Abstract
Aims
Nonkeratinizing morphology in oropharyngeal squamous cell carcinoma (NKSCC) strongly correlates with human papillomavirus and p16 status, but as a unique diagnostic entity is not widely recognized by pathologists. We sought to prospectively examine the performance of a new histological typing system during 1 year of routine clinical practice (Aim 1) and also its reproducibility amongst six head and neck pathologists using a 40 case test set (Aim 2).
Methods and Results
The three histological types were: Type 1 (keratinizing), Type 2 (nonkeratinizing with maturation) and Type 3 (nonkeratinizing). For Aim 1, there were 85 cases. p16 immunohistochemistry was positive in five of the 18 (27.8%) cases classified as Type 1, 18 of the 19 (94.7%) as Type 2, and 47 of the 48 (97.9%) as Type 3. For Aim 2, agreement among pathologists on the test cases was best for types 1 and 3 (kappa values 0.62 and 0.56; P < 0.0001) and lowest for type 2 (kappa 0.35; P < 0.0001). All 21 cases classified as NK SCC (type 3) by any of the reviewers was p16 positive.
Conclusions
Pathologists can recognize NK SCC with good agreement, and when a pathologist classifies a tumour as NK SCC, this reliably predicts p16 positivity.
Keywords: morphology, nonkeratinizing, oropharyngeal, p16, squamous cell carcinoma
Introduction
Oropharyngeal squamous cell carcinoma (SCC) is now recognized as distinct because of its strong association with high risk human papillomavirus (HPV).1,2 Conventional head and neck SCC is strongly associated with smoking, smokeless tobacco use, and/or heavy alcohol use while HPV-related oropharyngeal SCC is associated with higher numbers of sex partners and higher oral sex exposure.3 Conventional head and neck SCC commonly occurs in middle aged to older men without a significant race predilection, while HPV-related oropharyngeal SCC occurs in slightly younger patients, is even more common in men than women, is associated with lower smoke exposure, and is more common in Caucasians.4 While conventional head and neck SCC rates are dropping, those for oropharyngeal SCC are increasing so that oropharyngeal SCC is accounting for a larger percentage of all head and neck SCC.2,5,6
HPV-related oropharyngeal SCC is biologically distinct as well. The tumours are less genetically complex,7 less frequently harbour p53 mutations,8,9 and show differing global gene expression profiles compared to HPV-negative oropharyngeal SCC.7 HPV-related oropharyngeal SCC is characterized by transcriptionally-active virus with the E6 and E7 transcripts altering cell cycle functions and apoptosis. E6 binds to wild type tumour suppressor protein p53 through E6 associated protein, and E7 binds to retinoblastoma (Rb) protein, each leading to their degradation.10 Rb degradation results in aberrant overexpression of the tumour suppressor protein p1611–15 because Rb normally inhibits p16 transcription.5 This high level p16 expression in HPV-related carcinoma is in contrast to conventional head and neck SCC where the gene is frequently inactivated by methylation or deletion.16 As such, p16 is a very good surrogate marker for HPV-related oropharyngeal SCC,5 and it is easily detected by immunohistochemistry.
From a large amount of retrospective,15,17 and now prospective data,11,14,18,19 clinical outcomes have been shown to be markedly better for HPV-related oropharyngeal SCC despite their tendency to present with nodal metastases. The tumours are more treatment responsive but have also been shown to do better regardless of primary treatment modality, whether surgical or non-surgical.15,19,20 Patients also have lower rates of second primary tumours.21
While virtually all major types of head and neck SCC, by subsite or variation, have been defined by histopathologic features, this has not been the case with oropharyngeal SCC. Rather, the tumours have been identified by HPV presence and/or clinical and molecular changes. While it has been consistently noted that the HPV-related oropharyngeal SCC cases usually have characteristic morphologic features,22,23 many pathologists are still unfamiliar with them. The majority of these tumours have a “blue cell” appearance which has variably been described as ‘poorly differentiated’, ‘basal’, or ‘basaloid’.23–25 In fact, terminology amongst pathologists for oropharyngeal SCC has been extremely variable. And the guidance in classification has been variable. For example, the 2005 WHO classification of oral cavity and oropharynx tumours reports that for SCC, ‘findings in the oral cavity and oropharynx do not differ significantly from those of the larynx and hypopharynx.’ It goes on to further recommend that tumours be graded simply into well-, moderately-, and poorly differentiated categories but then state that ‘grading by differentiation is really of limited prognostic value.’26 There is no discussion of the specific morphologic features that we now know are unique in the oropharynx. Also, in our experience, oropharyngeal SCC classification varies widely amongst practicing pathologists, with widely varying terminology and with many commonly using the term basaloid, which is used for a distinct histological variant of SCC, thus generating confusion with the common patterns of oropharyngeal SCC.
HPV-related oropharyngeal SCCs tend to be submucosal and lobulated with large, well-circumscribed and smooth-edged nests of cells with little stromal reaction. The cells have indistinct cell borders, small to modest amounts of eosinophilic cytoplasm, and oval to spindled nuclei which are hyperchromatic and lack (or have inconspicuous) nucleoli. There is brisk mitotic activity and abundant apoptosis. Comedo-type necrosis is also common. Maturing squamous differentiation is typically focal or absent. This morphologic appearance has also been characterized as ‘nonkeratinizing’27 and the distinction clearly made between this morphologic tumour type and that of conventional head and neck SCC which is usually ‘keratinizing’ with irregular, stellate nests of tumour cells with abundant eosinophilic cytoplasm, oval nuclei with frequent nucleoli, and brisk stromal desmoplasia (Figures 1, 2, and 3).28
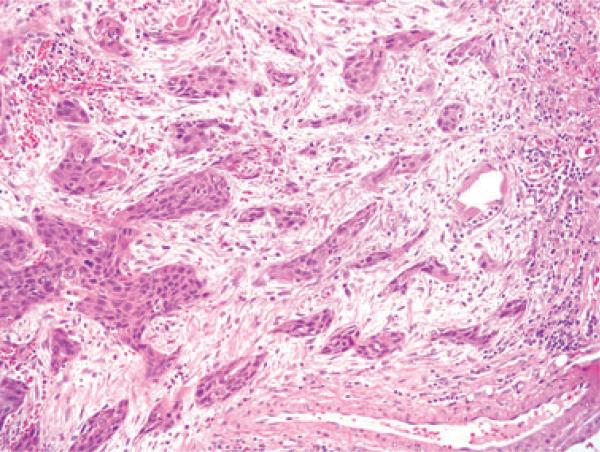
Figure 1.
Typical histological features of a keratinizing-type (Type 1) SCC with angulated nests of tumour cells which have abundant, eosinophilic cytoplasm, well-defined cell borders, and round nuclei with vesicular chromatin and occasional prominent nucleoli. There is also marked stromal desmoplasia (H&E; 100× magnification; SCC, squamous cell carcinoma).
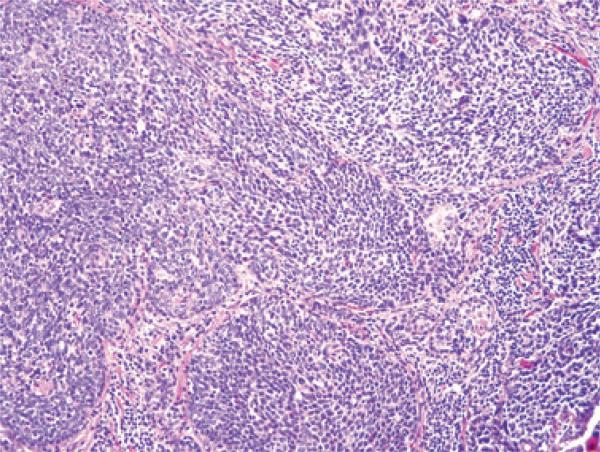
Figure 2.
Typical histological features of a nonkeratinizing (Type 3) SCC with well-circumscribed nests of tumour cells having round to oval, hyperchromatic nuclei, minimal cytoplasm, and abundant apoptosis and mitotic activity (H&E; 200× magnification; SCC, squamous cell carcinoma).
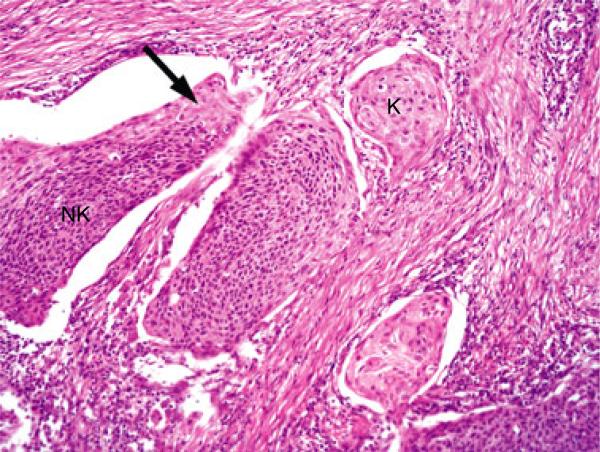
Figure 3.
Typical histological features of a nonkeratinizing SCC with maturation (Type 2) with large, smooth bordered nests of tumour cells, some of which have nonkeratinizing features (NK) with others having keratinizing-type maturation (K) with abundant eosinophilic cytoplasm. There is a focus of reverse maturation (arrow), and the tumour nests show ‘clefting’ artifact with separation from the stroma. Both of these latter features are common in Type 2 tumours (H&E; 200× magnification; SCC, squamous cell carcinoma).
We previously developed a histological typing system for oropharyngeal SCC and have shown the nonkeratinizing histological types to strongly correlate with HPV and p16 positivity and to predict for improved patient outcomes independent of all other clinical, pathologic, and treatment variables.12,28
In this study, we sought to assess the reproducibility of this histological typing system amongst head and neck pathologists naive to the system. We also sought to assess the relationship of the types to tumour p16 status in a prospective manner on routine clinical cases of oropharyngeal SCC.
Materials and methods
HISTOLOGICAL TYPING
A histological typing system for oropharyngeal SCC consisting of three types, as previously published,12,28 was utilized for this study.
KERATINIZING SCC (K SCC OR TYPE 1)
This tumour type consists entirely (or diffusely) of maturing squamous epithelium with no areas with NK SCC or ‘basal’ morphology (Figure 1). The cells have polygonal shapes with abundant, eosinophilic (keratinizing) cytoplasm, distinct cell borders, and intercellular bridges. The nests are usually angulated and irregular, and there is frequently marked stromal desmoplasia. Actual keratin formation is common but is not required as long as the cells have prominent eosinophilic cytoplasm along with the other features. This cytoplasm is filled with keratin intermediate filaments so despite sometimes lacking frank keratin formation, the cells still are ‘keratinizing’. These tumours can range from well to poorly differentiated.
NONKERATINIZING SCC (NK SCC OR TYPE 3)
This tumour type consists of sheets, nests, or trabeculae of oval and frequently spindled, hyperchromatic cells with indistinct cell borders and lacking prominent nucleoli (Figure 2). They have very little or only modest amounts of eosinophilic cytoplasm. Comedo-type necrosis and brisk mitotic activity are usually present. There is typically no (or minimal) stromal reaction to the invading tumour. Portions of the tumour can show squamous maturation, characterized by polygonal cells with mature, eosinophilic cytoplasm, distinct cell borders, intercellular bridges, and keratin pearls, but these mature areas should constitute <10% of the total surface area.
NONKERATINIZING SCC WITH MATURATION (‘HYBRID SCC’ OR TYPE 2)
This tumour type has features seen in both of the other two types, consisting of definitive areas with NK SCC morphology but also having maturing squamous differentiation comprising >10% of tumour surface area (Figure 3). These ‘maturing areas’ have cells with more abundant, eosinophilic cytoplasm, nuclei with open chromatin and/or prominent nucleoli, irregular, angulated nests with stromal desmoplasia, or areas of frank keratinization. They also frequently show ‘reverse maturation’, where the basal appearing cells are central in the nests but the cells at the periphery show squamous maturation. This is the opposite maturation pattern than what is seen in typical keratinizing type head and neck SCC.
Other rare histological types such as basaloid, spindle cell, undifferentiated, and adenosquamous carcinoma were diagnosed based on their published features,29–33 and were excluded. Basaloid SCC is frequently confused with nonkeratinizing SCC although, in our view, it is histologically distinct. It was specifically defined based on Wain's description29 of the histological features and based on the presence of rounded rather than spindled tumours cells, ‘jigsaw puzzle’ pattern nesting with molding of the nests to one another leaving thin lines of intervening stroma, central mucoid material in the nests, and hyalinized, basement membrane-like, pericellular stroma.
STUDY AIM 1
Aim 1 of this study was an evaluation of the histological typing system in prospective, routine, clinical practice. We prospectively captured one consecutive year of data (1/1/2010 to 1/1/2011) on in house oropharyngeal SCC cases at Washington University/Barnes Jewish Hospital. All cases were assigned a histological type utilizing our system by one of the three Washington University head and neck pathologists (JSL, RDC, SKE) simply depending on who was covering the head and neck surgical pathology service during the year and prior to any ancillary testing. During this time, we had also instituted a policy of performing p16 immunohistochemistry (methods below) on all new primary oropharyngeal SCC cases. We captured these immunohistochemical results on all patients and correlated them with the histological types. None of the cases represented recurrent disease, and none had undergone prior therapy.
STUDY AIM 2
Aim 2 of this study was an evaluation of the interobserver variation for the histologic typing in oropharyngeal SCC cases. We utilized a preexisting research database of 270 patients with oropharyngeal SCC which was established from clinical databases from the departments of Radiation Oncology and Otolaryngology Head and Neck Surgery at Washington University. The cases in this database were from 1997 to 2008, and 75% were from primary surgical resections. Corresponding blocks and slides had been retrieved from the files of the Barnes Jewish Hospital. Hematoxylin and eosin (H&E) slides had been previously reviewed by one of the authors (JSL) without knowledge of clinical follow up or outcome, and the tumours categorized using the previously described histological typing system. For the current study, we randomly selected 40 cases from this larger group to generate a study set. To reflect the frequency of each histological type in the larger database (which was 25% each for Types 1 and 2 and 50% for Type 3), we randomly selected 10 cases each of Types 1 and 2 and 20 of Type 3 to arrive at the total of 40. We picked representative blocks, generated 4 μm hematoxylin and eosin sections, and randomly labelled them one through 40. We also selected representative examples of each of the three histological types, generated single hematoxylin and eosin slides from them, and labelled them as typical examples of Types 1, 2, and 3 for the reviewers to view prior to categorizing the unknown cases. A single page description of the three histological types and their typical features was provided to the reviewers (Data S1). The study set was then circulated for review to six head and neck pathologists previously unfamiliar with the specifics of the histological typing system (MP, MBG, BPO, NSA, SM, and JBM), and their answers collected and collated. The handout and example slides were the only exchanged information between the central pathologists and the external study reviewers regarding tumour morphology and classification.
IMMUNOHISTOCHEMISTRY
Immunohistochemistry was performed for p16 on all database cases and all prospective clinical cases. This was performed on representative 4 μm sections cut from formalin-fixed, paraffin-embedded tissue blocks using a monoclonal antibody to p16 (MTM Laboratories; monoclonal; 1:1 dilution) on a Ventana Benchmark LT automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ, USA) according to standard protocols. Detection involved Ventana's ultraView Universal DAB Detection Kit that utilizes a cocktail of enzyme labelled secondary antibodies that locate the bound primary antibody. The complex is then visualized with a hydrogen peroxide substrate and a 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogen. No biotin is involved. Antigen retrieval, standard on the machine, utilized the Ventana CC1, EDTA-Tris, pH 8.0 solution. A known p16 expressing head and neck SCC case was used as the positive control and sections of normal tonsil used for negative controls with each run. Staining was nuclear and cytoplasmic in all cases and was graded in a quartile manner for its extent as follows: 0 = negative; 1 = 1–25% of cells positive; 2+ = 26–50%; 3+ = 51–75%; 4+ = 76–100% (Figure 4). Although partial p16 expression is quite uncommon (approximately 5% of oropharyngeal SCC),12 there is a growing consensus amongst head and neck pathologists that only extensive p16 expression in oropharyngeal SCC is associated with transcriptionally active (and thus biologically and clinically significant) HPV. For example, in the largest retrospective study on oropharyngeal SCC and prognosis, the definition for a positive p16 immunohistochemistry result was set at >70% tumour staining.11 In addition, Schlecht et al., in a recent publication, showed that strong p16 expression is more consistently associated with high risk HPV E6 and E7 mRNA by RT-PCR.34 For this reason, we divided our cases binarily into positive (3+ or 4+ or more simply stated, >50% tumour cell staining) and negative (0, 1+, or 2+ or more simply stated, no tumour cell staining or <50% staining) groups.
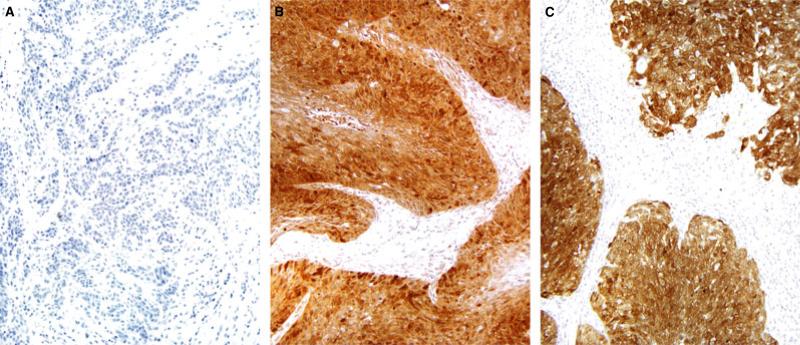
Figure 4.
Representative p16 immunohistochemistry in the three histological tumour types A) No staining for p16 in keratinizing SCC (Type 1) B) Strong and diffuse p16 positivity in nonkeratinizing SCC with maturation (Type 2) C) Strong and diffuse p16 positivity in nonkeratinizing SCC (Type 3) (SCC, squamous cell carcinoma).
STATISTICS
When examining associations between two categorical variables, for instance, p16 status and histological type, we used Pearson's Chi Square Test. In order to assess agreements among multiple reviewers, a macro code called ‘MAGREE’ from SAS Institute Company was executed. The script is created by SAS for computing estimates and tests of agreement among multiple reviewers when responses (classifications or typings) are on a nominal or ordinal scale. We reported kappa and P values for agreement evaluations. All tests were two sided, and the level for statistical significance was set at <0.05. SAS 9.1 was used for all major statistical calculations (SAS Institute Inc, Cary, NC, USA).
Results
STUDY AIM 1
Aim 1 consisted of 1 year of prospective oropharyngeal SCC cases in routine clinical practice. There were a total of 85 new cases of oropharyngeal SCC over this period, and data are presented in Table 1. Strong p16 expression was present in 70 of the 85 (82.4%) cases (Figure 4). Interestingly, nonkeratinizing SCC strongly predicted p16 status with 47 of 48 (97.9%) being strongly p16 positive. The vast majority of nonkeratinizing SCC with maturation were also p16 positive (18/19 or 94.7%) as were a significant minority of the keratinizing SCC (5/18 or 27.8%). The differences in p16 positivity rates between the three histological types and also for type 1 versus types 2 and 3 (combined) were statistically significant (P = 0.05 and 0.01, respectively.
Table 1.
Aim 1 results showing p16 expression by tumour histological type
| Type | Total cases | p16 positivity* |
|---|---|---|
| Keratinizing (1) | 18 | 5 (2.78%) |
| Nonkeratinizing with maturation (2) | 19 | 18 (94.7%) |
| Nonkeratinizing (3) | 48 | 47 (97.9%) |
| 85 | 70 (82.4%) |
The difference in p16 positivity rates amongst the three histological types was statistically significant (P = 0.005). The difference in p16 positivity rates for type 1 versus Types 2 and 3 combined was also statistically significant (P = 0.001).
As previously mentioned, partial p16 expression is uncommon in oropharyngeal SCC.12 For this Aim 1 cohort of 85 patients, 68 of the 70 (97.1%) p16 positive cases were 4+ and two of the 70 (2.9%) were 3+. Of the 15 p16 negative cases, 13 (86.7%) were completely negative, and 2 (13.3%) were focally positive, each showing <25% of the tumour staining. These latter two cases were both keratinizing SCC (Type 1).
STUDY AIM 2
Aim 2 consisted of a 40 case test set of oropharyngeal SCC circulated to six external head and neck pathologists to evaluate agreement in histological typing. The results for each case by reviewer are presented in Table 2. All six reviewers agreed on 14 cases (35.0%), at least five of six reviewers agreed on 25 cases (62.5%), and at least four of six agreed on 30 cases (75.0%). Kappa values for each histological type were as follows: Type 1, 0.62 (P < 0.0001), Type 2, 0.35 (P < 0.0001), and Type 3, 0.56 (P < 0.0001). For Types 1 and 3, these kappa values are generally considered to be ‘moderate’ or ‘good’ to ‘substantial’ agreement, and for Type 2, the kappa value is generally considered ‘fair’ or ‘poor’ agreement.35,36 Thirty three of the 40 study set cases (82.5%) were p16 positive. Correlation with p16 immunohistochemistry showed that all 21 cases that were classified as Type 3 (nonkeratinizing SCC) by any reviewer were p16 positive.
Table 2.
Aim 2 results showing reviewer typing results for each case
| Reviewer | |||||||
|---|---|---|---|---|---|---|---|
| Case | #1 | #2 | #3 | #4 | #5 | #6 | p16 |
| 1 | 3 | 3 | 3 | 3 | 3 | 3 | + |
| 2 | 3 | 2 | 2 | 2 | 2 | 2 | + |
| 3 | 3 | 3 | 2 | 3 | 3 | 3 | + |
| 4 | 2 | 2 | 2 | 2 | 3 | 2 | + |
| 5 | 3 | 3 | 2 | 3 | 3 | 3 | + |
| 6 | 2 | 2 | 2 | 2 | 2 | 2 | + |
| 7 | 2 | 3 | 2 | 2 | 2 | 2 | + |
| 8 | 2 | 2 | 1 | 1 | 2 | 1 | + |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| 10 | 3 | 3 | 3 | 3 | 3 | 3 | + |
| 11 | 2 | 2 | 1 | 1 | 2 | 2 | + |
| 12 | 2 | 2 | 1 | 1 | 2 | 1 | + |
| 13 | 1 | 2 | 1 | 2 | 3 | 2 | + |
| 14 | 3 | 3 | 2 | 3 | 2 | 3 | + |
| 15 | 2 | 2 | 1 | 1 | 1 | 1 | + |
| 16 | 1 | 2 | 1 | 1 | 1 | 1 | - |
| 17 | 1 | 1 | 1 | 1 | 1 | 1 | + |
| 18 | 2 | 2 | 2 | 2 | 2 | 2 | + |
| 19 | 3 | 3 | 2 | 3 | 2 | 2 | + |
| 20 | 3 | 3 | 2 | 2 | 2 | 3 | + |
| 21 | 3 | 3 | 3 | 3 | 3 | 3 | + |
| 22 | 2 | 2 | 2 | 2 | 2 | 2 | - |
| 23 | 3 | 3 | 3 | 3 | 2 | 2 | + |
| 24 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| 25 | 2 | 2 | 1 | 2 | 1 | 1 | + |
| 26 | 1 | 2 | 3 | 3 | 2 | 2 | + |
| 27 | 1 | 2 | 2 | 2 | 2 | 2 | + |
| 28 | 2 | 3 | 3 | 3 | 3 | 3 | + |
| 29 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| 30 | 3 | 2 | 2 | 3 | 1 | 2 | + |
| 31 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| 32 | 3 | 3 | 3 | 3 | 3 | 3 | + |
| 33 | 1 | 1 | 1 | 1 | 1 | 1 | + |
| 34 | 3 | 2 | 1 | 2 | 3 | 2 | + |
| 35 | 3 | 3 | 3 | 3 | 2 | 3 | + |
| 36 | 1 | 1 | 1 | 2 | 1 | 1 | + |
| 37 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| 38 | 2 | 2 | 2 | 2 | 1 | 2 | + |
| 39 | 3 | 2 | 2 | 2 | 1 | 2 | + |
| 40 | 3 | 2 | 2 | 2 | 1 | 1 | + |
Green highlighting indicates 100% agreement, yellow highlighting indicates agreement by five of six (83.3%), and orange highlighting indicates agreement by four of six (66.6%). Rows with no highlighting indicate <66.6% agreement. For p16 immunohistochemistry, all positive cases showed expression in >75% of the cells and all negative cases showed no expression in the tumour cells.
Discussion
The importance of HPV as a causative agent in oropharyngeal SCC has been emerging over the past several decades of investigation.37 Transcriptionally active HPV-related oropharyngeal SCCs present in clinically distinct ways, are genetically less complex, respond very well to treatment regardless of modality used, and have a favourable prognosis.19 Testing for HPV in such tumours is complicated, with myriad different testing types such as DNA-based PCR, RNA-based RT-PCR, DNA-based in situ hybridization, and more recently, slide-based RNA in situ hybridization.30 The sensitivities of these assays for transcriptionally active HPV vary considerably. p16 has been extensively documented as a surrogate marker of transcriptionally active HPV in oropharyngeal SCC. Overexpression of p16 has been consistently and repeatedly shown to be associated with better response to therapy and favourable clinical outcome in oropharyngeal SCC.11–14,38,39 It is widely available, easy to perform, and easy to interpret with staining either being strongly and diffusely positive (with cytoplasmic and nuclear staining) or completely absent. Traditionally in pathology and medicine, the microscopic features of tumours have been used as the basis for their classification. Nasopharyngeal carcinoma is a perfect example, where the morphology is utilized quite well to define subsets for association with Epstein-Barr virus, clinical behaviour, treatment, and prognostication. However, morphology not been widely used for HPV-related SCC of the oropharynx. Investigators have variably observed and reported that HPV-related tumours are ‘poorly differentiated’ or have ‘basal’ or ‘basaloid’ features, frequently lacking keratinization and having cells with only small amounts of cytoplasm giving the tumours a ‘blue-cell’ appearance.18,23–25 Terminology and descriptions have varied quite a bit, but more recently the term ‘nonkeratinizing’12,22,28 has been utilized in describing their appearance, and this term appears to be gaining more widespread acceptance.
We previously developed a histological typing system for routine oropharyngeal SCC cases consisting of three types: Type 1 (or keratinizing-type SCC), Type 2 (or nonkeratinizing SCC with maturation – representing an intermediate or ‘mixed’ category), and Type 3 (or nonkeratinizing SCC). We showed that the types strongly correlate with tumour HPV and p16 status and strongly predict patient outcomes.12,28 At Washington University in St Louis, we have been applying this typing system for the reporting of active clinical cases for several years. What had not been studied was the performance of this typing system for routine patient care nor the reproducibility of the system amongst other head and neck pathologists.
In Aim 1 (routine surgical pathology practice evaluation) of this study, we have validated the use of our histological typing system in routine practice. Virtually all Type 2 (94.7%) and Type 3 (97.9%) cases were strongly p16 positive by immunohistochemistry compared to 27.8% for Type 1, showing that nonkeratinizing morphology is an excellent surrogate marker for strong p16 expression and, by extension, biologically and clinically relevant HPV in the tumours.
In Aim 2 (interobserver variability evaluation) of this study, we have shown that external pathologists, provided only with simple descriptions of the morphologic types, can identify nonkeratinizing and keratinizing-type squamous cell carcinomas. However, the agreement was lower for nonkeratinizing SCC with maturation (Type 2). This latter histological type is one which has definitive nonkeratinizing histological features, but which has more than 10% of the tumour surface area showing squamous maturation, with cells with abundant, eosinophilic cytoplasm, angulated nest borders with stromal reaction, or keratinization. As an intermediate group, it is perhaps not entirely surprising that it had less agreement.
The significance and meaning of kappa values for data is difficult to classify and is dependent on many factors.34,35 Statistical significance (P values < 0.05), as was reached for the interobserver agreement for all three histological types in our study, is not a particularly good guideline for considering the practical significance of the agreement rates. Landis and Koch proposed descriptive terms for ranges of kappa values (based on personal opinion) such that <0 indicates no agreement and 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement.34 However, this is dependent on so many factors that a ‘good’ kappa value is very hard to assign. The higher the number of variables tested, given the same basic agreement levels, will give higher kappa values. In our case, having only three variables/types, and having kappas of 0.62 and 0.56 for keratinizing-type and nonkeratinizing SCC, respectively, would probably be considered relatively good agreement.
Why there is not higher agreement among the reviewers in histological typing of such tumours is not clear. The histological features of carcinomas, although they can be described generally, are not always consistent even within specific diagnostic types/categories. Variation amongst tumours within the three types certainly may explain the significant number of discrepant cases. Also, there is probably a tendency to ‘err’ on the side of putting tumours in the Type 2 category because it is intermediate between the two. Indeed, of the 26 study set cases that had discrepant typing by the reviewers, there were a total of 156 ‘typings’, of which 35 (or 23%) were called Type 1, 46 (or 30%) were called Type 3, and 74 (or 47%) were called Type 2. This overrepresentation of Type 2 amongst the discrepant cases argues that reviewers ‘fell back’ on Type 2 frequently in their typing. Our personal experience with utilizing this typing system in routine practice is that we become more confident with calling Types 1 and 3 with more experience.
The larger question in the current management of patients with oropharyngeal SCC is ‘where does histological typing potentially factor in?’ As risk stratification factors for patients with oropharyngeal SCC have become more clearly defined, routine HPV-specific testing has been recommended and instituted by many. However, p16 is a very good surrogate marker for HPV and is arguably a better single predictor of good outcome than any HPV-specific test. It strongly correlates with the presence of transcriptionally active HPV40 and consistently demonstrates equal or higher risk stratification between positive and negative cases.11,12,38,40 p16 immunohistochemistry is now utilized as the single risk stratification test in oropharyngeal SCC at many institutions. Nonkeratinizing morphology, with its very strong association with the presence of transcriptionally-active HPV, with p16 overexpression, and with improved patient outcomes, is also helpful in identifying HPV-related tumours. In our previous large oropharyngeal SCC study cohort,12 124 of 126 (98.4%) nonkeratinizing SCC (Type 3) cases were strongly p16 positive. In Aim 1 of the current study, 47 of 48 (97.9%) SCC cases that were classified as nonkeratinizing were p16 positive, and in Aim 2, all 21 of the cases from the study set that any of the reviewers classified as nonkeratinizing SCC were p16 positive. Based on this data, nonkeratinizing histology essentially implies p16 positivity. One could then reasonably propose that oropharyngeal SCC can be typed by the pathologist at the time of diagnosis, and if the tumour is Type 3, p16 testing could be omitted because positive staining is essentially assured. If the tumour is Type 1 or 2, p16 immunohistochemistry should be performed and reported. Although Type 2 tumours in our prospective (Aim 1) cohort were almost all p16 positive (95%), this is an intermediate histological category that overlaps with keratinizing-type (Type 1) tumours. We saw in the interobserver cohort (Aim 2) that many cases were variably classified as Type 1 and 2 by the various observers so p16 immunohistochemistry would still be indicated.
In smaller medical facilities or poor countries, where ancillary studies like p16 immunohistochemistry or HPV-specific testing might be difficult or impossible to obtain, pathologists can use nonkeratinizing histological features to still help predict which patients have better prognosis tumours. Nonkeratinizing histological features can also be very helpful in practice, otherwise. For example, in neck metastases (which are a common presentation specimen for oropharyngeal SCC because the primary tumours are frequently clinically occult), recognizing the nonkeratinizing (Type 3) or even nonkeratinizing with maturation (Type 2) morphology at frozen section can help one guide the surgeons to the oropharynx (or less commonly the nasopharynx) as the likely primary site. This morphology should prompt one to consider HPV as causative in such tumours and, if indicated, to do p16 immunohistochemistry or HPV-specific testing. And since WHO Type 2a (or differentiated nonkeratinizing) nasopharyngeal carcinoma can be histologically somewhat similar to oropharyngeal nonkeratinizing SCC, Epstein-Barr virus staining should also be considered, particularly if p16 immunohistochemistry or HPV-specific testing is negative.
Finally, our results add further credence to the idea that, as currently defined and utilized, grading and differentiation are not clinically useful in oropharyngeal SCC.41 The terms ‘poorly differentiated’,13,23 ‘high grade’, and ‘basaloid’,18,24 which are frequently applied to nonkeratinizing SCC in the literature and in clinical practice, imply (just as in almost every other organ system and tumour type) a more aggressive tumour with a poorer prognosis for the patient. However, this is the exact opposite of the case for oropharyngeal SCC, where the most ‘poorly differentiated’ looking tumours do exceptionally well clinically with the appropriate treatment.18 The most well-differentiated, mature keratinizing type SCCs are usually unrelated to HPV, present with larger primary tumours, and have worse prognosis.12,25,28 We have carefully avoided the term ‘grade’ in our study and in our proposed terminology for this very reason.
Pure basaloid SCC of the head and neck, a tumour type which occurs in the larynx, hypopharynx, and oropharynx and is frequently clinically aggressive, has some morphologic features which overlap nonkeratinizing SCC. Basaloid and nonkeratinizing SCC are frequently confused with each other. In the practice of seeing many ‘blue’ looking oropharyngeal SCC cases over the years, however, we have found that basaloid SCC and nonkeratinizing SCC, when clearly defined, are distinct. And, importantly, oropharyngeal basaloid SCC is a mixed variant with the HPV-related tumours having more favourable outcomes. We have been careful to exclude cases of true basaloid SCC from our study by strictly defining them with the histological features that are well-described in the literature.29,42,43
In summary, we have further validated a three-tiered histological typing system in oropharyngeal SCC. Nonkeratinizing histological morphology can be recognized by pathologists, can be utilized for diagnosis in routine practice, and correlates almost perfectly with strong p16 expression by immunohistochemistry. Our hope is that this well-characterized typing system for oropharyngeal SCC will help to eliminate some of the confusion about these tumours.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842. None of the authors has any financial disclosures to report.
Footnotes
This work was presented at the United States and Canadian Academy of Pathology Annual Meeting in San Antonio, TX on March 2, 2011.
Supporting Information
Additional Supporting information may be found in the online version of this article:
Data S1. Detailed instruction sheet which was provided to the six independent head and neck pathologists reviewing and typing cases for Aim 2.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 2.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int. J. Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Ridge JA, Gillison ML, et al. Head Neck; Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting; Washington, D.C.. November 9–10, 2008; 2009. pp. 1393–1422. [DOI] [PubMed] [Google Scholar]
- 6.Ernster JA, Sciotto CG, O'Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papillomavirus. Laryngoscope. 2007;117:2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 7.Jung AC, Briolat J, Millon R, et al. Biological and clinical relevance of transcriptionnally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int. J. Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 8.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2008;14:366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 10.Hafkamp HC, Manni JJ, Speel EJ. Role of human papillomavirus in the development of head and neck squamous cell carcinomas. Acta Otolaryngol. 2004;124:520–526. doi: 10.1080/00016480310016893. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am. J. Surg. Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger PM, Yu Z, Haffty BG, et al. Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin. Cancer Res. 2004;10:5684–5691. doi: 10.1158/1078-0432.CCR-04-0448. [DOI] [PubMed] [Google Scholar]
- 14.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J. Clin. Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int. J. Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 16.Nakahara Y, Shintani S, Mihara M, Ueyama Y, Matsumura T. High frequency of homozygous deletion and methylation of p16(INK4A) gene in oral squamous cell carcinomas. Cancer Lett. 2001;163:221–228. doi: 10.1016/s0304-3835(00)00699-6. [DOI] [PubMed] [Google Scholar]
- 17.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int. J. Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 18.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 19.Rich JT, Milov S, Lewis JS, Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong AM, Dobbins TA, Lee CS, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br. J. Cancer. 2010;103:1510–1517. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of Human papillomavirus-associated oropharyngeal cancer. J. Clin. Oncol. 2011;29:739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Ukpo OC, Pritchett CV, Lewis JE, Weaver AL, Smith DI, Moore EJ. Human papillomavirus-associated oropharyngeal squamous cell carcinomas: primary tumor burden and survival in surgical patients. Ann. Otol. Rhinol. Laryngol. 2009;118:368–373. doi: 10.1177/000348940911800509. [DOI] [PubMed] [Google Scholar]
- 24.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 25.Wilczynski SP, Lin BT, Xie Y, Paz IB. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am. J. Pathol. 1998;152:145–156. [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson N, Francheschi S, Ferlay J, et al. Squamous cell carcinoma of the oral cavity and oropharynx. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours – Pathology and Genetics Head and Neck Tumours. IARC Press; Lyon, France: 2005. pp. 168–175. [Google Scholar]
- 27.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2:163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum. Pathol. 1986;17:1158–1166. doi: 10.1016/s0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 30.Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ, Lewis JS., Jr Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5:108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am. J. Surg. Pathol. 2010;34:800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 32.Cardesa A. Spindle cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours - Pathology and Genetics Head and Neck Tumours. IARC Press; Lyon, France: 2005. pp. 127–128. [Google Scholar]
- 33.Cardesa A, Zidar N, Alos L. Adenosquamous carcinoma. In: Barnes EL, Eveson J, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours - Pathology and Genetics Head and Neck Tumours. IARC Press; Lyon, France: 2005. pp. 130–131. [Google Scholar]
- 34.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod. Pathol. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 36.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam. Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 37.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 2005;32(Suppl. 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 39.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High risk human papillomaviru E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am. J. Surg. Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 40.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J. Clin. Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 41.Westra W. The changing face of head and neck cancer in the 21st century: the impact of hpv on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am. J. Surg. Pathol. 2008;32:1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 43.Chernock RD, Lewis JS, Jr, Zhang Q, El-Mofty SK. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum. Pathol. 2010;41:1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.