Abstract
A rapid surface modification technique for the formation of self-assembled monolayers (SAMs) of alkanethiols on gold thin films using microwave heating in less than 10 min is reported. In this regard, SAMs of two model alkanethiols, 11-mercaptoundecanoic acid (11-MUDA, to generate a hydrophilic surface) and undecanethiol (UDET, a hydrophobic surface), were successfully formed on gold thin films using selective microwave heating in 1) a semi-continuous and 2) a continuous fashion and at room temperature (24 hours, control experiment, no microwave heating). The formation of SAMs of 11-MUDA and UDET were confirmed by contact angle measurements, Fourier–transform infrared (FT-IR) spectroscopy and X-ray photoelectron spectroscopy (XPS). The contact angles for water on SAMs formed by the selective microwave heating and conventional room temperature incubation technique (24 hours) were measured to be similar for 11-MUDA and UDET. FT-IR spectroscopy results confirmed that the internal structure of SAMs prepared using both microwave heating and at room temperature were similar. XPS results revealed that the organic and sulfate contaminants found on bare gold thin films were replaced by SAMs after the surface modification process was carried out using both microwave heating and at room temperature.
Keywords: Alkanethiols, self-assembled monolayers, gold thin films, surface plasmon resonance, surface plasmon fluorescence spectroscopy, microwave-induced temperature gradients
INTRODUCTION
Plasmonic materials have gained world-wide attention of researchers due to their ability to manipulate and transport electromagnetic energy at the nanoscale. Our ever increasing knowledge of the nature of plasmonic materials led to several commercially viable technologies, such as, Surface Plasmon Resonance (SPR) 1, 2, 3, 4, Surface Enhanced Raman Scattering (SERS) 5, 6, 7 and Surface Plasmon Fluorescence Spectroscopy (SPFS). 8, 9, 10 The interest in plasmonic materials is also due to their ability to directly interact with biological materials and report the changes in the environment of the biomolecules themselves. Plasmonic materials exist in many forms including as nanoparticles of different sizes,11 shapes12, 13 and types14, 15, 16 in solution and planar thin films deposited onto solid surfaces through thermal evaporation, etc. In the technologies mentioned above, the synthesis and/or construction of the plasmonic materials is followed by surface modification procedures. 17, 18
There are numerous reported techniques for the surface modification of plasmonic materials in literature, which include layer-by-layer assembly 17, 19, SAMs 17, 20, covalent attachment 17, 21, 22, 23 and sol-gels 24. One of the most commonly used surface modification techniques is the formation of SAMs of alkanethiols on plasmonic materials. The attachment of alkanethiols onto plasmonic materials is carried out via covalent attachment of the thiol group of the alkanethiols, where the tail end features another functional group. The functional groups in alkanethiols are: carboxylic acid (-COOH), hydroxyl and its derivatives (-OH), amine group and its derivatives (-NH2), which afford for further chemical modification and non-functional groups such as methyl (-CH3), just to name a few. The formation of SAMs on planar plasmonic thin films25, 26, 27, 28, 29 takes up to 24 hours due to the diffusion limited chemisorption of alkanethiols from an organic solvent onto plasmonic materials deposited onto a solid substrate. In order to overcome the long preparation times, Whitesides group developed a technique called microcontact printing,30 which affords for the transfer of SAMs of alkanethiols onto gold surfaces within a few minutes. However, the microcontact printing technique employs polymer stamps,31, 32 which requires a relatively tedious process and has inherent performance issues. Consequently, there is still a need to minimize the duration of the surface modification of plasmonic thin films on solid substrates with alkanethiols without the need of any additional tools.
Plasmonic gold thin films were previously used in conjunction with microwave heating for fast and sensitive bioassays for proteins33 and DNA hybridization. 34 In these reports, gold thin films were deposited onto standard glass microscope slides and then cut into pieces of 1.2×1.2 cm2. The use of smaller pieces of gold thin films prevented the destruction of gold thin films due to accumulation of electric on the surface (since the size of gold thin films are less than 1/10th of the wavelength of microwaves at 2.45 GHz, which is 12.2 cm).33, 34 These results were also corroborated via Finite-Difference Time-Domain calculations, which showed that the temperature of the gold thin films surface was not increased during microwave heating. 33 In addition, thermal images of the gold thin films during microwave heating showed that there is a microwave-induced temperature gradient between the bulk and the gold thin films. 33 These results provided us with the preliminary proof that plasmonic gold thin films can be used in a microwave-accelerated surface modification process.
In this work, we report the findings of our approach to rapid surface modification of gold thin films with alkanethiols using selective microwave heating. Our results revealed that the formation of SAMs of 11-MUDA and UDET using selective microwave heating were comparable to those SAMs formed at room temperature. FT-IR results confirmed the formation of well-ordered SAMs of 11-MUDA on gold thin films. Quantitative analysis of the SAMs were carried by XPS, which revealed that the organic and sulfate contaminants present on the bare gold thin films were replaced by SAMs of 11-MUDA or UDET after the surface modification process at both conditions. The results presented here prove the utility of our surface modification technique, which affords for the reduction of time for surface modification of gold thin films with SAMs from 24 hours (conventional technique) to less than 10 minutes without the use of any additional tools.
EXPERIMENTAL SECTION
Materials
3-Triethoxysilylpropylamine (APTES)-modified glass slides, 200-proof ethanol, 1-undecanethiol (UDET), 11-mercaptoundecanoic acid (11-MUDA), 30 wt% hydrogen peroxide, and sulfuric acid were obtained from Sigma-Aldrich. All chemicals were used without further purification.
Preparation of solutions
0.5 mM solutions of UDET and MUDA were prepared by dissolving appropriate amounts of either chemical in 200-proof ethanol. Solutions were stored in clean glass bottles at room temperature.
Preparation of gold thin films
A continuous gold thin film of 15 nm was applied to silanized slides using a sputter coater (EMS, model No: 150R S). Slides were then cut into pieces of 2.0×1.5 cm2 using a diamond tip glass cutter. Individual pieces were checked for an initial contact angle with water of ~66° before use.
Surface modification procedure
A 250 mL beaker was cleaned with piranha solution (3:7 30% hydrogen peroxide/concentrated sulfuric acid: CAUTION! piranha solution reacts violently with most organic materials and should be handled with extreme care) and filled with ~100 mL of either UDET or 11-MUDA solution. Six gold thin films were then arranged in the solution (Figure S1 and S3, Supporting Information) and the air-tight sealed beaker was left undisturbed for 24 hours at room temperature, or heated in a conventional microwave oven (900 Watts, 0.9 ft3, Frigidaire Model No. FCM09Z03KB, Figure S1, Supporting Information). Gold thin films were microwave heated for either 1–30 minutes in a semi-continuous fashion or continuously for 10 minutes at power level 1 (duty cycle: 3 seconds, Figure S2, Supporting Information). During semi-continuous heating, microwave heating is turned OFF and ON at the indicated times in Figure S4 (Supporting Information). Readers are referred to one of our previously published papers35 for detailed description of the duty cycle of the microwave oven used in this study. It is important to note that the evaporation of solvent was minimal under our experimental conditions, based on the observations made for the solution before and after microwave heating (Figure S3, Supporting Information). Upon completing the allotted time period, gold thin films modified with UDET were washed with deionized water twice (further rinse attempts resulted in the removal of the gold film from the glass surface); gold thin films modified with 11-MUDA were sequentially rinsed by deionized water-ethanol-deionized water.
Temperature measurements inside the microwave cavity
All temperature measurements reported in this study were carried out a using a UMI4 Universal Multi channel instrument from FISO Technologies, Inc. (Canada). A 1-mm thick fiber optic sensor (model no: FOT-L-SD-C1-F1-M2-R1-ST) was placed through the <5 mm hole created on top of the microwave cavity into the ethanolic solution of alkanethiols (Figure S1, Supporting Information). The temperature measurements were carried out according to the following configuration: 1) the solution when the fiber optic sensor was placed ~5 cm above the gold thin films for the temperature of solution and 2) directly onto the gold thin films for the temperature of gold thin films (Figure S2, Supporting Information).
Characterization of surface modified gold thin films
The contact angle of modified gold thin films was observed using a Kruss drop shape analysis system. A 20 ML drop of double distilled water was applied to the gold thin films and the contact angle with the slide was measured.
FT-IR spectroscopy measurements were carried out using a Cary 630 FT-IR with ATR accessory. Gold thin films with and without SAMs were placed on the ATR accessory and 256 scans at 2 cm−1 resolution were collected under atmospheric pressure. The raw was saved in. ascii format ans re-plotted using Sigmaplot without any modification.
X-ray photoelectron spectroscopy (XPS) measurements were carried out using a Kratos AXIS 165 spectrometer at the Surface Analysis Center at University of Maryland, College Park. Peak constraints for peak fit were: 2 pair of spin-orbit split components (area ratio 3/2 / 1/2 -> 2:1, spin orbit splitting fixed at 1.18 eV), all peaks restricted to have the same full width at height maximum. Quantification was done after application of a Shirley Background. All peaks were calibrated to the hydrocarbon at 284.8 eV. Only one peak in C region consistent with alkanes was present.
RESULTS AND DISCUSSION
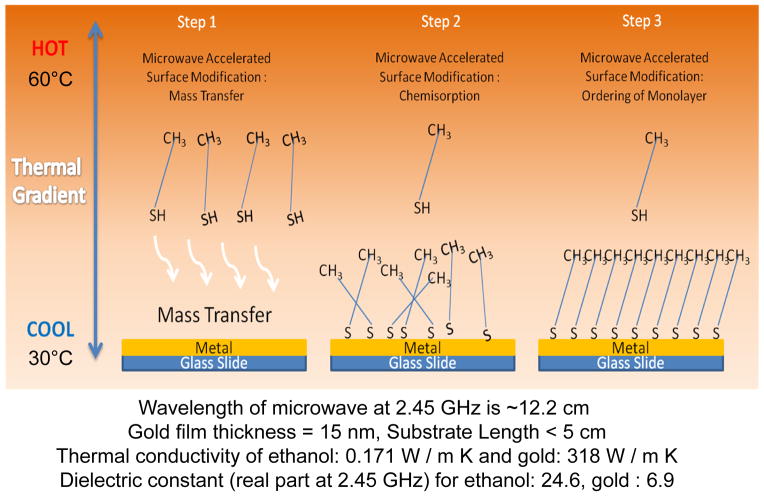
It is important to discuss the crux of the rapid surface modification methodology (depicted in Scheme 1) before the presentation of the experimental data. We hypothesize that in step 1, a thermal gradient is generated by microwave heating between the alkanethiol solution in ethanol and the gold thin film surface due to ~4 orders-of-magnitude difference in the thermal conductivity of ethanol and gold thin films (i.e., selective microwave heating. The thermal gradient results in the mass transfer of alkanethiols from the warmer solution to the cooler gold thin films.34 After the mass transfer process, alkanethiols molecules are chemisorbed onto the gold thin films (step 2). Finally, it is thought that the SAMs are formed in an ordered fashion as it would occur using the room temperature incubation of alkanethiol solution on gold thin films. The microwave-induced thermal gradients in our system are shown in Figure S4 (supporting Information). When the alkanethiol and the gold thin films are continuously heated the temperature gradient between these two components continuously increase up to ~30°C in 10 min. On the other hand, the heating of these two components in a semi-continuous fashion results in a temperature gradient of ~3°C in 10 min.
Scheme 1.
Schematic depiction of rapid surface modification of gold thin films with alkanethiols using selective microwave heating.
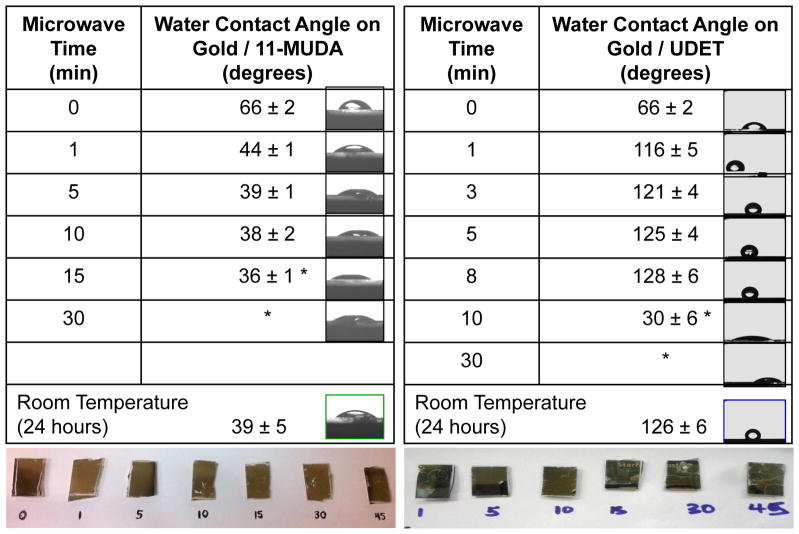
Table 1 shows the contact angle measurements for water placed on gold thin films before and after the application of the surface modification procedure at room temperature and using selective microwave heating. It is important to note the following experimental conditions for the surface modification procedures carried out here: 1) in the procedure involving selective microwave heating, to investigate the effect of microwave heating time, microwave heating was paused for 10 sec (i.e., semi-continuous heating) at the designated times given in Table 1 and a sample was removed each time from the beaker containing gold thin films and the alkanethiol solution, 2) in the surface modification procedure carried out at room temperature, the gold thin films were immersed in the identical solution continuously for 24 hours. In this study, to demonstrate the proof-of-principle of our rapid surface modification technique, two model alkanethiols that convert moderately hydrophobic bare gold thin films to a hydrophilic (11-MUDA) and a hydrophobic (UDET) surface were selected. The initial contact angle for water on bare gold thin films before the surface modification process (t = 0 min) was 66 ± 2°, which was deceased to 39 ± 1° after the formation of SAM of 11-MUDA at room temperature for 24 hours (i.e., no microwave heating, a control sample). The water contact angle on gold thin films modified with 11-MUDA using selective microwave heating showed a quantitative decrease as the time of microwave heating was increased up to 10 min. The continuation of microwave heating beyond 10 min resulted in the cracks on the gold thin films, and subsequently the contact angle measurements were deemed unusable, as corroborated by the real-color photographs (bottom of the table) and black and white photographs (right side of the table) obtained using the video camera of the Kruss drop shape analysis system. The contact angle for water on gold thin films modified with 11-MUDA using selective microwave heating for 10 min was 39 ± 1°, identical to that measured from 11-MUDA modified gold thin films prepared at room temperature. This implies that both the surface modification procedures (microwave heating and room temperature) produce identical results and the required time for the formation of SAMs of 11-MUDA was significantly reduced using selective microwave heating.
Table 1.
Contact angle of a 20 μL water droplet on gold thin films modified with 11-MUDA and UDET using selective microwave heating for up to 30 min. Microwave heating was paused at the indicated times and was continued after the removal of one of the gold thin films. Contact of angle of water on gold thin films modified with the identical alkane thiols at room temperature is also shown. RT-Room Temperature. *Surfaces were destroyed. Sample number is 5 for each data point.
|
Table 1 also shows that the water contact angle on gold thin films modified with UDET using selective microwave heating showed a quantitative increase from an initial value of 66 ± 2° up to 128 ± 6°, as the time of microwave heating was increased up to ~ 8 min. As observed during the surface modification of gold thin films with 11-MUDA, the prolonged microwave heating of the gold thin films in a UDET solution (>10 min) resulted in the destruction of the gold thin films as shown in the real-color images below Table 1. Subsequently, the water contact angle measurements on gold thin films heated longer than 10 min was unusable. The contact angle for water on gold thin films modified with UDET at room temperature was 126 ± 6°, which is similar to that measured on gold thin films modified with UDET using selective microwave heating for 8 min. These observations imply that while both the surface modification procedures produce identical results, the required time for the formation of SAMs of UDET was significantly reduced from 24 hours to ~ 8 min. These observations also imply that the order of SAMs formed on gold thin films by selective microwave heating were comparable with the SAMs formed by room temperature incubation of alkanethiols.
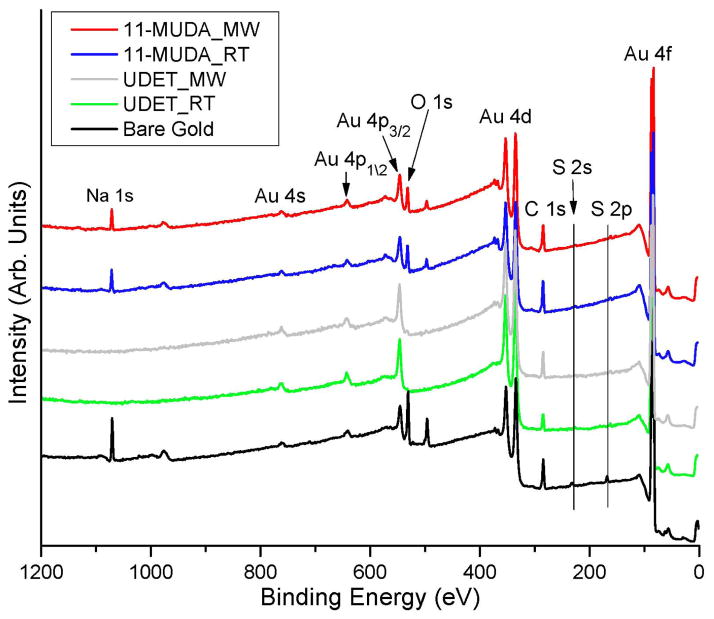
Although contact measurements are a useful tool in the characterization of SAMs, it is important to employ a more definitive characterization technique for SAMs of alkanethiols produced by selective microwave heating in order to make an appropriate comparison with those produced by room temperature incubation (i.e. conventional technique). In this regard, XPS analysis of SAMs of 11-MUDA and UDET on gold thin films and bare gold thin films (a control sample) was carried also out and the results are given in Figures 1–2 and Tables 2–3. Figure 1 shows the comparison of the XPS results for bare gold thin films and gold thin films modified with 11-MUDA and UDET using selective microwave heating (MW) and at room temperature (RT). One can observe that the most relevant peaks for 11-MUDA (Au 4f, S 2p, C 1s and O 1s) and for UDET (Au 4f, S 2p and C 1s) detected from both surfaces prepared by selective microwave heating and at room temperature appears to be identical. The same qualitative observation can be made for all other relevant signals as depicted in Figure 1. One can also see from Figure 1 that the strong XPS peak relevant to sodium (Na 1s) present in bare gold thin films was completely disappeared after the formation of SAMs of UDET both using selective microwave heating and at room temperature. This observation can be attributed to the displacement of sodium (sulfate) ions by the SAMs of UDET. In addition, the lack of sodium ions on the surface can also be attributed to the presence of unreactive –CH3 functional groups on UDET. On the other hand, reduced XPS peaks relevant to sodium were observable on SAMs of 11-MUDA on gold thin films as shown in Figure 1, which can be attributed to the presence of the –COOH functionality of 11-MUDA and the interactions of sodium ions with –COOH groups.
Figure 1.
Comparison of the XPS results for gold thin films modified with 11-MUDA and UDET using selective microwave heating (MW) and at room temperature (RT) and bare gold thin films.
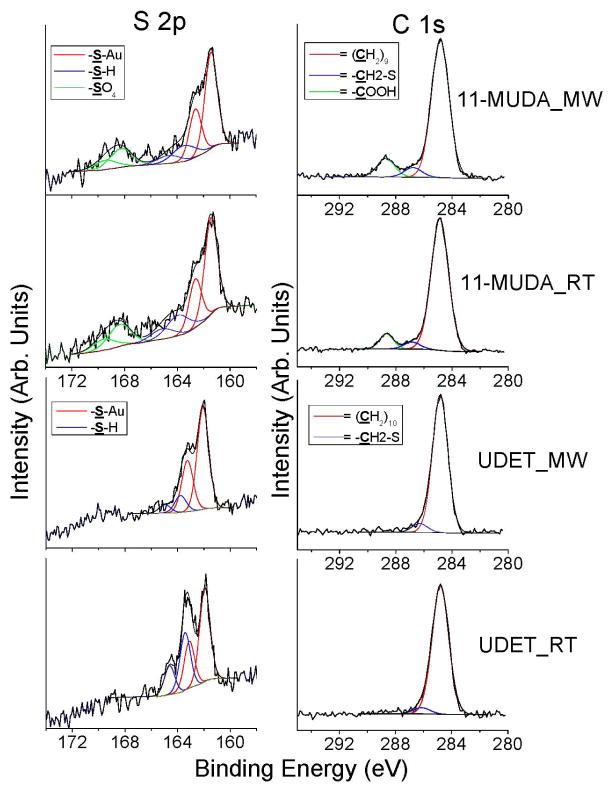
Figure 2.
Comparison of the selected regions (C 1s, S 2p) of the XPS results for gold thin films modified with 11-MUDA and UDET using selective microwave heating (MW) and at room temperature (RT).
Table 2.
Atomic percentage composition calculated from XPS results for gold thin films modified with alkanethiols using selective microwave heating (MW) and at room temperature (RT). ND- Not detected. Note all samples were produced and run in triplicate, except bare gold which was duplicate, means and standard deviations are reported.
| Atomic % | C / Au ratio | |||||
|---|---|---|---|---|---|---|
| C | O | Au | S | Na | ||
| Bare gold | 40.0 ± 1.8 | 23.3 ± 2.1 | 22.3 ± 4.7 | 7.5 ± 1.0 | 6.8 ± 0.2 | 1.8 ± 0.5 |
|
|
56.4 ± 1.6 | 15.0 ± 2.4 | 23.1 ± 3.1 | 2.6 ± 0.4 | 2.9 ± 0.6 | 2.5 ± 0.4 |
|
|
54.1 ± 3.1 | 14.5 ± 0.4 | 24.8 ± 3.7 | 2.6 ± 0.3 | 4.0 ± 0.8 | 2.2 ± 0.5 |
|
|
49.4 ± 0.3 | ND | 48.0 ± 0.5 | 2.6 ± 0.3 | ND | 1.0 ± 0.1 |
|
|
53.2 ± 3.3 | ND | 44.2 ± 3.2 | 2.6 ± 0.2 | ND | 1.3 ± 0.2 |
Table 3.
Summary of X-ray photoelectron spectroscopy results for gold thin films modified with alkanethiols using selective microwave heating (MW) and at room temperature (RT). Specific peak assignments (eV) and area under the curve for bound thiol, unbound thiol and sulfate is listed. ND- Not detected.
| % Area | Bound / Unbound Thiol | |||
|---|---|---|---|---|
| Bound Thiol | Unbound Thiol | Sulfate | ||
| Bare Gold | ND | ND | 100 | - |
|
|
55.6 ± 3.2 | 22.6 ± 1.7 | 22.1 ± 2.6 | 2.5 ± 0.2 |
|
|
56.6 ± 3.5 | 20.8 ± 2.3 | 22.6 ± 1.4 | 2.8 ± 0.5 |
|
|
60.6 ± 3.1 | 39.4 ± 3.1 | ND | 1.4 ± 0.2 |
|
|
75.0 ± 5.3 | 25.0 ± 5.3 | ND | 3.1 ± 1.0 |
In order to demonstrate the presence of 11-MUDA and UDET on the surface modified gold thin films using selective microwave heating and at room temperature, C 1s and S 2p peaks relevant to 11_MUDA (–(CH2)9, –CH2-S, –COOH, S-Au, -SH and –SO4) and UDET (–(CH2)9, –CH2-S, S-Au and –SH) were plotted and shown in Figure 2. The data shown in Figure 2 is discussed below in conjunction with the tabulated quantitative data (Table 2 and 3). Table 2 shows the atomic percentage composition for SAMs of 11-MUDA and UDET on gold thin films as compared to those measured from bare gold thin films. Since it is difficult to determine the surface coverage accurately from the XPS signals of each component of the SAMs, the calculation and comparison of the atomic percentage composition for SAMs on gold thin films and bare gold thin films can be considered as an appropriate approach. For example, the atomic percentage for C 1s can be miscalculated due to the possible surface contamination of organic chemicals during the transfer of bare gold thin films to the vacuum chamber in a laboratory setting. In this regard, Buck et. al. reported that the initial contamination of carbon species can be as high as 40% of that saturated SAMs on gold surface,36 which is comparable to our observation on bare gold thin films as shown in Table 2. Typical XPS peaks of C 1s can be attributed to carbon species both in SAMs and surface contamination by organic chemicals while XPS peaks of O 1s are mainly due to oxygen species in the surface contamination by organic chemicals. As shown in Table 2, the atomic percentage of C 1s on the bare gold thin films are approximately 43.5 ± 2.6%. The atomic percentage of C 1s in the 11-MUDA and UDET modified gold thin films were increased to 56.4 ± 1.6% and 49.4 ± 0.3% when selective microwave heating was used, respectively, which is due to the attachment of 11-MUDA and UDET onto the gold surface. Subsequently, the ratio of atomic percentage of C 1s to Au 4f is increased from 1.8 for bare gold to 2.2 and 2.5 for 11-MUDA-modified gold surface. The C 1s / Au 4f ratio for UDET-modified gold surfaces is close to 1 to 1.3, since no O 1s peak was detected as a result of the formation of SAMs of UDET on gold surfaces for both using selective microwave heating and at room temperature. Figure 2 shows that C 1s peaks exactly match those peaks relevant to 11_MUDA (–(CH2)9, –CH2-S and –COOH,) and UDET (–(CH2)9 and –CH2-S) on gold thin films prepared by selective microwave heating and at room temperature. Similar observations have also been made for SAMs of 11-MUDA and UDET on gold surfaces prepared at room temperature and for SAMs on gold thin films previously by other researchers.36
As shown in Table 2, the atomic percentage of O 1s on the bare gold thin films are approximately 23.3 ± 2.1%, which was significantly reduced to 14.5 to 15% after the formation of SAMs on 11-MUDA on gold thin films using selective microwave heating and at room temperature. Most noticeably, no XPS peak for O 1s was detectable on UDET modified gold thin films prepared using selective microwave heating and at room temperature, which provides additional evidence for the formation of SAMs alkanethiols on gold thin films.
In order to confirm that a chemical bond was formed between gold thin films and alkanethiols by replacing sulfate on the gold surface, we further investigated the XPS peaks observed between 160–172 eV, as depicted in Figure 2. Figure 2 shows that the binding energy of S 2p peak for SAMs at 161.5–161.9 eV on 11-MUDA and UDET modified gold thin films was clearly different from that observed on bare gold thin films at 169.2 eV, which is a characteristic peak for sulfate. However, the atomic percentage of S 2p on bare gold thin films (~7.5%, from sulfate) was significantly larger than those measured from 11-MUDA and UDET modified gold thin films (~2.6%). We attribute this observation to the deposition of sulfate (sodium) during the preparation of gold thin films while using our thermal evaporator. These peaks are clearly shown in Figure 2: sulfate peak observed on bare gold thin films were replaced by a small peak was observed clearly around 161.5 eV. The peak observed at 161.5 eV can be assigned to As–S bonding on the gold thin films after the surface modification by 11-MUDA and UDET using selective microwave heating and at room temperature.
Table 3 and Figure 2 also summarize the XPS results for sulfate, bound thiol and unbound thiol measured on bare gold thin films and gold thin films modified with 11-MUDA and UDET using selective microwave heating and at room temperature. As indicated earlier in the text, the observed peak in Figure 2 (S 2p) at 168.2 eV on bare gold films is consistent with sulfate, while the observed peaks between 161.3–163.1 eV and 163.5–164.6 eV are consistent with bound and unbound thiols, respectively. Bare gold thin films did not display any peaks for bound or unbound thiols but only for sulfate (Table 3). Table 3 also shows that the area under the peak assigned to bound thiols was measured to be identical for gold thin films modified with 11-MUDA using selective microwave heating and at room temperature. We also observed the presence of sulfate on 11-MUDA modified gold thin films using selective microwave heating and at room temperature, which was attributed to the to the presence of sodium and the –COOH functionality of 11-MUDA and the interactions of sodium ions with the –COOH groups and sulfates. The presence of unbound thiols on gold thin films are reported by others37 and can be attributed to the occupation of gold surfaces by other atoms/molecules present.
It is also important to note that in surface modification procedure involving alkane thiols, due to the high concentration of alkane thiols (~mM) in solution and comparatively small surface area on the plasmonic gold thin films, only a small fraction of the alkane thiols in solution can be adsorbed to the gold surface. Subsequently, some of the alkane thiols are associated in to the SAMs as “unbound thiols” due to the imperfections of the SAMs and one has to account for the unbound thiols. Table 3 also shows that the area under the peak assigned to sulfate was negligible after the surface modification of gold thin films with UDET in all conditions. In addition, the extent of area under the peaks for bound and unbound thiols showed an increase for gold thin films modified with UDET, which is consistent with the replacement of sulfate by thiols and the lack of interactions of sulfate with the SAMs of UDET. The comparison of the extent of bound and unbound thiols on gold thin films modified with UDET at room temperature and selective microwave heating reveals that the extent of bound thiols on SAMs prepared using microwave heating are significantly larger than those measured on SAMs prepared at room temperature. Although we do not have the full explanation for such an observation at this time, it can be partially attributed to our hypothesis that microwave heating accelerates the formation of chemical bonds between the gold thin films and alkanethiols and the organization of SAMs, such that, the extent of unbound thiols on the samples are reduced by shifting the equilibrium in favor of thiol adsorption. At room temperature, since the formation of SAMs require longer incubation times (24 hours), the equilibrium between the unbound and bound thiols are maintained at a higher value than that observed from microwaved heated samples. It is important to note that due to the proof-of-principle nature of this work, the comparison of the contact angle measurements and XPS results obtained using our rapid surface modification technique directly with those obtained using a well-established surface modification technique carried out at room temperature provides sufficient evidence for the validity of our technique.
In addition to contact angle measurements and XPS data, we have evaluated the SAMs using FT-IR spectroscopy (Figure S5, Supporting Information). FT-IR spectrum of the gold thin films modified with 11- MUDA showed a spectrum fundamentally similar to the absorbance spectrum of 11- MUDA in its isotropic sample; it showed C-H stretches in asymmetric and symmetric modes at 2920 cm−1 and 2850 cm−1, respectively. The comparison of these IR bands to those of a crystalline polymethylene chain (2920 cm−1 and 2850 cm−1) or in the liquid state (2928 cm−1 and 2856 cm−1) reveals that the 11-MUDA monolayers on our gold thin films are “solid-like” in nature. The C=O stretch at 1734 cm−1 associated with asymmetric (1647 cm−1) and symmetric (1542 cm−1) COO- stretches is characteristic of an organic carboxylic acid compound. The additional IR peaks at 1455 cm−1 and 1257 cm−1 can be attributed to C-H deformation and C-O stretch, respectively. The FTIR spectrum for UDET on gold thin films shows that there are three major vibrational modes around 2885, 2922–2925, and 2975 cm−1. They are assigned to the, -CH3 symmetric stretch, -CH2 asymmetric stretch, and -CH3 asymmetric stretch modes, respectively. The peak measured at 2922–2925 cm−1 (-CH2 asymmetric stretch) for SAMs of UDET on gold thin films indicates that the alkyl monolayer are as ordered and closely packed as those observed for SAMs of 11-MUDA on gold thin films. This observation is line with the variation in our contact angle measurements for SAMs prepared at room temperature and using microwave heating and can be attributed to lower surface coverage of SAMs of UDET on our gold thin films.
Based on the successful demonstration of rapid surface modification of gold thin films with alkanethiols using selective microwave heating applied in a semi-continuous manner, we subsequently investigated whether continuous microwave heating can also be utilized for rapid surface modification procedure. Table 4 shows the contact angle measurements for water on five different gold thin films modified with alkanethiols using continuous selective microwave heating. In these experiments, the microwave heating of 11-MUDA and UDET solutions containing gold thin films were carried out continuously for 10 and 8 min, respectively. The contact angle for water on gold thin films modified with 11-MUDA using continuous microwave heating was 33 ± 7°, which is similar to that observed using semi-continuous microwave heating (38 ± 2° at 10 min) and at room temperature (39 ± 5°). The real-color photographs of the gold thin films taken after continuous microwave heating show that gold thin films were not affected by the microwave heating (i.e., no visible cracks). These observation imply that one can both employ semi-continuous and continuous microwave heating for the rapid surface modification of gold thin films with 11-MUDA.
Table 4.
Rapid surface modification of gold thin films using continuous selective microwave heating. Contact angle of water on 11-MUDA-modified gold thin films (n=5) and on UDET-modified gold thin films (n=5) after 10 and after 8 minutes of continuous selective microwave heating, respectively.
| Sample No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Contact Angle | 28.5° | 31.0° | 26.1° | 34.3° | 43.1° |
|
|

|

|

|

|

|
| Average 33 ± 7° |

|

|

|

|

|
| Contact Angle | 114.01 | 116.38 | 118.29 | 120.68 | 113.19 |
|
|

|

|

|

|

|
| Average 116 ± 3° |

|

|

|

|

|
Similar observations were made for gold thin films modified with UDET using continuous microwave heating: the contact angle for water on gold thin films modified with UDET using continuous microwave heating was 116 ± 3°, which is slightly lower than that observed on UDET-modified gold thin films using semi-continuous microwave heating (126 ± 2° at 8 min) and at room temperature (126 ± 6°). The real-color photographs of the gold thin films modified with UDET taken after continuous microwave heating show the presence of multiple cracks in most of the gold thin films, which partially explains the slightly lower water contact angle observed on these surfaces. Based on these observations, for the successful and rapid surface modification of gold thin films with UDET, the use of microwave heating in a semi-continuous fashion is recommended. Our research laboratory is currently working on the application of selective microwave heating to rapid surface modification of other metal thin films using a wide range of alkanethiols. The results of these studies will be reported in due course.
CONCLUSIONS
A rapid surface modification procedure, based on selective microwave heating of gold thin films in the presence of alkanethiols solution, was demonstrated. SAMs of two model alkanethiols, namely, 11-MUDA and UDET, on 15 nm thick gold thin films were prepared within 10 min using selective microwave heating in a semi-continuous and continuous fashion, and was compared to SAMs of these alkanethiols prepared using room the conventional room temperature incubation technique, which took 24 hours to complete. Contact angle measurements were employed for the characterization of SAMs of alkanethiols prepared by our technique and the conventional technique. These experiments showed that the water contact angle on gold thin films (2.0 × 1.5 cm2) had a similar value after the surface modification process using both our technique and the conventional technique. More definite characterization techniques (i.e., FT-IR and XPS) were also employed to gain more insights into our surface modification technique. FT-IR studies revealed that SAMs of 11-MUDA on gold thin films were well ordered and closely packed, while SAMs of UDET were concluded to be less ordered, based on the location of the –CH2 asymmetric stretch peak occurring closer to that measured from UDET in solution. XPS results confirmed the presence of the chemical bond between gold and alkanethiols. The extent of surface contamination of bare gold thin films by organic chemicals and sulfates was observed to be similar to those previous reports found in the literature. One of these major contaminants in our samples, i.e., sodium sulfate, was found to be completely replaced by SAMs of UDET after the application of surface modification using selective microwave heating and at room temperature. Sulfates were still detected after the formation of SAMs of 11-MUDA using both surface modification techniques, which was attributed to the interactions of sodium with carboxylic acid functionality of 11-MUDA and sulfates. XPS peaks for bound and unbound thiols were clearly detected from 11-MUDA and UDET modified gold thin films using our technique and the conventional technique. The data presented in this work provides direct evidence for the utility of our surface modification technique, which afforded for the significant reduction of time for surface modification of gold thin films with SAMs from 24 hours (conventional technique) to less than 10 minutes.
Supplementary Material
Acknowledgments
The project described was partially supported by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Footnotes
Additional optical images of the experimental setup used in this work, plasmonic gold thin films before and after microwave heating, the actual temperatures of solvent and plasmonic gold thin films during microwave heating, FTIR results and raw XRD data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zheng R, Cameron BD. Surface plasmon resonance: recent progress toward the development of portable real-time blood diagnostics. Expert Rev Mol Diagn. 2012;12(1):5–7. doi: 10.1586/erm.11.91. [DOI] [PubMed] [Google Scholar]
- 2.Linman MJ, Yu H, Chen X, Cheng Q. Surface plasmon resonance imaging analysis of protein binding to a sialoside-based carbohydrate microarray. Methods Mol Biol. 2012;808:183–94. doi: 10.1007/978-1-61779-373-8_13. [DOI] [PubMed] [Google Scholar]
- 3.de Mol NJ. Surface plasmon resonance for proteomics. Methods Mol Biol. 2012;800:33–53. doi: 10.1007/978-1-61779-349-3_4. [DOI] [PubMed] [Google Scholar]
- 4.Pimkova K, Bockova M, Hegnerova K, Suttnar J, Cermak J, Homola J, Dyr JE. Surface plasmon resonance biosensor for the detection of VEGFR-1--a protein marker of myelodysplastic syndromes. Anal Bioanal Chem. 2012;402(1):381–7. doi: 10.1007/s00216-011-5395-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Han H, Luo Z. Streptococcus suis II immunoassay based on thorny gold nanoparticles and surface enhanced Raman scattering. Analyst. 2012;137(5):1259–64. doi: 10.1039/c2an15997j. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Wang Z, Zong S, Song C, Zhang R, Cui Y. Distinguishing breast cancer cells using surface-enhanced Raman scattering. Anal Bioanal Chem. 2012;402(3):1093–100. doi: 10.1007/s00216-011-5577-z. [DOI] [PubMed] [Google Scholar]
- 7.Guven B, Boyaci IH, Tamer U, Calik P. A rapid method for detection of genetically modified organisms based on magnetic separation and surface-enhanced Raman scattering. Analyst. 2012;137(1):202–8. doi: 10.1039/c1an15629b. [DOI] [PubMed] [Google Scholar]
- 8.Murakami T, Arima Y, Toda M, Takiguchi H, Iwata H. Effect of dielectric spacer thickness on signal intensity of surface plasmon field-enhanced fluorescence spectroscopy. Anal Biochem. 2012;421(2):632–9. doi: 10.1016/j.ab.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Feng CL, Yin M, Zhang D, Zhu S, Caminade AM, Majoral JP, Mullen K. Fluorescent core-shell star polymers based bioassays for ultrasensitive DNA detection by surface plasmon fluorescence spectroscopy. Macromol Rapid Commun. 2011;32(8):679–83. doi: 10.1002/marc.201000788. [DOI] [PubMed] [Google Scholar]
- 10.Huang CJ, Dostalek J, Sessitsch A, Knoll W. Long-range surface plasmon-enhanced fluorescence spectroscopy biosensor for ultrasensitive detection of E. coli O157:H7. Anal Chem. 2011;83(3):674–7. doi: 10.1021/ac102773r. [DOI] [PubMed] [Google Scholar]
- 11.Lu L, Burkey G, Halaciuga I, Goia DV. Core-shell gold/silver nanoparticles: synthesis and optical properties. Journal of colloid and interface science. 2013;392:90–5. doi: 10.1016/j.jcis.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Wan Y, Guo Z, Jiang X, Fang K, Lu X, Zhang Y, Gu N. Quasi-spherical silver nanoparticles: aqueous synthesis and size control by the seed-mediated Lee-Meisel method. Journal of colloid and interface science. 2013;394:263–8. doi: 10.1016/j.jcis.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Choi R, Choi SI, Choi CH, Nam KM, Woo SI, Park JT, Han SW. Designed Synthesis of Well-Defined Pd@Pt Core-Shell Nanoparticles with Controlled Shell Thickness as Efficient Oxygen Reduction Electrocatalysts. Chemistry. 2013 doi: 10.1002/chem.201203834. [DOI] [PubMed] [Google Scholar]
- 14.Deng D, Jin Y, Cheng Y, Qi T, Xiao F. Copper Nanoparticles: Aqueous Phase Synthesis and Conductive Films Fabrication at Low Sintering Temperature. ACS applied materials & interfaces. 2013 doi: 10.1021/am400480k. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Roy P, Mondal S, Bera T, Mukherjee A. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids and surfaces B, Biointerfaces. 2013;107:27–34. doi: 10.1016/j.colsurfb.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 16.Intartaglia R, Das G, Bagga K, Gopalakrishnan A, Genovese A, Povia M, Di Fabrizio E, Cingolani R, Diaspro A, Brandi F. Laser synthesis of ligand-free bimetallic nanoparticles for plasmonic applications. Physical chemistry chemical physics: PCCP. 2013;15(9):3075–82. doi: 10.1039/c2cp42656k. [DOI] [PubMed] [Google Scholar]
- 17.Abel B, Akinsule A, Andrews C, Aslan K. Plasmon-Enhanced Enzymatic Reactions: A Study of Nanoparticle-Enzyme Distance- and Nanoparticle Loading-Dependent Enzymatic Activity. Nano Biomed Eng. 2011;3(3):184–191. doi: 10.5101/nbe.v3i3.p184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Luna VH, Aslan K, Betala P. Colloidal gold. Encyclopedia of Nanoscience and Nanotechnology. 2004;2(1):27–49. [Google Scholar]
- 19.Brett CMA, Crespilho FN, Ghica ME, Florescu M, Nart FC, Oliveira ON. A strategy for enzyme immobilization on layer-by-layer dendrimer-gold nanoparticle electrocatalytic membrane incorporating redox mediator. Electrochem Commun. 2006;8(10):1665–1670. [Google Scholar]
- 20.Phadtare S, Shah S, Prabhune A, Wadgaonkar PP, Sastry M. Immobilization of Candida bombicola cells on free-standing organic-gold nanoparticle membranes and their use as enzyme sources in biotransformations. Biotechnol Progr. 2004;20(6):1817–1824. doi: 10.1021/bp049792h. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Kwon KY, Yang SB, Kong BS, Jung HT. High-performance biosensors based on enzyme precipitate coating in gold nanoparticle-conjugated single-walled carbon nanotube network films. Carbon. 2010;48(15):4504–4509. [Google Scholar]
- 22.Herne TM, Tarlov MJ. Characterization of DNA Probes Immobilized on Gold Surfaces. Journal of the American Chemical Society. 1997;119(38):8916–8920. [Google Scholar]
- 23.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382(6592):607–9. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 24.Brennan JD, Luckham RE. Bioactive paper dipstick sensors for acetylcholinesterase inhibitors based on sol-gel/enzyme/gold nanoparticle composites. Analyst. 2010;135(8):2028–2035. doi: 10.1039/c0an00283f. [DOI] [PubMed] [Google Scholar]
- 25.Azmi AA, Ebralidze, Dickson SE, Horton JH. Characterization of hydroxyphenol-terminated alkanethiol self-assembled monolayers: interactions with phosphates by chemical force spectrometry. J Colloid Interface Sci. 2013;393:352–60. doi: 10.1016/j.jcis.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Glebe U, Baio JE, Arnadottir L, Siemeling U, Weidner T. Molecular suction pads: self-assembled monolayers of subphthalocyaninatoboron complexes on gold. Chemphyschem. 2013;14(6):1155–60. doi: 10.1002/cphc.201300074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tantakitti F, Burk-Rafel J, Cheng F, Egnatchik R, Owen T, Hoffman M, Weiss DN, Ratner DM. Nanoscale clustering of carbohydrate thiols in mixed self-assembled monolayers on gold. Langmuir. 2012;28(17):6950–9. doi: 10.1021/la300444h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T, Tanaka Y, Koide Y, Tanaka M, Hara M. Mechanism underlying bioinertness of self-assembled monolayers of oligo(ethyleneglycol)-terminated alkanethiols on gold: protein adsorption, platelet adhesion, and surface forces. Phys Chem Chem Phys. 2012;14(29):10196–206. doi: 10.1039/c2cp41236e. [DOI] [PubMed] [Google Scholar]
- 29.Bould J, Machacek J, Londesborough MG, Macias R, Kennedy JD, Bastl Z, Rupper P, Base T. Decaborane thiols as building blocks for self-assembled monolayers on metal surfaces. Inorg Chem. 2012;51(3):1685–94. doi: 10.1021/ic202000b. [DOI] [PubMed] [Google Scholar]
- 30.Tien J, Xia Y, Whitesides GM. Microcontact printing of SAMs. in Self-Assembled Monolayers of Thiols. In: Ulman A, editor. Thin Films. Vol. 24. Academic Press; San Diego, CA: 1998. pp. 227–250. [Google Scholar]
- 31.Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Experimental cell research. 1997;235(2):305–13. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 32.Jackman RJ, Wilbur JL, Whitesides GM. Fabrication of submicrometer features on curved substrates by microcontact printing. Science. 1995;269(5224):664–6. doi: 10.1126/science.7624795. [DOI] [PubMed] [Google Scholar]
- 33.Aslan K, Malyn SN, Geddes CD. Microwave-Accelerated Surface Plasmon-Coupled Directional Luminescence: Application to fast and sensitive assays in buffer, human serum and whole blood. Journal of immunological methods. 2007;323(1):55–64. doi: 10.1016/j.jim.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Aslan K, Previte MJR, Zhang Y, Geddes CD. Microwave-accelerated surface plasmon-coupled directional luminescence 2: A platform technology for ultra fast and sensitive target DNA detection in whole blood. Journal of immunological methods. 2008;331(1):103–113. doi: 10.1016/j.jim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Alabanza AM, Mohammed M, Aslan K. Crystallization of L-alanine in the Presence of Additives on a Circular PMMA Platform Designed for Metal-Assisted and Microwave-Accelerated Evaporative Crystallization. CrystEngComm. 2012;14(24):8421–8431. doi: 10.1039/C2CE26363G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buck M, Eisert F, Fischer J, Grunze M, Träger F. Investigation of self-organizing thiol films by optical second harmonic generation and X-ray photoelectron spectroscopy. Appl Phys A. 1991;53(6):552–556. [Google Scholar]
- 37.Singhana B, Rittikulsittichai S, Lee TR. Tridentate Adsorbates with Cyclohexyl Headgroups Assembled on Gold. Langmuir. 2012;29(2):561–569. doi: 10.1021/la303101x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.