Abstract
Objectives
To evaluate the frequency of human papillomavirus–related oropharyngeal squamous cell carcinoma in African Americans and whites and to examine patient outcomes in these 2 groups.
Design
Retrospective study.
Setting
One tertiary care, university medical center.
Patients
Information on patients with stage III/IV oropharyngeal squamous cell carcinoma diagnosed between 1998 and 2007, and with primary surgical samples available for review, were selected from a radiotherapy database. One patient was Native American and was excluded from analysis; data on 174 patients were analyzed.
Results
One hundred forty-eight patients (85.1%) were white and 26 (14.9%) were African American. Human papillomavirus in situ hybridization–positive and p16-positive tumors were much more common in whites (63.5% and 83.1% of tumors, respectively) than in African Americans (11.5% and 34.6% of tumors, respectively) (P<.001). African Americans were also more likely to have received definitive (nonsurgical) rather than postoperative radiation therapy (P=.001) and had a higher frequency of T3/T4–stage tumors (P=.03) compared with whites. Disease-free survival was significantly shorter for African Americans (P=.02). In multivariate analysis, viral status (P=.006), T stage (P=.02), and treatment type (P=.002), but not race (P=.98), were significant factors contributing to disease-free survival.
Conclusions
In high-stage oropharyngeal squamous cell carcinoma, the proportion of human papillomavirus–related tumors is much higher in whites than in African Americans. African Americans also appear to develop higher T-stage tumors and are more likely to receive definitive therapy. The shorter disease-free survival observed in African Americans may be due to viral status, treatment type, and higher T stage, but does not appear to be due to race.
Many studies1-5 HAVE found outcome disparities between African Americans and whites with head and neck squamous cell carcinoma (SCC), with African Americans having shorter overall and disease-free survival. However, the reasons for these differences in outcome are unclear. A variety of factors have been suggested, including differences in access to health care, exposure to tobacco and alcohol, frequency of human papillomavirus (HPV)–related tumors, and/or unknown biological factors. Often, SCCs from different head and neck anatomic subsites have been grouped, complicating the analysis, since the etiologies and outcomes of SCC often differ according to site.
Human papillomavirus infection is well recognizedasanetiologicagentinalargesubset of SCCs that occur in the oropharynx. These HPV-related oropharyngeal tumors haveuniqueclinical,pathologic,andbiological features. Human papillomavirus is less commonlydetectedinoralcavity,larynx,and hypopharynx SCCs. At these sites, the significance of HPV detection is less clear.
Many of the pathologic and clinical characteristics of HPV-related SCC of the oropharynx have been well studied. Histologically, HPV-related SCCs are frequently nonkeratinizing and HPV-negative SCCs are typically keratinizing.6 Diffuse and strong p16 immunoreactivity, a surrogate marker for HPV-related SCC, is almostalwaysseen.7 Clinically,patientswith HPV-related SCC tend to be younger and of higher socioeconomic status, have a higher number of vaginal or oral sex partners, and are more likely to have a history of marijuana use.8-11 In contrast, HPV-negative SCC is more strongly associated with tobacco and alcohol use and poor dental hygiene.8,12 Most important, the clinical outcome in HPV-related SCC is significantly better than in HPV-negative SCC, with the former having longer disease-free and overall survival.11,13
Racial differences in the frequency of HPV-related SCC of the oropharynx and its effect on patient outcome have not been well studied. However, some reports have noted that HPV-related SCC occurs more frequently in whites than African Americans,9,14,15 although others have not found this to be true.16-18 Recently, Settle et al19 suggested that poorer outcomes for African Americans receiving chemoradiation for their stage III/IV head and neck SCC may be due to the lower frequency of HPV-related oropharyngeal SCC in African Americans compared with whites. This finding has not been validated in other patient populations or treatment groups.
The aims of this study were to evaluate the frequency of HPV-related oropharyngeal SCC in African American and white patients at a large university medical center and to examine patient outcomes in these 2 groups treated with primary surgery followed by adjuvant radiation therapy or definitive (nonsurgical) therapy.
METHODS
CASE SELECTION
The study was approved by the Human Research Protection Office of Washington University. Cases were identified from a radiotherapy database of all patients with head and neck SCC who received intensity-modulated radiation therapy (IMRT) at Washington University Medical Center. All patients with SCC of the oropharynx treated with either primary surgical procedures followed by IMRT or definitive IMRT between 1998 and 2007, and with primary surgical samples available for review, were initially selected (N=184). Only stage III or IV tumors were included owing to the low numbers of stage I and II tumors (n=9). Race information, based on self-reporting by the patients, was obtained from the medical record management software Clinical Desktop (BJC HealthCare, St Louis, Missouri). All but 1 patient were African American or white. One patient was Native American and was excluded from analysis. No patients from other racial groups were included. The total number of patients in the study was 174.
IN SITU HYBRIDIZATION FOR HPV
In situ hybridization (ISH) was performed on formalin-fixed, paraffin-embedded, 4-μm tissue sections, using the ISH I View Blue Plus Detection Kit (Ventana Medical System, Inc, Tucson, Arizona) according to the manufacturer's instructions. The probe hybridizes with the high-risk HPV (HR HPV) genotypes including types 16, 18, 33, 35, 45, 51, 52, 56, and 66. Ventana Red Counterstain II (Ventana Medical System, Inc) was used. Positive staining was identified as blue nuclear dots. Any definitive nuclear staining in the tumor cells was considered positive. Cases were classified in a binary manner as either positive or negative.
IMMUNOHISTOCHEMISTRY FOR p16
Immunoperoxidase staining was done on formalin-fixed, paraffin-embedded, 4-μm tissue sections using the DAKO LSAB2 horseradish peroxidase system (DAKO Corp, Carpentaria, California) according to the manufacturer's instructions. Antigen retrieval was done by microwave heating for 10 minutes in 10mM citrate buffer (pH 6.0); a p16 monoclonal antibody (1:40 dilution) was used (Novocastra Laboratories Ltd, Newcastle upon Tyne, United Kingdom). Cases were classified in a binary manner as either positive (any cells with nuclear and cytoplasmic staining) or negative for statistical analysis. Cases were further classified as diffuse (>50% of cells staining) or focal (≤50% of cells staining).
STATISTICS
Fisher exact tests were used to examine associations between race and the categorical variables HPV ISH, smoking history, sex, and T stage; χ2 tests were used to examine associations between race and p16, treatment strategy, and concomitant chemotherapy. For the continuous variables of age and follow-up length, t tests were performed to evaluate differences by race. Log-rank tests were used to determine differences in overall and disease-free survival by race, HPV ISH or p16 status, treatment strategy, and T stage. Overall survival was calculated from the start date of treatment to the date of death from any cause or the last known follow-up date. Disease-free survival was calculated from the start date of treatment to the date of disease recurrence, death, or last known follow-up if there was no recurrence. For multivariate analysis, proportional hazard regression model was used to adjust for the covariates race, HPV ISH or p16 status, treatment strategy, and T stage. All statistical tests were 2-sided, and the level for statistical significance was set at .05. SAS version 9.1 was used for all major statistical calculations (SAS Institute Inc, Cary, North Carolina).
RESULTS
A total of 174 patients were identified. Of these, 26 were African American (14.9%) and 148 were white (85.1%). The median age was 55 years (range, 32-83 years). In total, 159 were men (91.4%) and 15 were women (8.6%). Of these patients, 132 (78.1%) had a history of current or former tobacco use, while 37 (21.9%) did not. Data on tobacco use were not available for 5 patients (2.9%). Only patients with stage III (24 [13.8%]) and stage IV (150 [86.2%]) tumors were included in the study. However, 100 (58.1%) had low T-stage (T1 or T2) tumors, while 72 (41.9%) had high T-stage (T3 or T4) tumors. T stage was not available for 2 patients. Most patients (121 [69.5%]) were treated with primary surgical procedures followed by postoperative IMRT; the remaining 53 patients (30.5%) were treated with definitive IMRT. Data regarding receipt of concomitant chemotherapy with IMRT were available for all but 5 patients (2.9%). More than half of the patients (99 [58.6%]) received concomitant chemotherapy. The median length of follow-up was 28 months (range, 2-106 months).
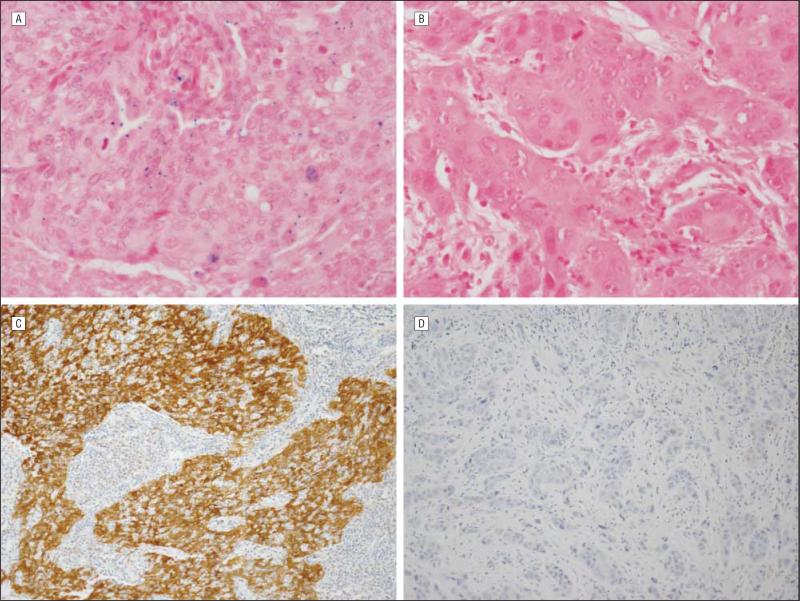
Overall, 97 (55.8%) of the tumors were HR HPV-positive by ISH and 132 (75.9%) of the tumors were p16-positive by immunohistochemistry. Examples of HR HPV ISH– and p16-positive and negative tumors are shown in Figure 1. There was a striking difference in the rates of HR HPV ISH–positive and p16-positive tumors in African Americans compared with whites (Table 1). While 63.5% (94 of 148) of the tumors were HR HPV ISH–positive and 83.1% (123 of 148) were p16-positive in whites, in African Americans, only 11.5% (3 of 26) of tumors were HR HPV ISH–positive and 34.6% (9 of 26) were p16-positive.
Figure 1.
Examples of high-risk (HR) human papillomavirus (HPV)–in situ hybridization (ISH) and p16 immunohistochemistry results. A, HR HPV–positive (blue nuclear dots) tumor by ISH (original magnification ×600). B, HR HPV–negative tumor by ISH (original magnification ×600). C, p16-positive tumor by immunohistochemistry showing strong nuclear and cytoplasmic staining (original magnification ×200). D, p16-negative tumor by immunohistochemistry (original magnification ×200).
Table 1.
HR HPV ISH and p16 Immunohistochemistry Results by Race
| No. (%) Positive |
||
|---|---|---|
| Race, No. (%)a | HR HPV ISHb | p16b |
| African American, 26 (14.9) | 3 (11.5) | 9 (34.6) |
| White, 148 (85.1) | 94 (63.5) | 123 (83.1) |
Abbreviations: HPV, human papillomavirus; HR, high-risk; ISH, in situ hybridization.
N = 174.
P < .001.
Almost all tumors that were HR HPV ISH positive were also p16 positive. Only 4.1% (4 of 97) of the HR HPV ISH–positive tumors were p16 negative and all of these tumors were from white patients. On the other hand, 29.5% (39 of 132) of tumors that were p16 positive were HR HPV ISH negative. While the vast majority of p16-positive tumors showed strong and diffuse staining (>50% of tumor cells) with nuclear and cytoplasmic immunoreactivity, 4 tumors showed focal (≤50% of tumor cells) nuclear and cytoplasmic staining. All 4 of these tumors were from the HR HPV ISH–negative group. Three of these cases with focal p16 staining were from African American patients, and 1 was from a white patient.
The clinical characteristics of patients and tumors from African Americans and whites are presented in Table 2. All patients had stage III or IV tumors as an inclusion criterion, and African Americans had a greater percentage of T3- or T4-stage tumors (61.5%; 16 of 26) compared with whites (38.4%; 56 of 146) (P=.03). African Americans were also more likely to have been treated with definitive IMRT (57.7%; 15 of 26) rather than surgery followed by postoperative adjuvant IMRT (P=.001). Only 38 white patients (25.7%) received definitive IMRT. There were no significant differences in age, sex, history of tobacco use, concomitant chemotherapy, or length of follow-up between the 2 groups.
Table 2.
Comparison of Clinical Characteristics by Race
| Race |
|||
|---|---|---|---|
| Characteristic | African American (n=26) | White (n=148) | P Value |
| Age, median (range), y | 54 (39-83) | 55 (32-73) | .85 |
| Sex, No. (%) | |||
| Male | 24 (92.3) | 135 (91.2) | >.99 |
| Female | 2 (7.7) | 13 (8.8) | |
| Smoking history, No. (%) | |||
| Ever | 23 (88.5) | 109 (76.2) | .20 |
| Never | 3 (11.5) | 34 (23.8) | |
| T stage, No. (%) | |||
| T1 or T2 | 10 (38.5) | 90 (61.6) | .03a |
| T3 or T4 | 16 (61.5) | 56 (38.4) | |
| Treatment strategy, No. (%) | |||
| Definitive IMRT | 15 (57.7) | 38 (25.7) | .001a |
| Postoperative IMRT | 11 (42.3) | 110 (74.3) | |
| Concomitant chemotherapy, No. (%) | |||
| Yes | 17 (68) | 82 (56.9) | .30 |
| No | 8 (32) | 62 (43.1) | |
| LOF, median (range), mo | 27 (5-68) | 29 (2-106) | .43 |
Abbreviations: IMRT, intensity-modulated radiation therapy; LOF, length of follow-up.
Significant at P< .05.
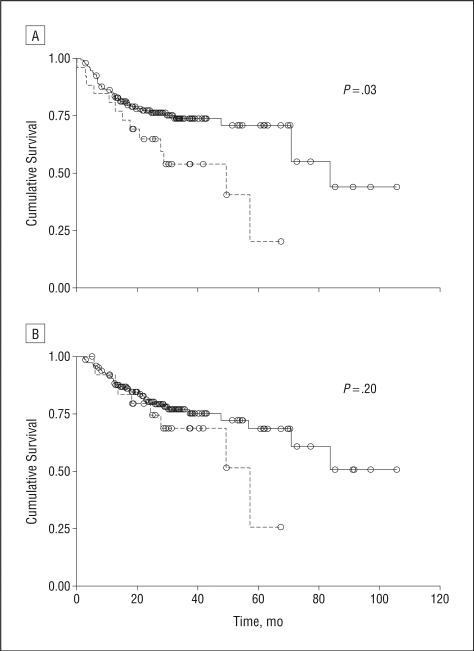
African Americans had significantly shorter disease-free survival compared with whites (Figure 2A, P=.02). Overall survival was not significantly different between the 2 groups (Figure 2B, P=.20), although there did appear to be a trend toward poorer overall survival for African Americans. In multivariate analysis, HR HPV status (P=.006), treatment type (P=.002), and T stage (P=.02), but not race (P=.70), were significant factors influencing disease-free survival (Table 3). When p16 status was included in multivariate analysis rather than HPV status, the findings were similar (Table 4). In addition, p16 status appeared to be a very strong predictor (P<.001) of disease-free survival in multivariate analysis (Table 4).
Figure 2.
Kaplan-Meier curves of disease-specific (A) and overall (B) survival in African American (dashed line) and white (solid line) patients. P values are unadjusted.
Table 3.
Multivariate Analysis of Disease-Free Survival With HR HPV ISH
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| White | 0.87 (0.44-1.75) | .70 |
| Postoperative IMRT | 0.40 (0.22-0.72) | .002a |
| High-risk HPV positive | 0.41 (0.22-0.78) | .006a |
| T stage 3 or 4 | 1.41 (1.05-1.89) | .02a |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high-risk; IMRT, intensity-modulated radiation therapy; ISH, in situ hybridization.
Significant at P< .05.
Table 4.
Multivariate Analysis of Disease-Free Survival With p16
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| White | 0.76 (0.38-1.50) | .42 |
| Postoperative IMRT | 0.40 (0.23-0.72) | .002a |
| p16 positive | 0.26 (0.14-0.48) | <.001a |
| T stage 3 or 4 | 1.43 (1.06-1.92) | .02a |
Abbreviations: CI, confidence interval; IMRT, intensity-modulated radiation therapy.
Significant at P< .05.
COMMENT
Several previous studies9,14,15 have found a higher frequency of HPV-positive oropharyngeal or head and neck SCCs in whites. In a small number of patients, Haraf et al15 found 7 of 9 tonsillar tumors in whites to be HPV-positive by polymerase chain reaction (PCR) or restriction fragment length polymorphism analysis compared with 2 of 9 tumors in African Americans. Fakhry et al14 found 5% (2 of 38) of HPV-positive oropharyngeal or laryngeal tumors and 22% (13 of 58) of HPV-negative tumors to be from nonwhite patients. Testing was done by ISH for HPV-16. In a case-control study9 of head and neck cancer, patients with HPV-16 ISH–positive tumors were more likely to be white (93 of 99) than were those with HPV-16 ISH–negative tumors (82 of 100) (P=.006). The latter 2 studies, however, included tumors from head and neck anatomic subsites other than the oropharynx where the etiologic relationship between HPV and tumor development is less clear. Fakhry et al14 did correlate HPV status with p16 immunohistochemistry in an attempt to provide supportive evidence that the HPV-positive tumors were in fact HPV driven.
A higher frequency of HPV-related tumors in whites has not been a universal finding. In a study of 253 patients with head and neck cancer, Gillison et al17 found no significant difference in HPV status by race (white/ Hispanic vs African American). Human papillomavirus was detected by PCR. The inclusion of subsites outside of the oropharynx, where HPV may be a bystander and not driving tumor development or affecting its clinical behavior, may have influenced the findings. However, Sedaghat et al18 recently found no significant difference in HPV-16 ISH status between African Americans and whites in a smaller number of only oropharyngeal SCCs. Two of 22 HPV-16 ISH–positive tumors and 2 of 17 HPV-16 ISH–negative tumors occurred in African Americans. Interestingly, in a review of data from the Surveillance Epidemiology and End Results program from 1973 to 2004 that included 45 769 patients, Chaturvedi et al16 found that so-called HPV-related oropharyngeal SCCs were actually more common in African American than in white patients. However, the SCCs were presumed to be HPV-related based on location (base of tongue, lingual tonsil, palatine tonsil, oropharynx, and Waldeyer ring) rather than actual HPV testing.16 Therefore, the validity of their findings is uncertain because they simply found that oropharyngeal SCCs, as a percentage of all head and neck SCCs, are more common in African American than in white patients.
The method of HPV detection, which is not uniform in studies throughout the literature, may be important as well. Although ISH may not be as sensitive as PCR, it allows for direct visualization of the virus in tumor cell nuclei.20 In addition, detection of HPV DNA, either by ISH or by PCR, is not necessarily proof that the virus played a role in the pathogenesis of that tumor. Additional molecular evidence is required. Positivity of p16 determined by immunohistochemistry has emerged as a sensitive, but not entirely specific, marker of HPV-related SCC that is often used in conjunction with ISH or PCR of the virus.21
In this study, with a large number of cases of stage III and IV oropharyngeal SCC treated either with definitive IMRT or surgery followed by postoperative IMRT, we found a marked difference in the frequency of HPV-related tumors in African Americans and whites using ISH for HPV detection as well as p16 immunohistochemistry. In African Americans, 11.5% (3 of 26) of SCCs were HR HPV ISH positive compared with 63.5% (94 of 148) of tumors in whites (P<.001). There was a similar disparity in the frequency of p16-positive SCCs between whites and African Americans. Only 34.6% (9 of 26) of SCCs in African Americans were p16 positive compared with 83.1% (123 of 148) of SCCs in whites (P<.001).
One may argue that the lower detected rate of HPV-positive tumors in African Americans was a sampling error. African Americans in our study were more likely to have received definitive IMRT and thus were more likely to only have biopsy material rather than surgical resections available for ISH and immunohistochemistry studies. In our experience, HPV ISH is typically patchy to focal. This, in theory, would make it less likely for HPV to be detected in biopsy material and consequently less likely to be detected in African Americans. However, the similar disparity in the frequency of p16-positive SCCs between whites and African Americans makes this less likely. The immunoreactivity of p16 is almost always diffuse and thus easily detectable in biopsy material. Furthermore, the diffuse pattern of p16 immunoreactivity is typically reported in HPV-related SCC. Despite the greater likelihood of testing being performed on biopsy material in African Americans, p16 was more frequently focal in African Americans (30.3%; 3 of 9) compared with whites (0.8%; 1 of 123). Correlating HPV with p16 status, as well as limiting analysis to tumors of the oropharynx, as we have done, helps to exclude cases in which the biological significance of HPV positivity is unclear (particularly tumors from the oral cavity, larynx, and hypopharynx) and helps to limit potential sampling errors from HPV ISH testing of biopsy material.
The reasons for the race disparity in HPV-related oropharyngeal SCC that we and others9,14,15,19 have found are unknown but are likely related to environmental differences, genetic differences, or a combination of these 2 factors. For example, it has been suggested that there may be racial differences in risk factors for HPV-related SCC.22 A survey23 of adolescents found that white males were more likely to engage in oral sexual activity, while African American males were more likely to engage in genitalto-genital sex. Brawley22 proposed that the reported higher rate of oral sexual activity as initial sexual behavior may lead to a higher prevalence of oral, rather than genital, HPV infections among whites. Genetic differences in host-virus interactions are also plausible. A polymorphism in the p73 gene, which is inactivated by HPV-16 E6 onco-protein, was recently found to correlate with HPV-16 tumor status among non-Hispanic white patients with head and neck SCC.24 However, this polymorphism has not been examined in other racial groups, and other host-viral genetic interactions have not been explored.
In addition to HPV tumor status, African Americans also differed significantly from whites in tumor T stage and treatment strategy, but not age, smoking history, receipt of adjuvant chemotherapy, or length of follow-up in our study. African Americans were more likely to have higher T-stage (3 or 4) tumors and be treated with definitive IMRT. The reasons for these differences are not entirely clear, although there are a few possible explanations.
Human papillomavirus–positive SCCs are typically of lower T stage than are HPV-negative tumors. Indeed, T1-or T2-stage tumors were much more likely to be HPV positive than were T3- or T4-stage tumors in our patients (P<.001). Although T stage of HPV-positive and HPV-negative SCCs is not frequently examined in the literature, others11,14 have found results similar to ours. The low frequency of HPV-related tumors in African Americans may, in part, explain the higher T-stage tumors in this group. However, alternative explanations cannot be excluded. It is also possible that decreased access to health care among African Americans could lead to delayed presentation and larger tumor size. Gourin and Podolsky2 found that African American patients were less likely to have insurance than were white patients, and even among African American patients, insurance status correlated with patient outcome. In our study, African Americans did not present with overall higher stage tumors, since all patients had stage III or IV tumors and there was no significant difference in stage III vs stage IV tumors by race (P> .99).
In terms of treatment strategy, others1,2 have similarly found that African Americans with head and neck cancer are more likely to receive nonsurgical treatment. T stage (tumor size) may be 1 factor contributing to this difference. Since HPV-positive tumors tend to be small, patients often present with lymph node metastases and an occult primary tumor. These tumors are often resected in the search for an unknown primary, particularly at institutions such as ours where transoral laser microsurgery with an operating microscope is used. This technique allows for the detection of very small primary tumors. If the primary tumor is not found, ipsilateral palatine and lingual tonsillectomies are typically performed. Therefore, white patients, who are more likely to have HPV-positive tumors, may be treated more frequently with surgical procedures. In addition, a portion of large, T4-stage tumors may be unresectable, necessitating nonsurgical treatment. There are probably other unknown, contributory factors that differ between African Americans and whites.
Very few studies have examined the effect of HPV status on survival disparities between African Americans and whites with oropharyngeal SCC. Recently, Settle et al19 found that a low prevalence of HPV-positive oropharyngeal SCC among African American patients explained overall survival differences between African American and white patients with stage III or IV head and neck cancer treated with chemoradiation. In a separate matched-pair analysis of race or ethnicity, African Americans had significantly worse survival from oropharyngeal cancer but not from cancer of other head and neck sites.25 While HPV testing was not done, the findings indirectly suggest a lower frequency of “good prognosis” HPV-related SCC of the oropharynx among African Americans.
We have similarly found that HPV, along with treatment strategy and T stage, explain survival differences between African Americans and whites with stage III or IV oropharyngeal SCC. In our study, disease-free survival was significantly shorter for African Americans com pared with whites (P=.02). In multivariate analysis, HPV ISH or p16 negativity, definitive IMRT, and high T stage, but not race, were associated with worse disease-free survival. Tumor p16 status appeared to be the strongest predictor of disease-free survival (P<.001).
Racial disparities in survival and the frequency of HPV-related oropharyngeal SCC need to be confirmed in prospective studies. However, our study and that of Settle et al19 suggest that differences in HPV-related SCC contribute to worse survival among African Americans with oropharyngeal SCC. Many other studies have found survival differences between African Americans and whites with head and neck cancer. However, these studies did not evaluate HPV status and often grouped multiple head and neck anatomic subsites together, making the effect of HPV-related oropharyngeal SCC on racial disparities in patient outcome difficult to assess.1-5,26
Our study population did not include any Asians or other racial groups other than 1 Native American who was excluded from analysis. This may simply be the result of low numbers of other racial groups in our patient population rather than truly indicative of a low rate of HPV-related oropharyngeal SCC among other racial groups. US Census Bureau (http://quickfacts.census.gov/qfd/states/29000.html) data indicate that Missouri, the site of our study, has a significantly lower rate of Asians and Hispanics than the national average. However, in a small study,27 HPV was detected in Australian but not Chinese patients with oropharyngeal SCC, suggesting that racial disparities among other racial or ethnic groups may exist. Further investigation is needed.
The reasons for racial disparity in the frequency of HPV-related oropharyngeal SCC that we and others have found are not clear. Further studies are needed to address specific environmental factors, genetic factors, or virus-host interactions that may play a role in HPV-related tumor development.
Acknowledgments
Funding/Support: Funding for this study was provided by the Department of Pathology and Immunology, Washington University School of Medicine. Support was also provided by the Biostatistics Core, Siteman Comprehensive Cancer Center, and support grant P30 CA091842 from the National Cancer Institute Cancer Center.
Previous Presentation: This study was presented at the United States and Canadian Academy of Pathology 99th Annual Meeting; March 23, 2010; Washington, DC.
Additional Contributions: We thank Rodney Brown, BA, ASCP, Kevin Keith, HT, and Jianping Li, BS, for their expert technical assistance with the immunohistochemistry and ISH experiments.
Footnotes
Author Contributions: Dr Chernock had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chernock and Lewis. Acquisition of data: Chernock, Thorstad, and Lewis. Analysis and interpretation of data: Chernock, Zhang, El-Mofty, and Lewis. Drafting of the manuscript: Chernock. Critical revision of the manuscript for important intellectual content: Zhang, El-Mofty, Thorstad, and Lewis. Statistical analysis: Zhang. Obtained funding: Chernock and Lewis. Administrative, technical, and material support: Thorstad and Lewis. Study supervision: El-Mofty and Lewis.
Financial Disclosure: None reported.
REFERENCES
- 1.Tomar SL, Loree M, Logan H. Racial differences in oral and pharyngeal cancer treatment and survival in Florida. Cancer Causes Control. 2004;15(6):601–609. doi: 10.1023/B:CACO.0000036166.21056.f9. [DOI] [PubMed] [Google Scholar]
- 2.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 3.Al-Othman MOF, Morris CG, Logan HL, Hinerman RW, Amdur RJ, Mendenhall WM. Impact of race on outcome after definitive radiotherapy for squamous cell carcinoma of the head and neck. Cancer. 2003;98(11):2467–2472. doi: 10.1002/cncr.11822. [DOI] [PubMed] [Google Scholar]
- 4.Miller CS, Henry RG, Rayens MK. Disparities in risk of and survival from oropharyngeal squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):570–575. doi: 10.1067/moe.2003.108. [DOI] [PubMed] [Google Scholar]
- 5.Arbes SJ, Jr, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Control. 1999;10(6):513–523. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 6.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klussmann JP, Gültekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 9.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 10.Lenselink CH, Melchers WJ, Quint WG, et al. Sexual behaviour and HPV infections in 18 to 29 year old women in the pre-vaccine era in the Netherlands. PLoS One. 2008;3(11):e3743. doi: 10.1371/journal.pone.0003743. doi:10.1371/journal.pone.0003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89(3):300–304. [PubMed] [Google Scholar]
- 12.Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992;70(3-4):320–327. doi: 10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann M, Görögh T, Gottschlich S, et al. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218(2):199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 15.Haraf DJ, Nodzenski E, Brachman D, et al. Human papillomavirus and p53 in head and neck cancer: clinical correlates and survival. Clin Cancer Res. 1996;2(4):755–762. [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 17.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 18.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119(8):1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 19.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2(9):776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 21.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 22.Brawley OW. Oropharyngeal cancer, race, and the human papillomavirus. Cancer Prev Res (Phila) 2009;2(9):769–772. doi: 10.1158/1940-6207.CAPR-09-0150. [DOI] [PubMed] [Google Scholar]
- 23.Gates GJ, Sonenstein FL. Heterosexual genital sexual activity among adolescent males: 1988 and 1995. Fam Plann Perspect. 2000;32(6):295–297, 304. [PubMed] [Google Scholar]
- 24.Ji X, Sturgis EM, Zhao C, Etzel CJ, Wei Q, Li G. Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer. 2009;115(8):1660–1668. doi: 10.1002/cncr.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LM, Li G, Reitzel LR, et al. Matched-pair analysis of race or ethnicity in out-comes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res (Phila) 2009;2(9):782–791. doi: 10.1158/1940-6207.CAPR-09-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murdock JM, Gluckman JL. African-American and white head and neck carcinoma patients in a university medical center setting: are treatments provided and are outcomes similar or disparate? Cancer. 2001;91(1)(suppl):279–283. doi: 10.1002/1097-0142(20010101)91:1+<279::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Thompson CH, Xin D, et al. Absence of human papillomavirus in tonsillar squamous cell carcinomas from Chinese patients. Am J Pathol. 2003;163(6):2185–2189. doi: 10.1016/S0002-9440(10)63576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]