Abstract
The ability to upregulate cognitive control in motivationally salient situations was examined in individuals with schizophrenia (patients) and healthy controls. Fifty-four patients and thirty-nine healthy controls were recruited. A computerized monetary response conflict task required participants to identity a picture, over which was printed a matching (congruent), neutral, or incongruent word. This baseline condition was followed by an incentive condition, in which participants were given the opportunity to win money on reward-cued trials. These reward-cued trials were interleaved with non-reward cued trials. Reaction times (RT) were examined for both incentive context effects (difference in RT between baseline and non-reward cue trials in the incentive condition) and incentive cue effects (difference in RT between non-reward and reward cue trials in the incentive condition). Compared to baseline, controls showed a speeding of responses during both the non-reward (incentive context effect) and reward cued (incentive cue effect) trials during the incentive condition, but with a larger incentive context than incentive cue effect, suggesting a reliance on proactive control strategies. Although patients also showed a speeding of responses to both non-reward and reward cued trials, they showed a significantly smaller incentive context effect than controls, suggesting a reduction in the use of proactive control and a greater reliance on the use of “just-in-time,” reactive control strategies. These results are discussed in light of the relationship between motivation and cognitive impairments in schizophrenia, and the potential role of impairments in prefrontally mediated active maintenance mechanisms.
Cognitive and motivational impairments are fundamental clinical features of schizophrenia (Barch, 2005a; Barch, Yodkovik, Sypher-Locke, & Hanewinkel, 2008; Dickinson, Ragland, Gold, & Gur, 2008; Gold, Waltz, Prentice, Morris, & Heerey, 2008; Medalia & Brekke, 2010; Meehl, 1962, 2001; Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009; Rector, Beck, & Stolar, 2005). The Diagnostic and Statistical Manual of Mental Disorders (APA, 2000) categorizes amotivation as a negative symptom, a category that also includes social withdrawal, blunted affect, and anhedonia. The goal of the current study was to examine the relationship between cognitive and motivational impairments in schizophrenia by examining the temporal dynamics of the influence of reward incentives on cognitive control.
The causal relationship between cognitive and motivational deficits in schizophrenia is currently under debate (Gard, Kring, Gard, Horan, & Green, 2007; Gold et al., 2008; Heerey & Gold, 2007; Herbener, Rosen, Khine, & Sweeney, 2007). It is possible that at least some cognitive dysfunctions commonly seen in the illness, including impaired intelligence, attention, executive control, and working memory, are secondary to motivational deficits (Barch, 2005b; Barch & Dowd, 2010; Barch et al., 2008). Alternatively, some aspects of cognitive dysfunction, such as difficulty retrieving prior information or experiences, or actively maintaining information about current or future events, may contribute to motivational impairments in schizophrenia as lack of self-reported anticipation of future rewards. Further, it may be that there are common mechanisms contributing to both cognitive and motivational deficits, such as deficits in the ability to represent goal information that may be important for both “cold” cognitive processing and for motivationally salient processing. Given that motivation impacts day-to-day functioning, such as maintaining relationships, seeking long-term employment, and learning the necessary skills to live on one’s own and provide self-care, our understanding of poor motivation is integral to designing better treatments for schizophrenia.
Untangling the nature of these motivational deficits has proven to be difficult. In interview-based testing and self-report measures, individuals with schizophrenia report experiencing lower levels of daily pleasure than do controls (Horan, Kring, & Blanchard, 2006). However, patients appear to experience equivalent amounts of pleasure in response to positive stimuli when compared to controls (Cohen & Minor, 2010; Kring & Moran, 2008), as well as similar levels of emotional experience in social role playing (Aghevli, Blanchard, & Horan, 2003). These seemingly contradictory findings can be clarified through an understanding of the motivational process. A motivationally salient situation can be divided into two distinct phases: “wanting,” the drive toward the stimulus, and “liking,” the pleasurable response that arises from its actualization (Berridge, 2004; Pecina, Cagniard, Berridge, Aldridge, & Zhuang, 2003). The liking function (“consummatory pleasure) in individuals with schizophrenia appears to be intact: enjoyment is experienced while engaging in pleasing activities. However, the ability to want an activity or object in the future, described by Gard et al.(Gard et al., 2007) as anticipatory pleasure, appears to be deficient. These findings suggest that individuals with schizophrenia are capable of experiencing pleasure in rewarding situations, but that they may not be able to anticipate such situations and thus may be not be motivated to seek them (Ursu et al., 2011).
The mesolimbic dopamine (DA) system is strongly implicated in reward, and may contribute to anticipatory pleasure (Hollerman, Tremblay, & Schultz, 1998; Schultz, 2002; Schultz, Apicella, & Ljungberg, 1993). DA dysfunction has long been considered a critical feature of schizophrenia (Braver, Barch, & Cohen, 1999) and recent studies have shown that both medicated (with typical antipsychotics) and unmedicated patients show decreased activation of the ventral striatum (VS), a major target of the mesolimbic DA system, in response to monetary incentives (Juckel et al., 2006; Kirsch, Ronshausen, Mier, & Gallhofer, 2007). Thus, it is possible that individuals with schizophrenia show deficits in anticipatory pleasure because of altered striatal DA function.
The dorsolateral prefrontal cortex (DLPFC) is also a critical component of the brain’s reward system. The DLPFC is thought to support many aspects of executive functioning, including cognitive control, the ability to maintain and focus attentional resources on task-relevant stimuli while filtering out task-irrelevant information (Braver, 2012; Miller & Cohen, 2001). Rewarding situations enhance this capability, potentially via a reward-mediated improvement in working memory or other aspects of cognitive control (Beck, Locke, Savine, Jimura, & Braver, 2010; Jimura, Locke, & Braver, 2010), an effect referred to as “motivated” cognitive control. Schizophrenia has long been associated with structural (Cullen et al., 2006; Ho et al., 2003; Kasparek et al., 2007; Pierri, Volk, Auh, Sampson, & Lewis, 2001; Rajkowska, Selemon, & Goldman-Rakic, 1998; Selemon & Rajkowska, 2003; Selemon, Rajkowska, & Goldman-Rakic, 1995; Shenton, Dickey, Frumin, & McCarley, 2001; Sigmundsson et al., 2001) and functional deficits in the PFC (Glahn et al., 2005; Minzenberg, Laird, Thelen, Carter, & Glahn, 2009). Importantly, this DLPFC dysfunction has been associated with deficits in the ability to proactively maintain internal representations that can be used to guide behavior, which could include representations about motivational incentives (Edwards, Barch, & Braver, 2010; Ursu et al., 2011). As such, it is possible that patients’ failure to use internal representations of reward to motivate their behavior, as reported by Gold et al. (Gold et al., 2008), may be related to impaired DLPFC functioning.
Importantly, relatively few investigations have explored the relationship between motivational incentives and cognitive control in schizophrenia, and the existing studies have provided mixed evidence. Several studies have shown evidence for improvements in some aspects of cognition (affect processing, visual masking, span of apprehension) with the provision of reward (Kern, Green, & Goldstein, 1995; Penn & Combs, 2000; Rassovsky, Green, Nuechterlein, Breitmeyer, & Mintz, 2005). However, other studies suggest that individuals with schizophrenia are not able to improve their performance on cognitive control tasks (e.g., Wisconsin Card Sorting) when offered monetary incentives (Green, Satz, Ganzell, & Vaclav, 1992; Hellman, Kern, Neilson, & Green, 1998; Vollema, Geurtsen, & van Voorst, 1995). However, none of these studies were designed to distinguish between the potential roles of DA versus PFC function in motivated cognitive control.
One way to distinguish between the potential contributions of altered striatally DA function versus impaired DLPFC function to motivated cognitive control in schizophrenia is to examine the temporal dynamics of incentive influences on cognitive control, as evidenced by incentive context versus incentive cue effects (Beck et al., 2010; Jimura et al., 2010). In healthy individuals, performance during cognitive control tasks is enhanced on trials in which participants are explicitly signaled that they can win rewards for better performance, referred to as an incentive cue effect (Beck et al., 2010; Jimura et al., 2010). This enhanced performance is accompanied by transient increases in activity in a number of reward related regions, including the midbrain and the dorsal striatum, as well as transient increases in DLPFC and parietal cortex activation (Beck et al., 2010). However, healthy participants also improve their performance on trials in which they cannot earn reward, when those trials are embedded in task conditions with available reward (e.g., non-reward cued trials interleaved with reward-cued trials), termed the incentive context effect. This effect is associated with sustained increases in activity in DLPFC and parietal cortex that are maintained across both reward cued and non-reward cued trials (Beck et al., 2010; Jimura et al., 2010), and may reflect active maintenance of information about the availability of motivational incentives and enhanced maintenance of task sets. If motivated cognitive control impairments in schizophrenia primarily reflect DA mediated impairments in reward anticipation, one would expect reductions in both incentive cue and incentive context effects. However, if such impairments are primarily due to deficits in the ability to actively maintain internal representations of reward to motivate behavior, then individuals with schizophrenia should show intact incentive cue effects, as these do not require maintenance of reward information, but should show impaired incentive context effects.
The current study was designed to test these hypotheses by examining the integrity of incentive cue and incentive context effects in schizophrenia during a cognitive control task. We used a modification of the response-conflict task developed by Padmala and Pessoa (Padmala & Pessoa, 2011), which requires participants to respond correctly to visual stimuli (pictures of houses or buildings) by filtering irrelevant stimuli (the words “house,” “building,” or the letters “xxxxx” imposed over the pictures). Padmala and Pessoa (2011) demonstrated that monetary incentives enhanced healthy controls’ abilities to direct their attention to task-relevant information, reducing conflict associated with incongruent words, an effect that was accompanied by enhanced activity in DLPFC.
Methods
Participants
The protocol used in this study was approved by the Washington University Human Research Protection Office. Participants included 54 medicated individuals with schizophrenia or schizoaffective disorder (SCZ), and 39 healthy controls (CON). The SCZ and CON were matched on average for age, gender, ethnicity and parental education (as a proxy for developmental exposure to educational opportunities). See Table 1 for demographic and clinical characteristics of the two groups. All participants received drug screening on the day of testing. If an individual tested positive for marijuana, cocaine, amphetamine, methamphetamine, or opiates, he or she was not allowed to participate. In addition, if a participants blood alcohol levels above 0.0002 g/mL (0.02%) he or she was excluded from participation.
Table 1.
Participant Demographic, Clinical, and Self-Report Measures
| Characteristics | Group
|
p | |||
|---|---|---|---|---|---|
| Healthy controls (N = 39) | Individuals with schizophrenia (N =54) | ||||
|
| |||||
| M | SD | M | SD | ||
| Demographics | |||||
| Age (years) | 36.46 | 9.12 | 38.85 | 8.13 | 0.19 |
| Sex (% male) | 48.7% | 61.1% | 0.24 | ||
| Ethnicity (% non-Caucasian) | 66.7% | 59.3% | 0.47 | ||
| Education (years) | 14.24 | 2.11 | 12.83 | 2.06 | 0.002 |
| Parental education (years) | 13.97 | 1.81 | 13.96 | 3.41 | 0.99 |
| Medication status | N/A | N/A | |||
| Typical antipsychotics (%) | 7.4% | ||||
| Atypical antipsychotics (%) | 83.3% | ||||
| Both typical and atypical (%) | 9.3% | ||||
| Clinical Ratings | |||||
| Positive symptoms | 0.03 | 0.16 | 3.73 | 2.77 | <.001 |
| Disorganization symptoms | 1.37 | 1.26 | 3.06 | 2.72 | <.001 |
| Negative symptoms | 1.58 | 2.33 | 7.78 | 2.90 | <.001 |
| Personality trait measures | |||||
| Social Anhedonia | 8.72 | 6.27 | 16.41 | 15.50 | <.001 |
| Physical Anhedonia | 10.33 | 5.49 | 18.62 | 9.45 | <.001 |
| SHAPS | 52.77 | 3.16 | 47.79 | 9.62 | .003 |
| TEPS Consummatory | 39.00 | 5.77 | 35.94 | 8.16 | .049 |
| TEPS Anticipatory Pleasure | 49.62 | 5.06 | 46.36 | 9.13 | .047 |
Note. The clinical ratings are based on Structured Clinical Interviews for the DSM-IV-TR (2000) 4th ed., text rev. (First et al., 2001). The Revised Social Anhedonia Scale is from Chapman et al. (1976), and the Revised Physical Anhedonia Scale is from Eckblad et al. (1982). The Snaith-Hamilton Pleasure Scale (SHAPS) is from Snaith et al. (1995). The Temporal Experiences of Pleasure Scale (TEPS) Anticipatory Pleasure is from Gard et al. (2005).
Clinical ratings
A Master’s-level clinician diagnosed participants, based on a Structured Clinical Interview for the DSM–IV–TR (First, Spitzer, Gibbon, & Williams, 2001). Exclusion criteria for the study were mental retardation, a major depressive episode or dysthymia within the past year, a substance abuse/dependence disorder within the past 6 months, or a head injury event with neurological sequelae and/or loss of consciousness. In addition, healthy controls were excluded for any personal or family history of psychosis, as well as bipolar disorder. Clinical symptoms were assessed using the Scales for Assessment of Negative Symptoms (Andreasen, 1983a) and the Scales for Assessment of Positive Symptoms (Andreasen, 1983b). Scores were categorized as follows: positive symptoms, including hallucinations and delusions; negative symptoms, including anhedonia, amotivation, blunted affect, and social withdrawal; and disorganization, including bizarre behavior, positive thought disorder, and inappropriate affect.
Self-report Measures
Self-reports of anhedonia
Participants completed the Revised Social Anhedonia Scale (Eckblad, Chapman, Chapman, & Mishlove, 1982) and the Revised Physical Anhedonia Scale (L. J. Chapman & Chapman, 1978). These scales use true/false questions to assess pleasure derived from social situations (e.g., A car ride is much more enjoyable if someone is with me) and physical stimuli (e.g., Beautiful scenery has been a great delight to me). High scores implicate high levels of anhedonia. Both measures have previously demonstrated strong internal reliability (coefficient α ranging from 0.79 and 0.89) as well as strong test-retest reliability (correlation coefficient ranging from 0.75 to 0.84) (J. P. Chapman, Chapman, & Kwapil, 1995). These scales have been widely used to assess symptoms of SCZ clinically and in the lab (Horan et al., 2006).
Patients also completed the 14-item Snaith-Hamilton Pleasure Scale, which assesses hedonic tone (Snaith et al., 1995). Participants responded with a 4-point Likert scale (1 = definitely disagree, 4 = definitely agree) to statements such as “I would enjoy seeing other people’s smiling faces.” Hence, low scores suggest anhedonia. The SHAPS has been shown to have a high internal consistency (Cronbach’s α = 0.91–0.94), adequate test-retest reliability (r = 0.70), high convergent validity, and adequate discriminant validity (Franken, Rassin, & Muris, 2007).
Self-reports of experiences of pleasure
Patients completed the 95-item Temporal Experience of Pleasure Scale (TEPS) to measure individual differences in anticipatory pleasure (e.g., I look forward to a lot of things in my life) and consummatory pleasure (e.g., The smell of freshly cut grass is enjoyable to me)(Gard, Germans, Kring, & John, 2006). Participants responded with a 6-point Likert scale (1 = very false for me, 6 = very true for me); low scores suggest a lack of pleasure. TEPS has previously demonstrated internal consistency, temporal stability, and convergent and discriminant validity (Gard et al., 2006). This scale has been used to examine deficits in patients’ anticipatory pleasure when compared to controls (Gard et al., 2007), and it has been used to gauge improvements after anticipatory pleasure skills training (Favrod, Giuliani, Ernst, & Bonsack, 2010).
Procedure
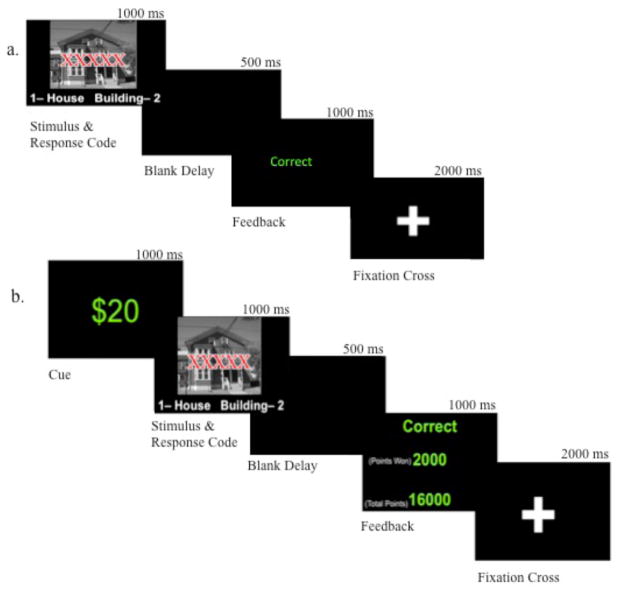
The response conflict task stimuli consisted of a picture of a house or a building shown for 1000 ms. The text “HOUSE,” “BLDNG,” or “XXXXX” was overlaid on the pictures, creating congruent, incongruent, or neutral trials (see Figure 1). The task was divided into two sections: a baseline section and a monetary incentive section (see Figure 2). In both sections, participants were asked to ignore the text and respond to the picture by pressing “1” for a house (with the right index finger) or “2” for a building (with the right middle finger) on a standard keyboard. A 500 ms blank delay screen followed the stimulus, creating a total response time limit of 1500 ms. A fixation cross was displayed between trials for 2000 ms. In the baseline section, a feedback screen, shown for 1000 ms, informed participants if their response was correct or incorrect. This section consisted of three blocks of 48 trials each, separated by pauses, allowing participants to rest as long as needed.
Figure 1.

Examples of the three different task trial types (congruent, incongruent, and neutral).
Figure 2.
Schematic of the Response Conflict Task Design: a) baseline condition and b) monetary incentive section. Schematic represents one complete trial for each section. Adapted from “Reward Reduces Conflict by Enhancing Attentional Control and Biasing Visual Cortical Processing,” by S. Padmala and L. Pessoa, 2011, Journal of Cognitive Neuroscience, 23, p. 3420. Copyright 2011 by the Massachusetts Institute of Technology.
Once the baseline section was completed, the monetary incentive section was initiated. Prior to viewing the stimuli, participants were shown a cue of either $20 or $0 for 1000 ms. A $20 cued trial, referred to as an incentive trial, informed participants that they had the ability to earn 2000 points on that trial. If participants responded accurately and more quickly than their median response time on blocks two and three of the baseline section, they were rewarded 2000 points. Otherwise, they received no points. Points were tallied and converted into actual money, which was given upon completion of the task. A $0 cue informed participants that there was no way to earn reward on that trial. On all trials, the feedback screen indicated whether the response was correct or incorrect, whether points had been won, and the total number of points won. The incentive section consisted of seven blocks of 36 trials each, separated by pauses for rest. Once the section was completed, participants were rewarded between $0 and $20 (M = 13.88, SD = 4.02) depending on the number of correct responses (in addition to the nominal amount they received for participating).
Analyses
Variables and measures
Both reaction times (correct trials only) and accuracy were examined. The analyses used repeated measures ANOVA with group (CON, SCZ) as a between subject factor and trial type (congruent, incongruent, and neutral) and reward condition (baseline section, $0 reward cue, and $20 reward cue) as within subject factors. Greenhouse-Geisser corrections were applied to adjust for non-sphericity. Relationships between task performance and individual difference measures of anhedonia were examined using Pearson’s Product Moment Correlations.
Results
As shown in Table 1, the two groups did not differ in age, gender, ethnicity, or parental education, though as expected the SCZ had lower personal education. In addition, the SCZ self reported significantly higher social and physical anhedonia, significantly lower pleasure scores on the SHAPS, and significantly lower consummatory and anticipatory pleasure on the TEPS.
Accuracy
The ANOVA for accuracy indicated significant main effects of group, F(1,91) = 5.30, p < 0.05, reflecting lower accuracy in SCZ (see Figure 3), and trial type, F(2, 182) = 31.1, p < 0.00, reflecting lower accuracy in the incongruent compared to neutral and congruent conditions. We found a marginally significant main effect of reward condition, F(2, 182) = 3.18, p = .064, reflecting lower accuracy in $0 trials (incentive context trials) compared to both the baseline condition, F(1, 92) = 4.44, p < 0.05, and the $20 trials in the incentive condition, F(1, 92) = 4.21, p < 0.05, but no significant difference between baselines and $20 trials. Finally, there was a marginally significant interaction between reward condition and trial type, F(4, 364) = 2.26, p =.066, indicating that the influence of reward condition on accuracy held for incongruent trials, F(2, 182) = 4.41, p < 0.05, but not for congruent or neutral trials.
Figure 3.
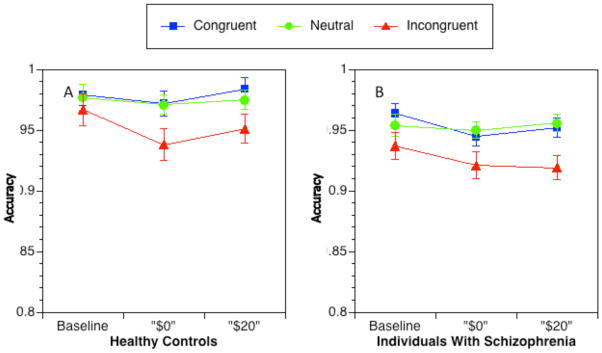
Accuracy for each trial type across reward conditions: controls (a) and patients (b). Error bars represent ± SEM.
Reaction Time
The ANOVA for RTs indicated significant main effects of group, F(1,91) = 18.80, p < 0.001, reward condition, F(2,182) = 53.75, p < 0.001, and trial type F(2,182) = 55.74, p < 0.001. The main effect of reward condition indicated overall faster RTs in the incentive cue trials compared to incentive context trials F(1, 92) = 49.54, p < 0.001, as well as incentive context trials compared to baseline F(1, 92) = 30.58, p < 0.001. The main effect of trial type reflected slower RTs in the incongruent trials compared to neutral trials, F(1, 92) = 14.24, p < 0.001, and slower RTs on neutral trials compared to congruent trials, F(1, 92) = 68.73, p < 0.001. As shown in Figure 4, the main effect of group reflected overall slower RTs in SCZ.
Figure 4.
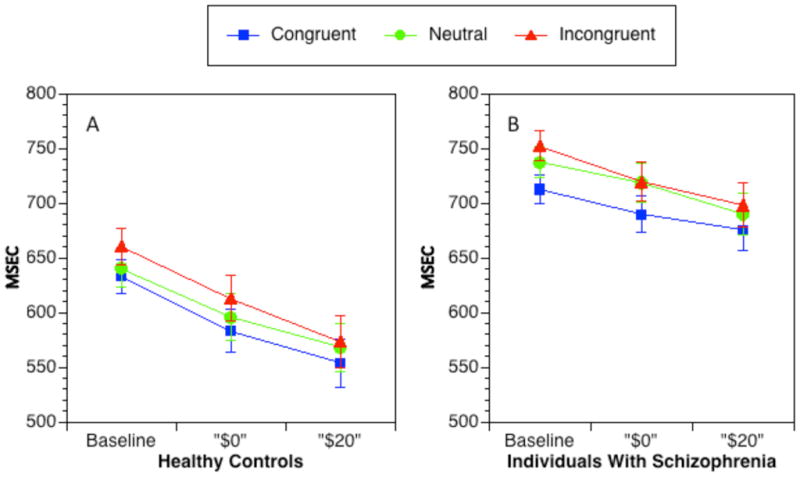
Reaction times for each trial type across reward conditions: controls (a) and patients (b). Error bars represent ± SEM.
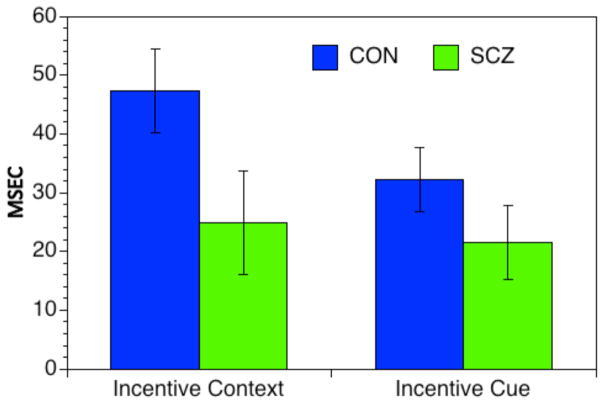
A significant interaction between group and reward condition, F(2,182) = 3.87, p < 0.05 was also found (see Figure 5), indicating that SCZ showed a significantly smaller overall speeding of response time in incentive context trials compared to baseline, t(91) = 1.88 p < 0.05, 1-tailed, but the groups did not differ in the degree of speeding between incentive cue and incentive context trials, t(91) = 1.23, p=.11, 1-tailed.
Figure 5.
Incentive Context and Cue Effects. Error bars represent ± SEM.
There was also a trend level interaction between reward condition and trial type, F(4,364) = 2.06, p =.09. To follow up on this finding, we computed interference scores for each condition by subtracting RTs in the congruent condition from RTs in the incongruent condition. This interference effect was significantly smaller, (t(93) = 2.62, p<.05), for $20 trials (M = 21.8, SD = 40.6) than for the baseline trials (M = 34.5, SD = 39.3), with the $0 trials falling in between (M = 29.8, SD = 42.6).
Psychometric Issues
Individuals with SCZ typically have longer RTs, and differences between conditions can be artifactually altered by the effects of longer RTs (L. J. Chapman & Chapman, 1973; L. J. Chapman, Chapman, Curran, & Miller, 1994). To address this concern, we used the recommended approach of converting RT scores into normal mean-deviate (i.e., Z-scores) of the mean RT across all conditions as the measure of RT in each condition for each participant (Faust, Balota, Spieler, & Ferraro, 1999). The logic for this approach is that the standard deviation (SD) across the conditions for SCZ is typically larger than SD for controls. This was true in the current data set (139 vs. 81 msec SD, Levene’s Test = 4.34, p <.001). Z-scores are calculated as a function of the magnitude of the SD. Thus, if the magnitude of incentive context or incentive cue effects in SCZ is influenced by their overall longer RTs, the use of Z-scores should reduce this effect. We computed the same ANOVA described above using Z-scores. This ANOVA again indicated a significant two-way interaction between Group and Reward Condition (F(2,116) = 6.01, p < 0.01). Follow up contrasts again indicated that the SCZ showed a significantly smaller overall speeding of response time in incentive context trials compared to baseline, t(91) = 2.41 p < 0.05, but the groups did not differ in the degree of speeding between incentive cue and incentive context trials, t(91) = 1.23, p=.11, 1-tailed. Thus, the significant group differences in the incentive context effect remained even when accounting for overall longer RTs in the SCZ.
Correlations with clinical measures
To examine whether individual differences in either incentive cue or incentive context effects were associated with either the severity of clinical symptoms or self reports of amotivation/anhedonia, we computed Pearson’s Product Moment Correlations between clinical symptom scales, the self-report measures of hedonic function, and the magnitude of incentive context and incentive cue effects in the individuals with schizophrenia. There were no significant correlations between either the incentive cue or incentive context effects and any of the self-report measures. There was a negative correlation between the severity of positive symptoms and the magnitude of incentive cue effects (r = −.30, p<.05), but not with incentive context effects (r = −.13, p=.35). Negative and disorganization symptoms were not significantly correlated with either incentive context or cue effects (all ps>.27).
Discussion
The goal of the current study was to examine whether individuals with schizophrenia were able to upregulate cognitive control in a motivationally salient context. Consistent with prior research, we found that participants speeded their reaction times both to explicit cues of reward ($20 vs. $0 trials) and to the context of reward ($0 vs. baseline trials). Further, we found a trend for a reduction in the interference effect – an even stronger index of cognitive control – for both incentive cues and incentive context. We had also hypothesized that if impairments in motivated cognitive control in schizophrenia primarily reflect DA-mediated impairments in reward anticipation, we would expect reductions in both incentive cue and incentive context effects. However, if such impairments are primarily due to deficits in the ability to actively maintain internal representations of reward to motivate their behavior, then we predicted that individuals with schizophrenia might show intact incentive cue effects as these do not require maintenance of reward information, but would show impaired incentive context effects. Consistent with this second hypothesis, we found that individuals with schizophrenia showed intact incentive cue effects on overall speeding of reaction times, but reduced incentive context effects on the overall speeding of reaction times. In addition, we found that the individuals with schizophrenia self-reported higher levels of anhedonia across a broad array of measures. Each of these results is discussed in more detail below.
Our findings replicate prior research showing that both explicit cues for incentives and information that incentives will be available in the current context allow individuals to enhance their cognitive control function (Jimura et al., 2010; Padmala & Pessoa, 2011). We saw this most strongly in overall speeding of reaction times, but we also saw evidence for a reduction in interference effects (incongruent – congruent reaction times), an even stronger index of cognitive control. Participants did show some evidence of a speed accuracy trade off in the incentive context trials ($0) as compared to baseline, as their accuracy in the interference condition was reduced on the $0 trials. However, they did not show any further reduction in accuracy from the $0 to the $20 trials, despite the significant speeding of responses. Thus, in the incentive context condition, individuals speeded their reaction times and reduced their interference effects, but at the cost of some reductions in accuracy. However, in the $20 trials, participants were able to speed their responses and reduce interference effects without any costs in accuracy. Thus, these results contribute to the growing literature demonstrating that various types of incentives can upregulate cognitive control.
The individuals with schizophrenia showed a significant reduction in the incentive context effect on overall reaction times, though they showed intact incentive cue effects on overall reaction times. These results may be related to the distinction between wanting and liking deficits observed previously in individuals with schizophrenia (Gard et al., 2007). When incentive cues are immediately available in the environment (e.g., $20 trials), individuals with schizophrenia are able to use such cues to speed their reaction times and to reduce their interference effects. However, in conditions where such cues are not immediately available (potentially akin to “wanting” or anticipatory condition), the individuals with schizophrenia were significantly less influenced by reward context. These results are also consistent with the hypothesis that at least some of the motivational impairments experienced by individuals with schizophrenia may reflect deficits in the ability to maintain representations about future rewards or incentive in order to guide ongoing behavior. On $20 trials, no maintenance of incentive information is needed, as the trial includes an explicit reminder about rewards. However, enhancing performance even on $0 trials may necessitate some maintained internal representation of the fact that incentives are available in the current context, even if not on the current trials. As described above, in prior work, these incentive context effects were associated with sustained activity in DLPFC.
Additional work will be needed to determine whether reductions in incentive context effects in schizophrenia are associated with reduced sustained activity in DLPFC. These results are also generally consistent with hypotheses about impaired proactive control functions in schizophrenia (Barch & Ceaser, 2012; Edwards et al., 2010). Braver and colleagues (Braver, 2012; Braver, Gray, & Burgess, 2007) proposed the dual mechanisms of control theory, which argues that cognitive control is supported by at least two dissociable mechanisms. Proactive control is anticipatory, goal-oriented control sustained throughout a task. It is responsible for active maintenance of contextual information, making it the likely mode of cognitive control responsible for context effects. The neural substrates for proactive control include the lateral PFC and the midbrain DA system. The PFC is hypothesized to represent context information, whereas the midbrain DA system is hypothesized to regulate this process via a gating mechanism (Braver et al., 1999; Braver & Cohen, 1999), ensuring that only task-relevant information is maintained. Reactive control, on the other hand, retrieves task relevant information and initiates “just-in-time” responses to some type of cue or stimulus. The neural substrates of reactive control include transient increases in activity in lateral PFC, the anterior cingulate cortex and the medial temporal lobe (Braver, 2012; Braver et al., 2007). In the current task, incentive context effects may reflect the operation of proactive control mechanisms that prepare an individual to better deal with upcoming conflict, with prior evidence that such mechanisms are impaired in individuals with schizophrenia (Edwards et al., 2010; A. MacDonald et al., 2005; Macdonald, 2008; MacDonald & Carter, 2003; A. W. MacDonald, 3rd et al., 2005). In contrast, incentive cue effects, which are associated with transient increases in DLPFC and striatal activity, may reflect more reactive control mechanisms that are engaged upon presentation of the reward cue. Such reactive control processes may be relatively more intact in individuals with schizophrenia.
These results are also consistent with the “Immediacy Mechanism”, an early theory proposed by Salzinger (K. Salzinger, 1984; K. Salzinger, Portnoy, Pisoni, & Feldman, 1970; Kurt Salzinger, 2006). The Immediacy Hypothesis states that individuals with schizophrenia respond preferentially to stimuli in their immediate environment, out of context of the “bigger” picture. This hypothesis has been used to explain schizophrenia-specific speech variations, such as the tendency for the speech of individuals with schizophrenia to consist of short strings of words related to one another, instead of using words related to one another across greater temporal distances, as is more typical in the speech of individuals without schizophrenia (K. Salzinger et al., 1970). The Immediacy Hypothesis has been broadly applied to schizophrenia research, including studies testing learning and memory, executive functioning, and decision making (K. Salzinger & Serper, 2004). Interestingly, the dual mechanisms of control theory described above may be a means of articulating at least some of the neural mechanisms that might be contributing to the “immediacy” effects that have long been apparent in the behavior of individuals with schizophrenia.
We did not find any relationships between individual differences in self-report or clinical ratings of negative symptoms or anhedonia and the magnitude of either incentive cue or incentive context effects, though we did see a relationship between positive symptoms and reduced incentive cue effects. This lack of relationship to individual differences in negative symptoms is unlikely to reflect low power given our relatively large sample size. Previous research has attempted to correlate negative symptom scores in patients with various measures of reward processing, including representations of value, decision making, and learning (Gold et al., 2008), with quite mixed results. There may be several explanations both for our current lack of individual difference findings, and for the mixed results in the literature in general. For example, it may be that self-report and laboratory measures assess different time scales, with self-report integrating over a longer time scale (e.g., a person’s heuristic judgment about themselves) and laboratory measures assessing behavior as a specific point in time. Second self-report/clinical measures may assess fundamentally different constructs than laboratory-based measures. For example, self-report or clinical measures may assess a combination of factors, such as the ability of a person to articulate their inner feelings, the ability of the individual to recall details about their past, and/or the types of individuals the clinician uses as a comparison in order to make ratings (e.g., other individuals with schizophrenia versus healthy individuals). In contrast, laboratory based measures may isolate a specific process or mechanism that might only be related to some of the constructs being assessed by self-report or clinical assessment, reducing the ability to detect individual difference relationships. An important issue for future research will be a comparison of the degree to which self-report, clinician assessment, and/or laboratory based measures of reward processing and motivation predict real world function, as it many ways this will be the gold standard by which to judge the relatively utility of different assessment approaches.
There were several limitations to the current study. First, all of our participants with schizophrenia were taking antipsychotic medications, which could impact the processing of reward information via blockade of subcortical DA receptors. However, one might have expected such blockade to have the strongest effect on incentive cue trials ($20) if it served to blunt processing of reward information. Given that the individuals with schizophrenia showed intact responses on reward cue trials, it seems less likely that our results reflect medication effects. However, it will be important to examine these same processes in future studies in either unmedicated individuals with schizophrenia or in individuals who share genetic liability to schizophrenia (e.g., siblings) in order to more definitively rule out medication effects. Another limitation is that patients may have responded to the monetary reward differently because their reduced socioeconomic status. We matched patients and controls on parental education to control for developmental socioeconomic status, but patients had a lower current socioeconomic status than controls, as is typically for this illness. This might have caused $20 to have a greater subjective value or expected utility to patients as compared to controls. However, one might have expected this to enhance the motivational effects of money in patients, whereas we either saw no differences (incentive cue) or reduced effects (incentive context). One other possibility is that lower socioeconomic status enhanced the subjective value of $20 to a point that counteracted deficits in putatively DA-mediated reward responsivity, protecting patients from the appearance of incentive cue deficits. We cannot test this possibility in the current study, but it could be addressed in future studies by titrating the amount of money provided on an individual basis using some type of subjective value or expected utility procedure. A final limitation is the possible interaction between the various cognitive deficits seen in schizophrenia (e.g., sustained attention, set switching) and the nature of task, which required participants to shift between interleaved trials ($20 and $0). However, the fact that we saw intact incentive cue effects in patients argues against a role for these factors, as deficits in set switching or sustained attention might have predicted reduced incentive cue effects as these were the trials that required a “switch” and which came later in the course of the experiment.
Second, we did not find any relationships between individual differences in self-report or clinical ratings of negative symptoms or anhedonia and the magnitude of either incentive cue or incentive context effect, though we did see a relationship between positive symptoms and reduced incentive cue effects. This lack of relationship to individual differences in negative symptoms is unlikely to reflect low power given our relatively large sample size, and thus further work will be needed to clarify whether heterogeneity in symptom presentation in schizophrenia is associated with variability in the influence of incentives on cognitive control.
In summary, both patients and controls demonstrated a robust ability to motivate cognitive control in response to monetary incentive, resulting in faster RTs across all trial types in response to explicit cues that a reward could be won on that trial. However, individuals with schizophrenia showed a significant reduction in incentive context effects. Such deficits may reflect impairments in proactive control mechanisms that allow individuals to maintain internal representations of goal or incentive information over time in a way that can modify ongoing behavior. Future research will be needed to determine whether such deficits are associated with reductions in sustained activity in DLPFC regions, and to determine whether such deficits predict impairments in real world functions associated with motivational functions. Given the impact of amotivation on functional impairments in schizophrenia (Foussias et al., 2009; Gard et al., 2007; Mathews & Barch, 2010; Velligan et al., 2006; Waltz et al., 2009), gaining an understanding of proactive control deficits and their role in amotivation may be a critical step in designing more effective behavioral and occupational therapies. For example, if at least some of the deficits in motivation in schizophrenia are due to proactive control deficits, we may consider interventions that involve environmental support for the maintenance of goals, such as the provision of prompts and reminders about goals and future rewards that do not require internal maintenance on the part of the individual. One highly speculative idea is that this may be an intriguing area where electronic devices could play a role, as a mechanism by which to provide such environmental reminders and supports that might help bridge the temporal gap between anticipation and experience.
Acknowledgments
The authors would like to thank the participants in this study, who gave generously of their time. Funding for this study was provided by NIMH MH066031.
Contributor Information
Claire L. Mann, Department of Psychology, The Colorado College
Owen Footer, Department of Psychiatry, Washington University.
Yu Sun Chung, Department of Psychology, Washington University.
Lori L. Driscoll, Department of Psychology, The Colorado College
Deanna M. Barch, Departments of Psychology, Psychiatry, and Radiology, Washington University
References
- Aghevli MA, Blanchard JJ, Horan WP. The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Res. 2003;119(3):261–270. doi: 10.1016/s0165-1781(03)00133-1. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS). The scale for the assessment of negative symptoms (SANS) University of Iowa; 1983b. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Press; 2000. revised ed. [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. In: Cannon T, Mineka S, editors. Annual Review of Clinical Psychology. Vol. 1. Washington, D.C: American Psychological Association; 2005a. pp. 321–353. [DOI] [PubMed] [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophrenia Bulletin. 2005b;31(4):875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser AE. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Science. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. sbq068 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: relationships to cognitive function, depression, anxiety, and personality. J Abnorm Psychol. 2008;117(4):776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PloS one. 2010;5(2):e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in cognitive sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: The gating model. Progress in Brain Research. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway AR, Jarrold C, Kane MJ, Miyake A, Towse J, editors. Variation in Working Memory. Oxford: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine T, Lencz T, Mednick S, editors. Schizotypal personality. New York: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The revised physical anhedonia scale. Madison: University of Wisconsin; 1978. unpublished test. [Google Scholar]
- Chapman LJ, Chapman JP, Curran TE, Miller MB. Do children and the elderly show heightened semantic priming? How to answer the question. Developmental Review. 1994;14:159–185. [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia bulletin. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. British Journal of Psychiatry. 2006;188:26–31. doi: 10.1192/bjp.bp.104.008169. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The revised social anhedonia scale 1982 [Google Scholar]
- Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Favrod J, Giuliani F, Ernst F, Bonsack C. Anticipatory pleasure skills training: a new intervention to reduce anhedonia in schizophrenia. Perspectives in psychiatric care. 2010;46(3):171–181. doi: 10.1111/j.1744-6163.2010.00255.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-−IV-−TR Axis I disorders. Washington, D. C: American Psychiatric Press; 2001. [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of affective disorders. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gard DE, Germans M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Personality Research. 2006;40:1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Satz P, Ganzell S, Vaclav JF. Wisconsin Card Sorting Test performance in schizophrenia: remediation of a stubborn deficit. Am J Psychiatry. 1992;149(1):62–67. doi: 10.1176/ajp.149.1.62. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Hellman SG, Kern RS, Neilson LM, Green MF. Monetary reinforcement and Wisconsin Card Sorting performance in schizophrenia: why show me the money? Schizophrenia Research. 1998;34(1–2):67–75. doi: 10.1016/s0920-9964(98)00088-7. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol. 2007;116(1):43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward rexpectation on behavior-related neuronal activity in primate striatum. Journal of Neurophysiology. 1998;80(2):947–963. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):151–157. doi: 10.1016/j.pnpbp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Goldstein MJ. Modification of performance on the span of apprehension, a putative marker of vulnerability to schizophrenia. J Abnorm Psychol. 1995;104(2):385–389. doi: 10.1037//0021-843x.104.2.385. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Cohen JD. Specificity of prefrontal dysfunction and context processing deficts to schizophrenia in a never medicated first-episode psychotic sample. American Journal of Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Macdonald AW., 3rd Building a Clinically Relevant Cognitive Task: Case Study of the AX Paradigm. Schizophr Bull. 2008;34(4):619–628. doi: 10.1093/schbul/sbn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Goghari VM, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19(6):814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- Medalia A, Brekke J. In search of a theoretical structure for understanding motivation in schizophrenia. Schizophrenia Bulletin. 2010;36(5):912–918. doi: 10.1093/schbul/sbq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. J Abnorm Psychol. 2001;110(1):188–193. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. 2009-05986-005 [pii] [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of cognitive neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Combs D. Modification of affect perception deficits in schizophrenia. Schizophr Res. 2000;46(2–3):217–229. doi: 10.1016/s0920-9964(00)00005-0. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Archives of General Psychiatry. 2001;58(5):466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington’s disease. Archives of General Psychiatry. 1998;55(3):215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. Am J Psychiatry. 2005;162(8):1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- Rector NA, Beck AT, Stolar N. The negative symptoms of schizophrenia: a cognitive perspective. Can J Psychiatry. 2005;50(5):247–257. doi: 10.1177/070674370505000503. [DOI] [PubMed] [Google Scholar]
- Salzinger K. The immediacy hypothesis in a theory of schizophrenia. Nebraska Symposium on Motivation Nebraska Symposium on Motivation. 1984;31:231–282. [PubMed] [Google Scholar]
- Salzinger K, Portnoy S, Pisoni DB, Feldman RS. The immediacy hypothesis and response-produced stimuli in schizophrenic speech. Journal of Abnormal Psychology. 1970;76(2):258–264. doi: 10.1037/h0029887. [DOI] [PubMed] [Google Scholar]
- Salzinger K, Serper MR. Schizophrenia: The immediacy mechanism. International Journal of Psychology and Psychological Therapy. 2004;4:397–409. [Google Scholar]
- Salzinger Kurt. Schizophrenia and the immediacy mechanism. American Psychologist. 2006;61(1):74. doi: 10.1037/0003-066X.61.1.74. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. The Journal of Neuroscience. 1993;13(3):900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med. 2003;3(5):427–436. doi: 10.2174/1566524033479663. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. The American journal of psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry : the journal of mental science. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. The American journal of psychiatry. 2011;168(3):276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollema MG, Geurtsen GJ, van Voorst AJ. Durable improvements in Wisconsin Card Sorting Test performance in schizophrenic patients. Schizophr Res. 1995;16(3):209–215. doi: 10.1016/0920-9964(94)00079-n. [DOI] [PubMed] [Google Scholar]