001 | P = .0007 |
| Binary intensity cutoff (high vs low) a | P < .0001 | P < .0001 | P = .0002 |
| Combination of image analysis 10% distribution cutoff and binary intensity |
P < .0001 | P < .0001 | P = .005 |
| Combination of image analysis 30% distribution cutoff and binary intensity |
P < .0001 | P < .0001 | P = .0002 |
| HPV RNA ISH–positive cohort | |||
| Image analysis 5% distribution cutoff | P = .34 | P = .70 | P = .38 |
| Visual 10% distribution cutoff | P = .89 | P = .82 | P = .23 |
| Image analysis 10% distribution cutoff | P = .55 | P = .22 | P = .30 |
| Visual 30% distribution cutoff | P = .58 | P = .52 | P = .69 |
| Image analysis 30% distribution cutoff | P = .066 | P = .12 | P = .28 |
| Binary intensity cutoff (high vs low) a | P = .038 | P = .014 | P = .015 |
| Combination of image analysis 10% distribution cutoff and binary intensity |
P = .13 | P = .10 | P = .057 |
| Combination of image analysis 30% distribution cutoff and binary intensity |
P = .029 | P = .02 | P = .025 |
NOTE. P values are calculated from log-rank tests.
Negative, no staining or staining of less than 50% of tumor cells; positive, staining of more than 50% of tumor cells.
Fig. 2.
Kaplan-Meier survival curves for cyclin D1 expression for the entire OSCC cohort using visual or digital analysis with a 10% cutoff.
Fig. 3.
Kaplan-Meier survival curves for cyclin D1 expression for the entire OSCC cohort using visual or digital analysis with a 30% cutoff.
Univariate survival analysis by cyclin D1 image analysis intensity score (dichotomized at median into high and low categories) also showed it to statistically significantly correlate with poorer survival (Table 3). When combining binary intensity scores with the 10% and 30% distribution scores, univariate survival analysis showed worse survival for patients with high distribution and high intensity cyclin D1 expression. However, the significance of the associations (ie, the P values) was not more than those for the binary distribution scores alone. In other words, for the entire cohort, intensity of cyclin D1 expression did not significantly increase the ability of cyclin D1 to predict survival beyond the distribution of expression alone (Table 3).
We examined cyclin D1 expression by all methods and cutoffs in just the HPV RNA–positive patients and separately in just the p16 immunohistochemistry–positive patients, and none of the distribution cutoffs showed statistically significantly different overall, disease-free, or disease-specific survival. Among these 149 HPV RNA ISH–positive patients, 15 (12.3%) of 122 with digital analysis cyclin D1 10% or less versus 6 (22.2%) of 27 with 10% or greater cyclin D1 expression developed recurrent disease. This difference was not statistically significant (P = .18). Using the digital analysis 30% cutoff, for the HPV RNA ISH–positive patients with cyclin D1 30% or less, 20 (13.9%) of 144 developed recurrent disease of any kind versus 1 (20.0%) of 5 with 30% or greater expression (not statistically significantly different, P = .70). Kaplan-Meier survival curves for the digital analysis with the 10% cutoff for just the HPV RNA–positive patients are presented in Fig. 4. In a follow-up analysis (data not shown), we also examined a 5% binary cutoff by digital analysis in just the HPV RNA–positive patients. Again, we found no significant differences in overall, disease-free, or disease-specific survival.
Fig. 4.
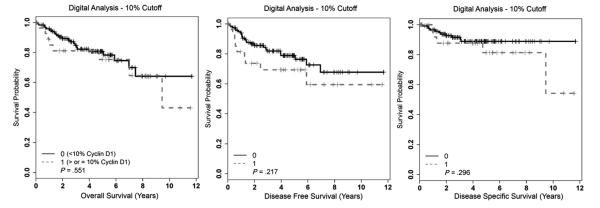
Kaplan-Meier survival curves for cyclin D1 expression for just the HPV RNA ISH–positive OSCC cohort using digital analysis with a 10% cutoff.
However, when we examined intensity of expression in just the HPV RNA–positive patients, we found that binary intensity score was statistically significantly associated with overall, disease-free, and disease-specific survival (Table 3). The data here suggested that intensity of cyclin D1 expression, rather than distribution of expression, was important in just the HPV RNA–positive OSCC patients.
We also examined cyclin D1 expression by all methods and cutoffs in just the HPV RNA–negative patient cohort (39 patients), and none of the methods or cutoffs showed statistically significantly worse overall, disease-free, or disease-specific survival for the cyclin D1 overexpressing tumors (data not shown).
To control for other variables, multivariate analysis was performed for the entire cohort using just the digital 30% distribution cutoff because this method and cutoff combination had the highest magnitude of association with patient survival in univariate analysis. Controlling for other major clinical and pathologic variables, cyclin D1 expression using this cutoff was not statistically significantly correlated with overall, disease-free, or disease-specific survival (Table 4). However, as expected for a contemporary cohort of OSCC patients, T stage and p16 expression were highly associated with overall, disease-free, and disease-specific survival.
Table 4.
Multivariate analysis of digital analysis using a 30% cutoff for cyclin D1 expression controlling for other variables
| Variable | Hazard ratio | Lower 95th | Upper 95th | P |
|---|---|---|---|---|
| Overall survival | ||||
| Race (white vs other) | 0.35 | 0.14 | 0.83 | .02 |
| Smoking (yes vs no) | 2.32 | 0.90 | 5.97 | .08 |
| HPV RNA ISH (− vs +) | 1.73 | 0.40 | 7.42 | .47 |
| p16 (+ vs −) a | 0.20 | 0.06 | 0.69 | .01 |
| Treatment (surgery vs postoperative vs definitive) | 0.54 | 0.29 | 1.02 | .06 |
| T stage (T3/T4 vs T1/T2) | 3.27 | 1.80 | 5.94 | <.001 |
| Digital analysis at ≥30%cutoff (+ vs −) | 1.30 | 0.44 | 3.84 | .63 |
| Disease-specific survival | ||||
| Race (white vs other) | 0.53 | 0.16 | 1.68 | .28 |
| Smoking (yes vs no) | 2.35 | 0.60 | 9.19 | .22 |
| HPV RNA ISH (− vs +) | 5.04 | 0.52 | 48.76 | .16 |
| p16 (+ vs −) a | 0.19 | 0.04 | 0.92 | .04 |
| Treatment (surgery vs postoperative vs definitive) | 0.31 | 0.13 | 0.76 | .01 |
| T stage (T3/T4 vs T1/T2) | 5.04 | 2.07 | 12.28 | <.001 |
| Digital analysis at ≥30% cutoff (+ vs −) | 2.33 | 0.34 | 15.87 | .39 |
| Disease−free survival | ||||
| Race (white vs other) | 0.36 | 0.15 | 0.87 | .02 |
| Smoking (yes vs no) | 2.06 | 0.86 | 4.91 | .10 |
| HPV.RNA.ISH (− vs +) | 1.38 | 0.35 | 5.43 | .64 |
| p16 (+ vs −) a | 0.25 | 0.07 | 0.86 | .03 |
| Treatment (surgery vs postoperative vs definitive) | 0.53 | 0.28 | 0.99 | .05 |
| T stage (T3/T4 vs T1/T2) | 2.89 | 1.62 | 5.14 | <.001 |
| Digital analysis at ≥30% cutoff (+ vs −) | 1.24 | 0.4 | 3.52 | .68 |
Negative, no staining or staining of less than 50% of tumor cells; positive, staining of more than 50% of tumor cells.
Because binary cyclin D1 intensity significantly correlated with survival in univariate analysis in just the HPV RNA–positive population and most of these patients had cyclin D1 less than 10%, we performed multivariate survival analysis combining binary cyclin D1 intensity and digital image analysis distribution with the 10% cutoff. Among just the less than 10% cyclin D1 distribution patients, those with high intensity expression still had significantly poorer overall, disease-free, and disease-specific survival than those with low-intensity expression, independent of all other major variables including T stage, treatment type, and HPV RNA and p16 status (Supplementary Table 1).
4. Discussion
The search for prognostic markers in head and neck cancers has been ongoing for 50+ years. As a cell cycle regulator, cyclin D1 is an attractive potential marker of tumor aggressiveness and has emerged as one of a pool of molecules that is consistently prognostic in head and neck SCC across anatomic subsites, whether by gene amplification or by immunohistochemical expression [7-11,15,16]. It is overexpressed by gene amplification or by signaling dysregulation in more than 80% of non–HPV-related head and neck SCC [10,26].
The relationship between HPV-related OSCC and cyclin D1 expression is not totally clear, however. Few studies have evaluated cyclin D1 OSCC cases, specifically [13,15,17,18], and some of these have found it to be an independent prognostic marker. Only 4 have controlled for tumor HPV/p16 status [14,17,18,27]. These studies have shown that HPV-related OSCC is strongly associated with low cyclin D1 expression and that most HPV-negative tumors have elevated cyclin D1 expression [28]. Although cyclin D1 has been prognostic in univariate analysis, they have had somewhat conflicting results on whether there is independent prognostic value after controlling for HPV status. Some have shown cyclin D1 overexpression to be associated with poorer prognosis in the HPV-related SCC cases [17,18], whereas others have not [13,27]. Furthermore, these studies have used patients with different primary treatment approaches, have used numerous different primary antibodies for cyclin D1 immunohistochemistry, and have also used different cutoffs for positivity, ranging from 5% to 50%. These studies have controlled for HPV by DNA polymerase chain reaction or ISH along with p16 overexpression or have controlled just for p16 overexpression as a surrogate marker for HPV. None have actually evaluated or controlled for the presence HPV E6/E7 mRNA.
In this study, cyclin D1 overexpression does predict poorer survival, by most methods and cutoffs used. However, this predictive ability is closely linked to, and complicated by, its strong correlation with tumor HPV RNA status. There is a remarkably strong inverse relationship between cyclin D1 expression and tumor HPV and p16 status [17,18]. In our study, only 14.4% of tumors with detectable HPV E6/E7 mRNA had cyclin D1 expression above 10%, and only 2.7% had expression above 30%.
Why tumors with transcriptionally active HPV have low cyclin D1 has not been clearly elucidated. By some studies, p16 overexpression, by a variety of mechanisms, should result in high cyclin D1 levels [29]. However, p16 also blocks cyclin D1 from forming complexes with cyclin-dependent kinases. This leaves cyclin D1 free and unbound in the cell, and some have suggested that this then causes it to be rapidly degraded [30]. This theory would help explain why cyclin D1 levels are low in these tumors.
Our results support the literature that suggests that cyclin D1 overexpression is prognostically adverse in OSCC. However, given that most HPV RNA–positive OSCC patients have very low cyclin D1 expression, staining intensity must also be assessed to risk stratify patients. As such, interpretation of the immunohistochemistry slides visually by pathologists likely will not be effective for substratification of staining into clinically different groups, thus limiting any potential application of cyclin D1 staining in routine clinical practice in OSCC.
In summary, low cyclin D1 expression strongly correlates with improved patient survival in OSCC, but in our study, this was predominantly due to its strong association with the presence of transcriptionally active HPV. HPV RNA–positive tumors consistently have low cyclin D1 expression but still can be segregated into prognostic subgroups by the intensity of staining.
Supplementary Material
Acknowledgments
The authors would like to thank Jianping Li, BS, and Autumn Watson, BA, for their expert technical assistance with the immunohistochemistry studies and tissue microarray construction, respectively. We would also like to thank Xiao-Jun Ma, PhD; John J. Flanagan, PhD; and Yuling Luo, PhD, at Advanced Cell Diagnostics, Inc, for performing the HPV RNA ISH studies. The authors also would like to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center, and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Presented at the United States and Canadian Academy of Pathology, 101st Annual Meeting, Vancouver, British Columbia, Canada 2012.
Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.humpath.2013.01.021.
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;36:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119:81–9. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–94. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 5.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BD, Haffty BG, Sasaki CT. Molecular markers in head and neck squamous cell carcinoma: their biological function and prognostic significance. Ann Otol Rhinol Laryngol. 2001;110:221–8. doi: 10.1177/000348940111000304. [DOI] [PubMed] [Google Scholar]
- 8.Meredith SD, Levine PA, Burns JA, et al. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch Otolaryngol Head Neck Surg. 1995;121:790–4. doi: 10.1001/archotol.1995.01890070076016. [DOI] [PubMed] [Google Scholar]
- 9.Akervall JA, Michalides RJ, Mineta H, et al. Amplification of cyclin D1 in squamous cell carcinoma of the head and neck and the prognostic value of chromosomal abnormalities and cyclin D1 overexpression. Cancer. 1997;79:380–9. [PubMed] [Google Scholar]
- 10.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 11.Fracchiolla NS, Pruneri G, Pignataro L, et al. Molecular and immunohistochemical analysis of the bcl-1/cyclin D1 gene in laryngeal squamous cell carcinomas: correlation of protein expression with lymph node metastases and advanced clinical stage. Cancer. 1997;79:1114–21. [PubMed] [Google Scholar]
- 12.Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck. 2004;26:1–9. doi: 10.1002/hed.10335. [DOI] [PubMed] [Google Scholar]
- 13.Perisanidis C, Perisanidis B, Wrba F, et al. Evaluation of immunohistochemical expression of p53, p21, p27, cyclin D1, and Ki67 in oral and oropharyngeal squamous cell carcinoma. J Oral Pathol Med. 2011;41:40–6. doi: 10.1111/j.1600-0714.2011.01071.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar RV, Kadkol SS, Daniel R, Shenoy AM, Shah KV. Human papillomavirus, p53 and cyclin D1 expression in oropharyngeal carcinoma. Int J Oral Maxillofac Surg. 2003;32:539–43. [PubMed] [Google Scholar]
- 15.Yu Z, Weinberger PM, Haffty BG, et al. Cyclin d1 is a valuable prognostic marker in oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2005;11:1160–6. [PubMed] [Google Scholar]
- 16.Kyomoto R, Kumazawa H, Toda Y, et al. Cyclin-D1-gene amplification is a more potent prognostic factor than its protein over-expression in human head-and-neck squamous-cell carcinoma. Int J Cancer. 1997;74:576–81. doi: 10.1002/(sici)1097-0215(19971219)74:6<576::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Hong AM, Dobbins TA, Lee CS, et al. Use of cyclin D1 in conjunction with human papillomavirus status to predict outcome in oropharyngeal cancer. Int J Cancer. 2010;128:1532–45. doi: 10.1002/ijc.25479. [DOI] [PubMed] [Google Scholar]
- 18.Hafkamp HC, Mooren JJ, Claessen SM, et al. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009;22:686–98. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 19.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–94. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JS, Jr, Scantlebury JB, Luo J, Thorstad WL. Tumor cell anaplasia and multinucleation are predictors of disease recurrence in oropharyngeal squamous cell carcinoma, including among just the human papillomavirus-related cancers. Am J Surg Pathol. 2012;36:1036–46. doi: 10.1097/PAS.0b013e3182583678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis JS., Jr p16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6:75–82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao G, Chernock RD, Gay HA, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132:882–90. doi: 10.1002/ijc.27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JS, Jr, Chernock RD, Ma XJ, et al. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012;25:1212–20. doi: 10.1038/modpathol.2012.79. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZW, Weinreb I, Kamel-Reid S, Perez-Ordonez B. Equivocal p16 immunostaining in squamous cell carcinoma of the head and neck: staining patterns are suggestive of HPV status. Head Neck Pathol. 2012;6:422–9. doi: 10.1007/s12105-012-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smeets SJ, Braakhuis BJ, Abbas S, et al. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25:2558–64. doi: 10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 27.Rahimi AS, Wilson DD, Saylor DK, et al. p16, cyclin D1, and HIF-1alpha predict outcomes of patients with oropharyngeal squamous cell carcinoma treated with definitive intensity-modulated radiation therapy. Int J Otolaryngol. 2012;2012:685951. doi: 10.1155/2012/685951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrone F, Suardi S, Pastore E, et al. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2006;12:6643–51. doi: 10.1158/1078-0432.CCR-06-1759. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khalaf HH, Colak D, Al-Saif M, et al. p16(INK4a) positively regulates cyclin D1 and E2F1 through negative control of AUF1. PLoS One. 2011;6:e21111. doi: 10.1371/journal.pone.0021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–7. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.