Abstract
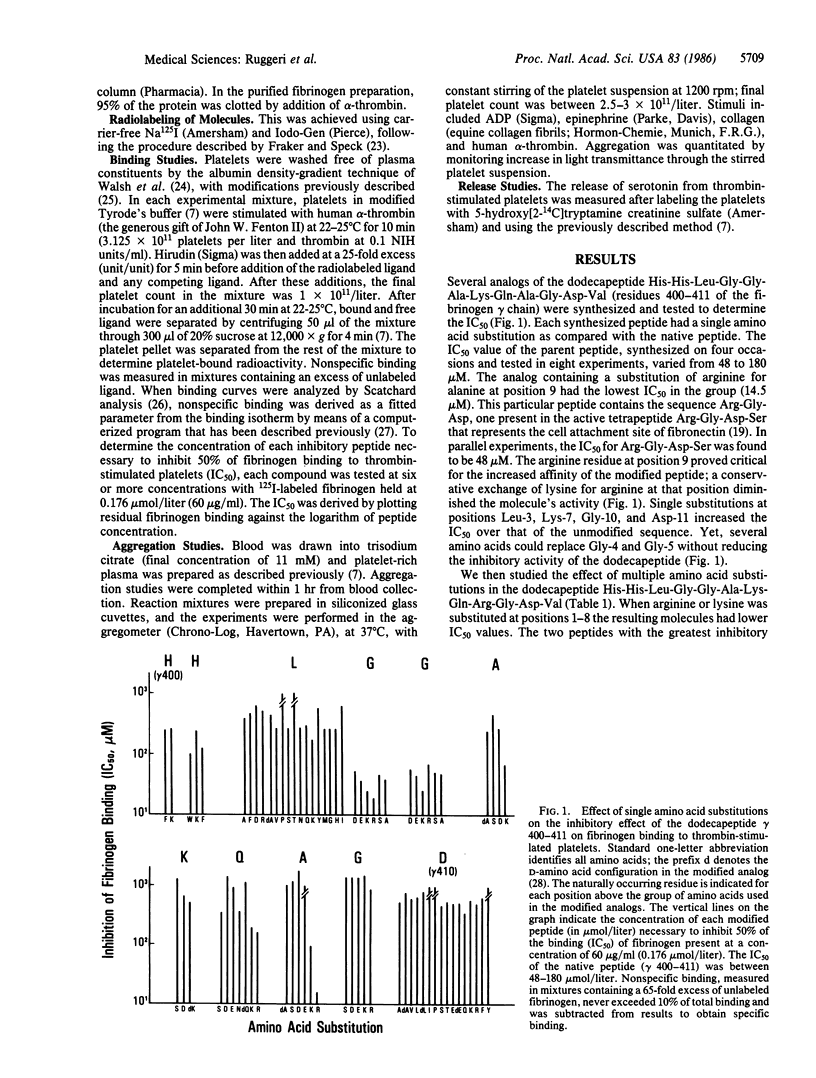
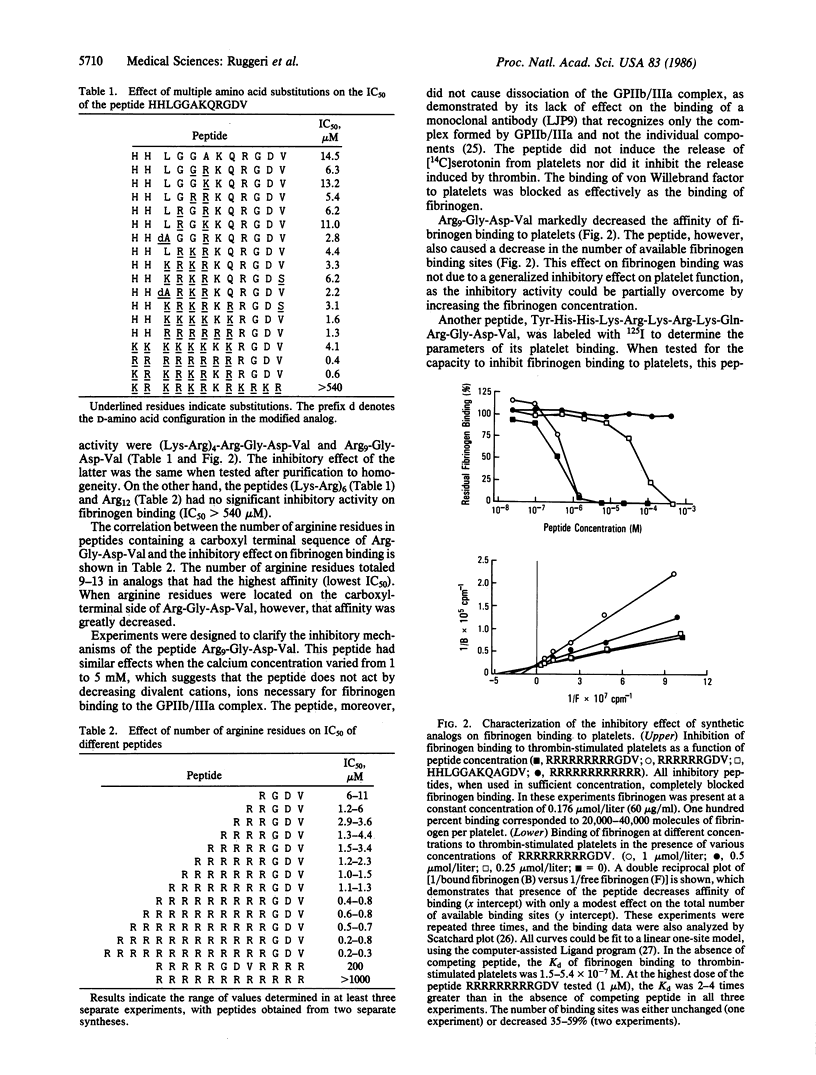
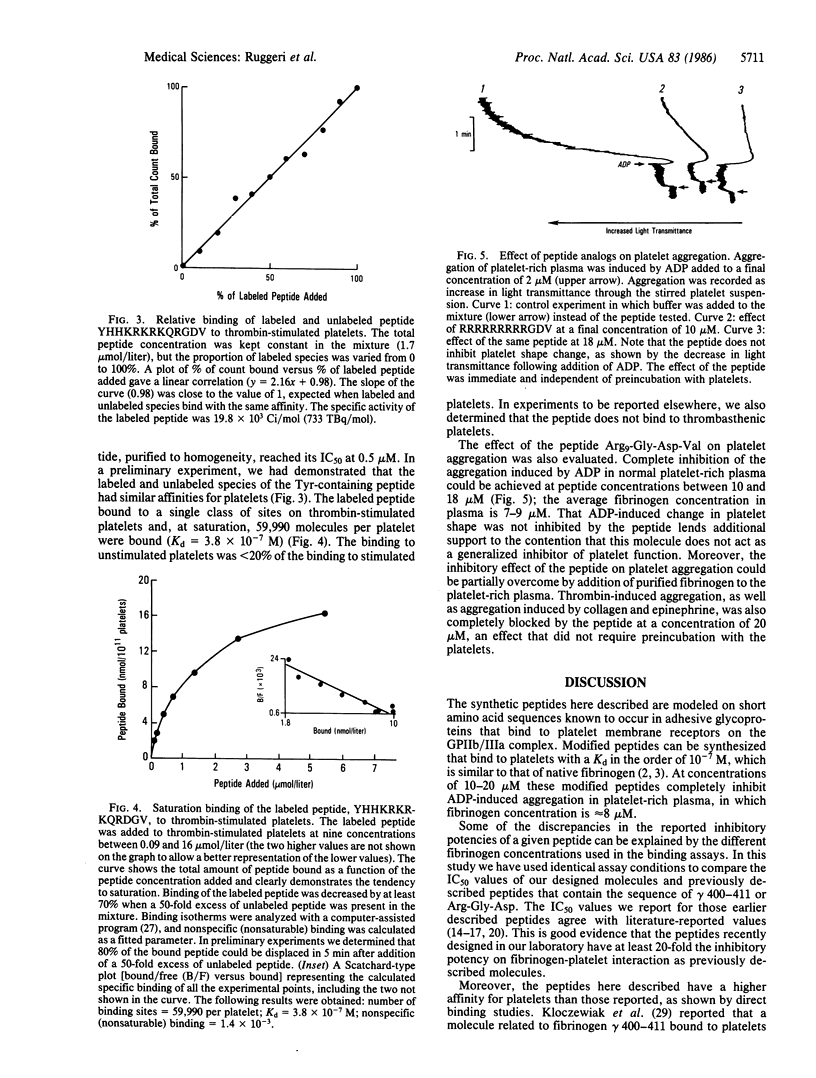
We have constructed synthetic peptides modeled on the sequences of (i) Arg-Gly-Asp, present in fibrinogen, fibronectin, and von Willebrand factor, and of (ii) the fibrinogen gamma chain (gamma 400-411) His-His-Leu-Gly-Gly-Ala-Lys-Gln-Ala-Gly-Asp-Val. The concentration of each peptide that inhibits 50% of 125I-labeled fibrinogen binding to thrombin-stimulated platelets (IC50) was then determined. The IC50 for (gamma 400-411) was 48-180 microM at a fibrinogen concentration of 60 micrograms/ml. A substitution of arginine for alanine at position 9 decreased the IC50 to 14.5 microM. Arginine substitutions for all other residues on the amino-terminal side of the peptide Arg9-Gly-Asp-Val resulted in an IC50 of 0.4-0.8 microM, and the IC50 of the peptide Arg13-Gly-Asp-Val was 0.2-0.3 microM. This contrasts with an IC50 of 200 microM for Arg5-Gly-Asp-Val-Arg4 and an IC50 greater than 1 mM for the peptide Arg12. The inhibitory effect resulted primarily in a decreased affinity of fibrinogen binding to platelets, although the number of available binding sites had also decreased. Binding was completely inhibited. At concentrations between 10 and 18 microM, Arg9-Gly-Asp-Val blocked all ADP-induced aggregation in citrated platelet-rich plasma. The peptide Tyr-His-His-Lys-Arg-Lys-Arg-Lys-Gln-Arg-Gly-Asp-Val was labeled with 125I to quantitate its binding to thrombin-stimulated platelets; at saturation, 59,990 molecules were bound per cell (Kd = 3.8 X 10(-7) M). These modified synthetic peptides bind to platelets with the same affinity as does intact fibrinogen and inhibit platelet function. The increased affinity of these modified peptides is greater than 20-fold that of peptides comprised of only native sequences and is a prerequisite for the potential antithrombotic use of these agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- De Marco L., Girolami A., Zimmerman T. S., Ruggeri Z. M. von Willebrand factor interaction with the glycoprotein IIb/IIa complex. Its role in platelet function as demonstrated in patients with congenital afibrinogenemia. J Clin Invest. 1986 Apr;77(4):1272–1277. doi: 10.1172/JCI112430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K., Hennessy S. W., Broekelmann T. J., McDonald J. A., Grant G. A., Santoro S. A., Frazier W. A. Inhibition of platelet aggregation by a monoclonal antibody against human fibronectin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3844–3848. doi: 10.1073/pnas.82.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Bennett J. S. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985 Oct 5;260(22):11891–11894. [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Gugler E., Lüscher E. F. Platelet function in congenital afibrinogenemia. Thromb Diath Haemorrh. 1965 Nov 15;14(3-4):361–373. [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Lombardo V. T., Hodson E., Roberts J. R., Kunicki T. J., Zimmerman T. S., Ruggeri Z. M. Independent modulation of von Willebrand factor and fibrinogen binding to the platelet membrane glycoprotein IIb/IIIa complex as demonstrated by monoclonal antibody. J Clin Invest. 1985 Nov;76(5):1950–1958. doi: 10.1172/JCI112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L., Perry D. W., Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978 Aug;52(2):453–466. [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. Inhibition of fibrinogen binding to human platelets by the tetrapeptide glycyl-L-prolyl-L-arginyl-L-proline. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3711–3715. doi: 10.1073/pnas.79.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons S., Kloczewiak M., Hawiger J. ADP-dependent common receptor mechanism for binding of von Willebrand factor and fibrinogen to human platelets. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4935–4939. doi: 10.1073/pnas.81.15.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T., Baumgartner H. R. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate--dependent decrease of adhesion in von Willebrand's disease and the Bernard-Soulier syndrome. J Lab Clin Med. 1978 Nov;92(5):750–764. [PubMed] [Google Scholar]



