Abstract
Rationale
Molecular imaging is useful for longitudinal assessment of engraftment. However, it is not known which factors, other than cell number can influence the molecular imaging signal obtained from reporter genes.
Objective
The effects of cell dissociation/suspension on cellular bioenergetics and the signal obtained by firefly luciferase(fluc) and human Na-I symporter(hNIS) labeling of cardiosphere-derived cells (CDCs) was investigated.
Methods and Results
18FDG uptake, ATP levels, 99mTc-pertechnetate uptake and bioluminescence were measured in vitro, in adherent and suspended CDCs. In vivo dual isotope SPECT-CT imaging or bioluminescence imaging (BLI) were performed 1hr and 24hrs following CDC transplantation. SPECT quantification was performed using a phantom for signal calibration. Cell loss between 1hr & 24hrs post-transplantation was quantified by qPCR and ex vivo luciferase assay.
Cell dissociation followed by suspension for 1hr resulted in decreased glucose uptake, cellular ATP, 99mTc uptake and BLI signal by 82%, 43%, 42%, and 44% respectively, when compared to adherent cells, in vitro. In vivo 99mTc uptake was significantly lower at 1hr, when compared to 24hrs following cell transplantation in the non-infarct (p<0.001, n=3) and infarct (p<0.001, n =4) model, despite significant cell loss during this period. The in vivo BLI signal was significantly higher at 1hr than at 24hrs (p<0.01), with the BLI signal being higher when CDCs were suspended in glucose-containing medium compared to saline(PBS).
Conclusion
Adhesion is an important determinant of cellular bioenergetics, 99mTc-pertechnetate uptake and BLI signal. BLI and NIS imaging may be useful for in vivo optimization of bioenergetics in transplanted cells.
Keywords: Molecular imaging, metabolism, stem cell
INTRODUCTION
Cellular cardiomyoplasty is plagued by low engraftment and small functional benefit. Molecular imaging facilitates the study of in vivo stem cell biology and optimization of cell therapies by permitting in vivo quantification of stem cell engraftment. For in vivo visualization, transplanted cells need to express a reporter gene or they must be labeled ex vivo.1 Reporter gene strategies (e.g. bioluminescence imaging of firefly luciferase gene expression2 and PET/SPECT imaging of herpes simplex virus thymidine kinase (HSV-tk)3 or human sodium-iodide symporter (hNIS)4 gene expression) are superior to ex vivo labeling of cells with tracers (e.g. 18FDG5, 6 or Indium7) or nano-particles for longitudinal assessment of engraftment,8 because transplanted cell viability is a prerequisite for reporter gene expression. However, it is not known which factors, other than cell number can influence the molecular imaging signal obtained from reporter genes.
Cell metabolism is an important determinant of cell survival, proliferation and function.9–11 Studies in cancer cells reveal that they primarily utilize glucose via glycolysis, rather than oxidative phosphorylation for ATP generation, despite the availability of oxygen – this phenomenon is referred to as the Warburg effect.12 However, very little is known about the determinants of metabolism and its relationship to function in stem cells.
In this study, we examined the effect of cell dissociation/suspension on cellular bioenergetics and the molecular imaging signal obtained by hNIS (SPECT) and firefly luciferase (fluc) labeling (bioluminescence imaging or BLI) of cardiosphere-derived cells (CDCs). CDCs are adherent cells composed of a mixture of cardiac-derived progenitor and supporting cells, and have recently completed a phase1 clinical trial.13 NIS promotes cellular uptake of iodide or 99mTc-pertechnetate, driven by the trans-membrane sodium gradient,14 which is maintained by Na+-K+-ATPase, whereas BLI is based on oxidation of the injected substrate D-luciferin by luciferase, a reaction that requires oxygen, Mg2+, and ATP, and results in emission of photons. Since signal generation by luciferase and NIS is linked to cellular ATP, we hypothesized that cellular bioenergetics would influence the molecular imaging signal obtained from hNIS and fluc-labeled cells. We studied hNIS labeling, because it is a human gene, that could be useful for longitudinal follow up of cell engraftment in small4, 15 and large animal models and patients; and luciferase labeling because it is widely used for quantification of engraftment in rodent models of cell transplantation.2 Our results illustrate that cell dissociation which is an essential first step in most clinical and experimental studies of cell transplantation impairs cellular bioenergetics and the molecular imaging signal derived from NIS and luciferase.
METHODS
Cell isolation and culture
Cardiosphere-derived cells (rCDCs) were isolated from syngeneic, male Wistar Kyoto rat hearts as previously described.16, 17 rCDCs were cultured in IMDM medium (Invitrogen) containing 10% FBS, 10% glutamine and 0.1mM mercaptoethanol, and expanded to 3–5 passages prior to experiments. Please see supplemental data section for details.
Lentivirus synthesis
A third-generation lentiviral vector system (kindly supplied by Professor Inder Verma, Salk Institute, USA) was used to label the rCDCs. Please see supplemental data section for details.
In vitro glucose uptake (18FDG) uptake
One day before the experiment, rCDCs were plated at a density of 1×105 cells/well. Prior to labeling, cells were washed twice with PBS and the medium was changed to glucose free-DMEM for 1hr. 18FDG (74 kBq/ml) was added to half the plated cells for 30min to measure glucose uptake in adherent cells; the remainder of the cells were trypsinized and suspended in medium containing 18FDG (74 kBq/ml) for 30min in order to measure glucose uptake in cell suspension. Subsequently, cells were washed twice with cold PBS to remove any remaining free 18FDG, suspended in 1ml 20% sodium dodecylsulfate to lyse the cells, and transferred to 5ml vials. Counts were recorded in a gamma-counter (LKB Wallac, Turku, Finland). After the gamma counting, 4ml of ice cold acetone was added to each sample, and samples were kept at 4°C to allow for radiotracer decay, prior to performing the Bradford protein assay.
ATP measurements
ATP estimation was performed using the ATP Determination Kit (A22066, Molecular Probes) using a luminometer (Turner BioSystem Veritas, Madison, WI, USA). All experiments were performed using 1 × 104 rCDCs (non-transduced) per well in a 96 well plate following 18–24 hrs of culture for the adherent condition, and following trypsinization and 1hr of suspension for the suspension condition. For suspension conditions, the wells were coated with poly-2hydroxyethyl methacrylate (polyHEMA), overnight, prior to cell plating. PolyHEMA was chosen because it is known to prevent cell attachment and spreading18, 19. Cell lysis Buffer (Cell Signaling Technology) was used to lyse the cells in each well for 20min; standard reaction solution was injected through the automated injector. The signal was normalized to protein content using the Bradford Assay. ADP/ATP ratio was assessed using ApoSENSOR ADP/ATP Ratio Assay Kit.
Flow cytometry
Annexin V and Propidium iodide (Invitrogen) were used to quantify apoptotic and dead cells, respectively immediately after cell dissociation and after suspension in cell culture medium for 1hr and 6hrs. Please see supplemental data section for detailed methods.
Measurements of cellular metabolism
Seahorse Bioscience XF96 instrument20 was used to measure the rate of change of dissolved O2 in each well (termed OCR or oxygen consumption rate) which reflects oxidative phosphorylation, and change in pH in the media which reflects glycolysis, the 2 main sources of ATP in cells. Oxygen concentration and pH were measured over 5 min periods with a mixing time of 2min in each cycle with three cycles in total for adherent CDCs (plated for 18–24hrs) and rCDCs suspended for 1hr in Seahorse medium containing glucose (25mM) in a specialized 96 well plate (n=3). Restoration of metabolism following cell suspension was assessed by re-plating viable rCDCs in a specialized 96 well plate (Seahorse biosciences) following suspension for 0, 3hrs and 6hrs in cell culture medium; metabolic measurements (OCR and ECAR) were performed at 24hrs following cell dissociation.
The effect of Oligomycin (2µM), an inhibitor of the mitochondrial ATP synthase was used to assess dependence of CDCs on oxidative phosphorylation for ATP synthesis. Iodoacetate (600µM), an inhibitor of the glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to assess the contribution of glycolysis to ATP synthesis. Cells were treated with these compounds for 30min prior to measurements.
The respiratory rates in each well were normalized to protein content using Bradford Assay. These experiments were performed at least in triplicate and repeated 2–3 times.
In vitro 99mTc-Pertechnetate uptake
rCDCs were transduced with a 3rd generation lentivirus expressing the human sodium-iodide symporter (Lv-CMV-hNIS) at an MOI of 20. In vitro 99mTc-pertechnetate uptake was measured by incubating hNIS+ rCDCs cultured for 18–24 hrs in a 6 well plate at a density of 105 cells/well for the adherent condition, or trypsinized and suspended in PBS (phosphate buffered saline) or IMDM medium (Invitrogen); 99mTc-pertechnetate (11.1 kBq/mL) was added for 1hr, immediately after generation of cell suspensions. The effect of perchlorate (100µM), a specific NIS blocker on 99mTc-pertechnetate uptake was measured by adding perchlorate to some wells prior to the addition of 99mTc-pertechnetate. At the end of 1hr, cells were rinsed twice with ice cold PBS and lysed with 20% sodium dodecylsulfate. Counts were recorded in a gamma-counter (LKB Wallac, Turku, Finland) and the Bradford protein assay was performed to normalize 99mTc uptake by protein content. We investigated cell suspension in PBS (phosphate buffered saline) and IMDM medium because PBS/saline, which lacks substrates, has been extensively used in experimental16, 21 and clinical studies13 of CDC transplantation; IMDM medium (Invitrogen) contains metabolic substrates like D-glucose, Ca2+, Mg2+ and is used for CDC culture.
In order to investigate the influence of trypsin on 99mTc-pertechnectate uptake, hNIS+ rCDCs were dissociated either using 0.05% trypsin (Invitrogen), which usually takes ~2min, or non-enzymatic cell dissociation solution (Sigma-Aldrich; contains EDTA and other proprietary reagents) which takes ~ 20–30min (for complete dissociation into single cells), following which they were suspended in IMDM medium containing 99mTc-pertechnetate (11.1 kBq/ml) for 1hr. Subsequently, cells were rinsed twice using ice cold PBS, lysed, and analyzed using the gamma counter; Bradford assay was performed to normalize uptake results to protein content. These experiments were performed in triplicate and repeated twice.
All in vitro studies were performed at 37°C.
In vivo 99mTc uptake (SPECT/CT Imaging)
Dual isotope imaging was performed. Data was acquired in list mode and post-processed by applying two energy windows (“75 keV +10%/−10%” and “140 keV +10%/−10%”) to obtain 201Tl and 99mTc projections separately. Please see supplemental methods section for details of animal surgery and imaging. Three million NIS+ rCDCs suspended in 100µl of IMDM (Invitrogen) were injected directly into the myocardium at three sites in the anterior wall of the left ventricle using a 30G needle. In vivo dual isotope SPECT imaging was performed 1hr after injection of 99mTc-pertechnetate (555–740 MBq) and 201Tl (37–74 MBq);4 CT imaging was performed prior to SPECT imaging. Animals were allowed to recover in their cages after completion of imaging on day 0. After 24hrs, the same rats were re-injected with 99mTc-pertechnetate (555–740 MBq) and 201Tl (37–74 MBq) via the tail vein and in vivo dual isotope SPECT-CT imaging was performed. The rats were euthanized after completing the 24hr imaging protocol.
Ex-vivo SPECT imaging
In order to validate the results obtained by in vivo imaging and to confirm the origin of the in vivo signal, a high resolution ex vivo SPECT scan was performed in hearts excised from a separate set of animals at 1hr (n=2) and 24hrs (n=2) following transplantation with NIS+ rCDCs. Please see supplemental data section for details.
Image quantification
For absolute quantification of in vivo 99mTc-pertechnetate uptake, we generated a dose response plot by dual isotope SPECT imaging of a rat size phantom containing several concentrations of 99mTc-pertechnetate and 201Tl. Calibration factor (MBq/i.i.) was defined as the quotient of the known activity concentration (MBq/ml) within the radioactive sphere in the phantom divided by the measured mean image intensity (i.i/cm3) within a ROI drawn over the small sphere in the SPECT image of the phantom. For absolute quantification, regions of interest (ROI) were manually defined, image intensity within the ROI was multiplied by the calibration factor (CF) to give the radioactivity (MBq), and further divided by the ROI volume to get the uptake concentration (MBq/ml). Please see supplemental data section for details.
In vivo bioluminescence imaging
For in vivo BLI, rCDCs were labeled with a 3rd generation lentivirus expressing firefly luciferase (Lv-CMV-fLuc) at an MOI of 20, which did not affect CDC survival, proliferation or differentiation.22 One million rCDCs transduced with Lv-CMV-fLuc were trypsinized, suspended in 100µl of PBS/saline (n=4) or IMDM (n=4) and then injected intra-myocardially into 3 sites in the anterior wall of the left ventricle of non-infarcted male WK rats using a 30G needle, following a left sided thoracotomy. In vivo BLI was performed using the Xenogen IVIS 200 system 1hr and 24hrs after cell transplantation. We studied non-infarcted rats in order to reduce signal variability resulting from the location of the transplanted cells (infarct versus border-zone) given the small size of the left ventricle and known dependence of the BLI signal upon the availability of oxygen.
In-vitro BLI
In vitro BLI was performed in a 96 well plate using a luminometer (Turner VERITAS Microplate, Promega, Madison, WI, USA) using fluc-transduced rCDCs. Ten thousand cells (104cells/well) were plated for 18–24 hrs for the adherent cell condition and for 1hr on polyHema coated wells (suspension condition) in PBS/saline or PBS/saline containing Ca2+/Mg2+ (1mM) and D-glucose (5.6mM). We used Ca2+/Mg2/glucose-containing PBS rather than IMDM in order to avoid the possible effects of phenol red, a component of IMDM medium, on the bioluminescence signal. The signal (RLU) was measured in suspended and adherent cells after injection of 30µg/ml of D-Luciferin (sodium salt, Gold Biotechnology, Saint Lois, MO, USA) into each well using the automated injector. Cells were lysed using the lysis buffer contained in the Dual-Luciferase® Reporter Assay System (E1910). The signal was normalized by protein content using the Bradford assay. All experiments were performed at least in triplicate and repeated twice.
Quantification of engraftment
We used the ex vivo luciferase assay to quantify in vivo engraftment of fluc+ rCDCs, and quantitative PCR for the male rat-specific SRY gene to quantify engraftment of NIS+ rCDCs in the first 24hrs following transplantation. Please see supplemental data section for detailed methods and rationale for the 2 methods.
Statistical analysis
For matched comparisons of continuous variables at different time points, the paired t-test was used (or repeated measures ANOVA, in case of more than 2 groups). For comparisons of continuous variables between two independent groups the Student’s t-test was used. A p<0.05 was chosen for statistical significance. Values are reported as mean ± SD.
RESULTS
Bioenergetics in CDCs
Since cell trypsinization/dissociation is an important step in most experimental and clinical studies of cell transplantation in the heart, we initially compared glucose uptake, ATP levels and cellular metabolism in non-transduced rCDCs that were trypsinized and suspended in culture media for 1hr, and CDCs that were plated on an adherent surface (tissue culture treated wells) for 18–24 hrs.
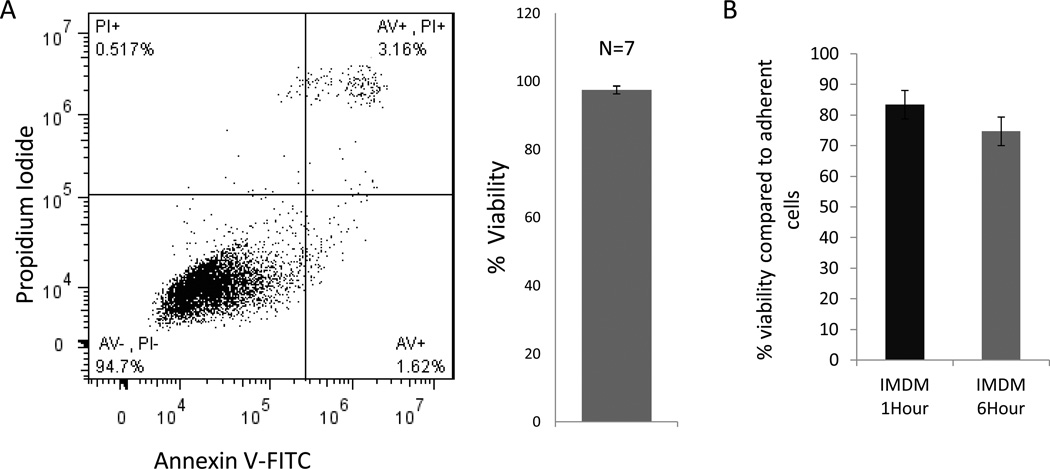
Cell viability was consistently >96% immediately post-dissociation, when assessed by trypan blue staining and flow cytometry (Fig 1A, B). However, CDC viability was reduced to 83±4% and 74±3% (n=3), respectively, following 1hr and 6hrs of suspension (Fig 1C).
Fig 1. Cell viability following suspension.
(A) CDC viability: representative flow plot and bargraph (B) immediately following cell dissociation reveals high CDC viability (97.5 ± 1.1; n=7). (C) Cell viability is reduced following suspension for 1hr and 6hrs (n=3)
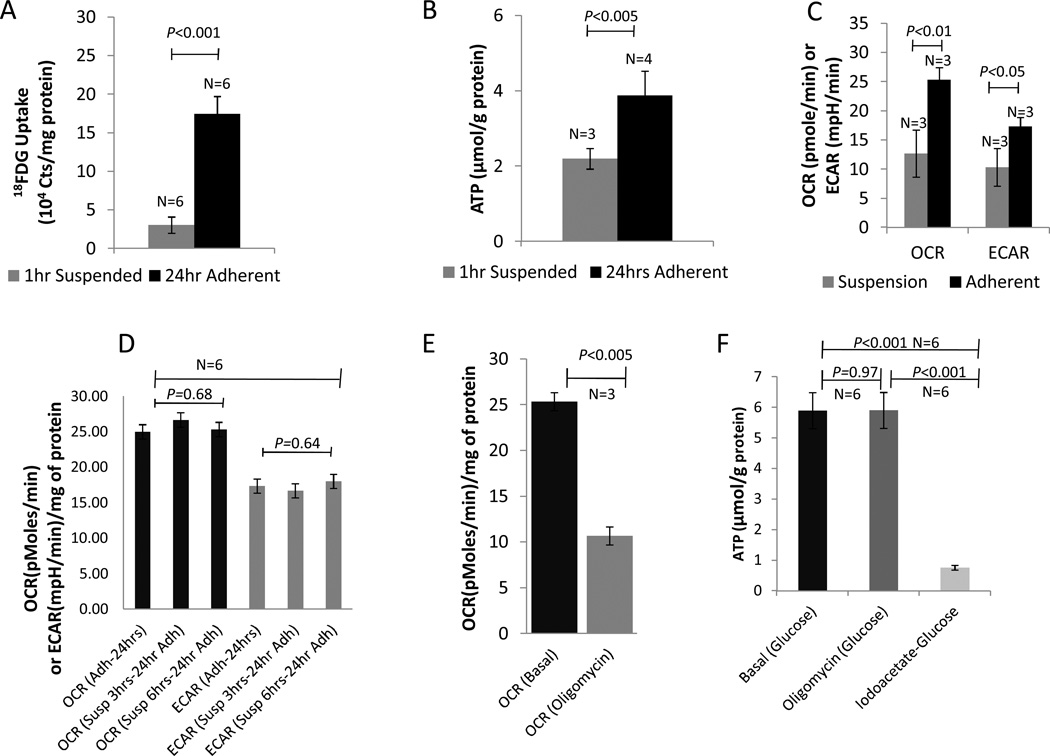
Cellular glucose (18FDG) uptake and ATP levels were decreased by 82% and 43% respectively, following 1hr of cell suspension, when compared to adherent cells. (Fig 2A: 18FDG: 3.03±1.06 × 104 vs. 1.74±0.23 × 105 counts/mg protein in suspended and adherent cells respectively, p<0.001; Fig 2B: ATP: 2.19±0.27 vs. 3.87±0.65 nmol/mg protein in suspended and adherent cells respectively; p<0.005). The ADP/ATP ratio increased from 0.27 in adherent rCDCs, to 1.1 and 1.8 in suspended rCDCs at 1hr and 3hrs respectively, indicating progressive bioenergetic deficits with increasing periods of cell suspension.
Fig 2. Cell dissociation impairs bioenergetics.
(A) 18FDG uptake and (B) ATP levels in 18–24 hr adherent and 1hr suspended CDCs following dissociation using trypsin(C) OCR and ECAR are significantly reduced in suspended CDCs (D) Re-plating after suspension for 1hr and 6hrs results in restoration of cellular metabolism, when assessed at 24hrs (n=6) (E) Oligomycin reduces OCR without affecting ATP levels in adherent CDCs (n=3) (F) Iodoacetate but not Oligomycin reduced ATP levels in adherent CDCs, indicating dependence on glycolysis for ATP generation (n=6)
Oxygen consumption rate (OCR) which reflects oxidative phosphorylation was significantly reduced following 1hr of cell suspension (25±3 pmol/min in adherent rCDCs vs. 12±5 pmol/min in suspended rCDCs; n=3, p<0.01). Extracellular acidification rate (ECAR), which reflects glycolysis was also significantly reduced upon 1hr of cell suspension (16±2 mpH in adherent rCDCs vs. 10±3mpH in suspended rCDCs; n=3; p<0.05), indicating that cell suspension impairs cellular bioenergetics (Fig 2C).
Oxygen consumption rate and extracellular acidification rate returned to normal at 24hrs, following re-plating of viable cells suspended for 3hrs and 6hrs, indicating that the metabolic derangements following cell suspension are reversible and linked to cell adhesion (Fig 2D).
Oligomycin, an inhibitor of mitochondrial F1-F0 ATP synthase, reduced OCR by 59 ± 2% (p<0.001; n=3) in adherent CDCs (Fig 2E), without affecting cellular ATP levels (Fig 2F); in contrast, iodoacetate, an inhibitor of glycolysis reduced ATP levels by 88±3% (p<0.001; n=6) despite the presence of glucose (Fig 2F), indicating dependence of CDCs on glycolysis for ATP synthesis, evidence for the Warburg effect.
In vitro studies
We measured the effect of cell dissociation/suspension on the signal derived from hNIS and firefly luciferase labeling of rCDCs in vitro, in the presence of either IMDM medium (Invitrogen) or PBS containing glucose/Ca2+/Mg2+ and PBS/saline.
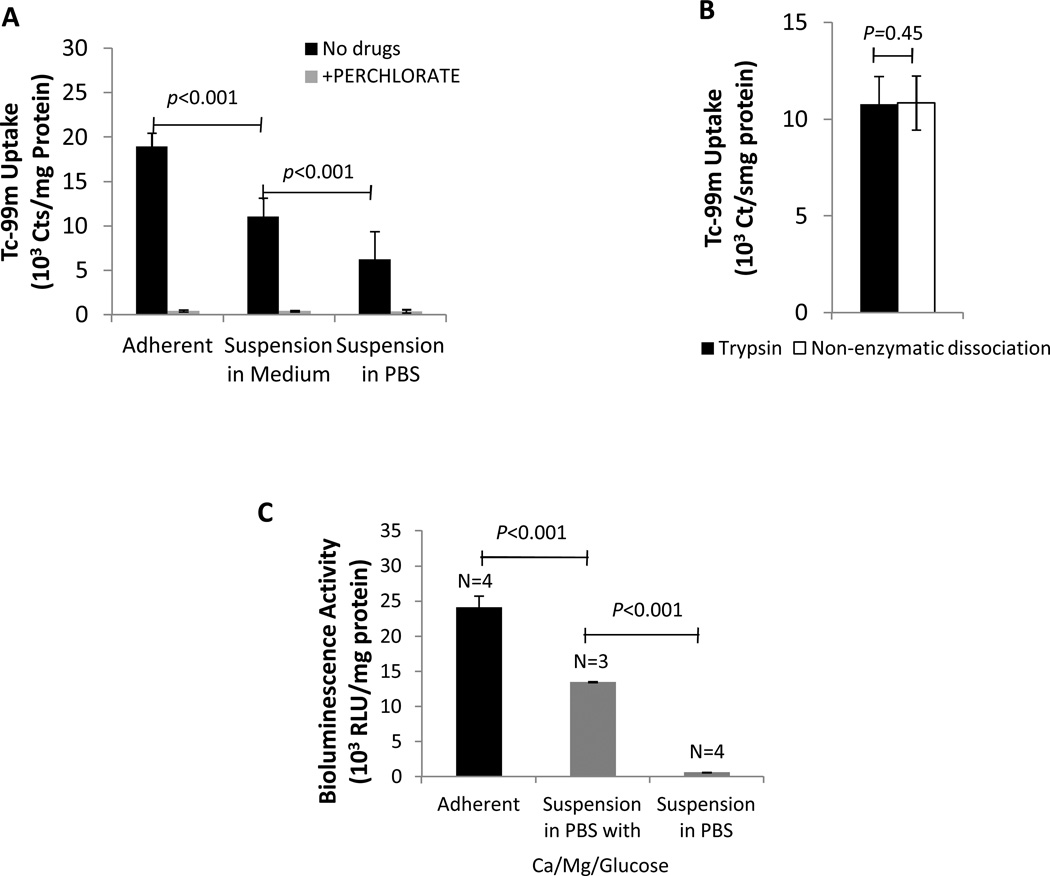
Trypsinization followed by suspension of NIS+ CDCs for 1hr in regular culture medium (IMDM) decreased 99mTc-pertechnetate uptake by 42% (1.10±0.21 × 104 vs. 1.89±0.15 × 104 counts/mg protein in suspended and adherent rCDCs respectively; p<0.001) (Fig 3A); suspension in PBS resulted in an additional decrease in 99mTc-pertechnetate uptake by 43% (6.23±3.14 × 103 counts/mg protein; p<0.001 vs. rCDCs suspended in IMDM, Fig 3A). Since hNIS is a surface protein that may be degraded by trypsin, we measured 99mTc-pertechnetate uptake in suspended hNIS+ rCDCs dissociated using trypsin or non-enzymatic dissociation solution that contains EDTA, but no enzymes. We found that 99mTc-pertechnetate in suspended rCDCs dissociated using trypsin was similar to that obtained by non-enzymatic dissociation (1.08±0.14 × 104 vs. 1.07±0.14 × 104 counts/mg protein, p=0.45), indicating that trypsin-mediated NIS protein degradation does not underlie the decreased 99mTc-pertechnetate uptake observed in NIS+ rCDCs following suspension (Fig 3B).
Fig 3. In vitro 99mTc-pertechnectate uptake and BLI.
(A) 99mTc-pertechnectate uptake is reduced by cell dissociation/suspension and inhibited by perchlorate (B) 99mTc-pertechnectate uptake was similar in suspended cells dissociated using trypsin and non-enzymatic dissociation solution (C) In vitro BLI signal is significantly higher in adherent cells, when compared to suspended cells. Suspension in PBS containing Ca2+, Mg2+, glucose results in a significantly higher signal than suspension in PBS.
In vitro BLI revealed a 44% reduction in signal (compared to adherent rCDCs) following suspension of fluc+ rCDCs for 1hr in saline/PBS containing glucose, Ca2+and Mg2+ (1.35±0.008 × 104 vs. 2.41±0.16 × 104 RLU/mg protein in suspended and adherent cells respectively; p< 0.01), and further reduction by 95% when suspended in PBS/saline (6.21±0.23 ×102 RLU/mg protein, p<0.01) (Fig 3C).
In vivo studies
Since cell dissociation/suspension decreased 99mTc uptake and BLI in vitro, we investigated the effect of cell dissociation/suspension on in vivo 99mTc uptake and BLI, acutely following cell transplantation, when the transplanted cells are likely to still be in suspension,23 and at 24hrs following transplantation, when live cells are expected to have attached to the cardiac extracellular matrix,24 and non-adherent cells have undergone anoikis (and hence are unable to generate a molecular imaging signal).
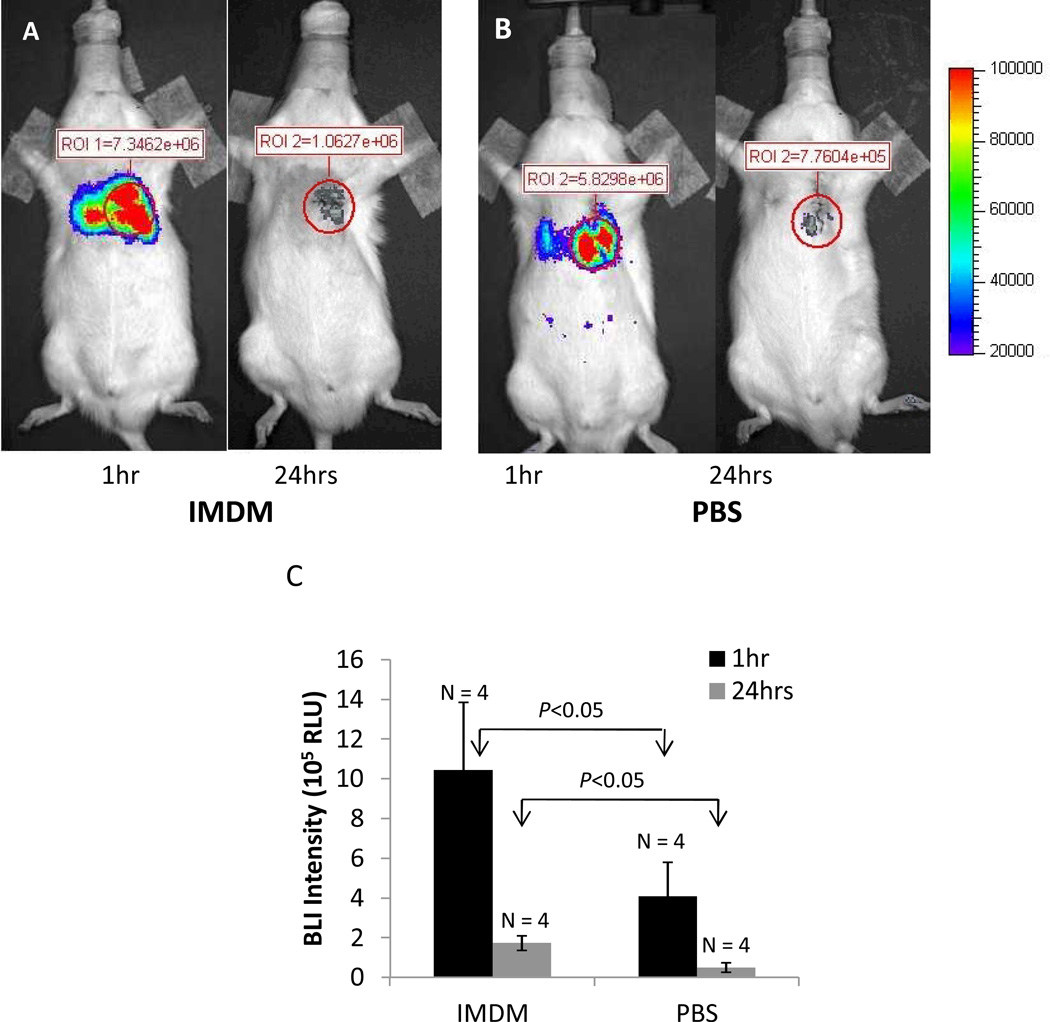
The in vivo BLI signal was higher at 1hr than at 24hrs. Importantly, at both time points, the in vivo BLI signal was lower when rCDCs were suspended in PBS/saline, when compared to the glucose-containing medium IMDM (4.09 ±1.73 × 105 vs. 1.05±0.34 × 106 RLU at 1hr for PBS and IMDM respectively, p<0.05; and 5.04±2.40 × 104 vs. 1.73±0.37 × 105 RLU for PBS and IMDM respectively at 24hrs; p<0.05) (Fig 4 A, B, C), indicating that presence of substrates in the cell suspension medium improves the sensitivity of in vivo BLI.
Fig 4. Bioluminescence imaging (BLI) following intra-myocardial transplantation of fLuc+ CDCs.
Representative in vivo BLI images in rats reveal higher signal when CDCs are suspended in IMDM medium (A) when compared to PBS (B) at 1hr and 24hrs following cell transplantation (AP view). (C) Bar graphs summarizing in vivo BLI results at 1hr and 24hrs following transplantation reveal that signal is significantly higher at 1hr, when compared to 24hrs. Suspension in IMDM medium results in a significantly higher signal at both time points
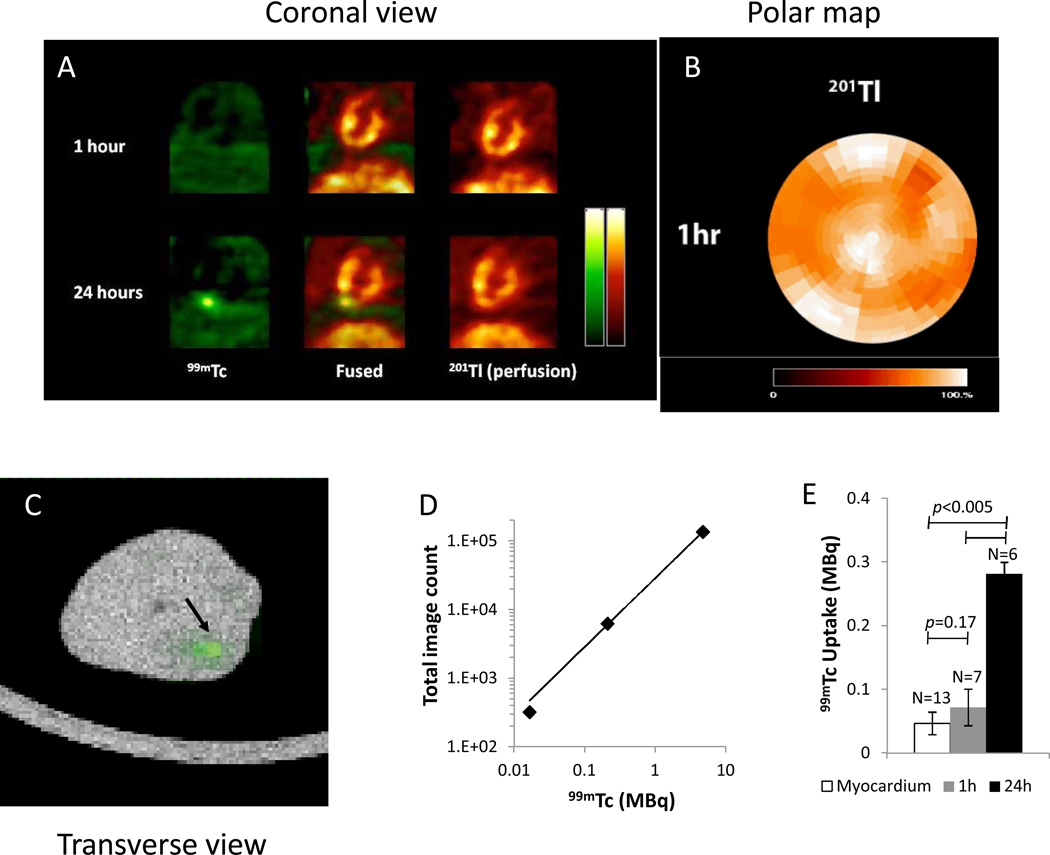
NIS+ rCDCs were suspended in IMDM to optimize their metabolism in suspension. In vivo dual isotope SPECT-CT imaging of intra-myocardially-injected NIS+ rCDCs suspended in IMDM revealed that NIS+ rCDCs were clearly visible 24hrs post-transplantation, but not 1hr post-transplantation in infarct and non-infarct models of cell transplantation (Fig 5A); perfusion imaging confirmed good perfusion at the site of cell injection in the non-infarcted animals (Fig 5B) and ex vivo SPECT imaging confirmed the presence of cells at the 1hr time point (Fig 5C). Calibration factor was calculated to be 3.37×10−5 MBq/i.i. from the dose response plot (Fig 5D). Signal quantification revealed that the in vivo 99mTc-pertechnetate uptake ratio was significantly greater at 24hrs, compared to 1hr in both non-infarct (0.05±0.01MBq at 1hr vs. 0.23±0.05MBq at 24 hrs) and infarct (0.09±0.02MBq at 1hr vs. 0.33±0.12MBq at 24 hrs) rat models (p<0.005; Fig 5E). Similarly, the ex-vivo 99mTc-pertechnetate uptake ratio was higher at 24hrs (0.01MBq at 1hr vs. 0.12±0.001MBq at 24 hrs).
Fig 5. In vivo dual isotope SPECT/CT imaging following intra-myocardial transplantation of NIS+CDCs.
(A) Representative coronal images of in vivo imaging (perfusion/201Tl in red and 99mTc-pertechnectate in green), 1hr and 24hrs following cell transplantation into normal myocardium reveals distinct 99mTc-pertechnectate uptake signal from transplanted cells at 24hrs, but not at 1hr. (B) Corresponding polar plot of 201Tl perfusion reveals normal perfusion 1hr post-transplantation into normal myocardium. (C) Representative SPECT/CT co-registered image of explanted rat heart at 1hr following transplantation reveals an area of 99mTc-pertechnetate uptake (indicated by arrow). (D) Dose response plot for 99mTc was obtained by dual isotope SPECT scanning, using a rat size phantom containing several doses of 99mTc-pertechnetate and 201Tl. (E) Summary of in vivo 99mTc-pertechnetate uptake in infarct and non-infarct animals, imaged at 1hr and 24hrs following cell transplantation confirms significantly increased signal at 24hrs (0.07±0.03MBq at 1hr vs. 0.28±0.18MBq at 24h). Cell-derived signal at 1hr is similar to the background signal from myocardium (0.05±0.02MBq).
Longitudinal, in vivo SPECT/CT imaging of NIS+ rCDCs and BLI of fluc+ rCDCs over 7days revealed progressive cell loss from day1 to 7 post-transplantation (Supplemental Fig I). Based on our in vitro studies which reveal that cellular metabolism is stable after adhesion for 24hrs (Supplemental Fig II), we believe that factors other than cell adhesion play a role in cell loss at later time points following transplantation.
Confirmation of Engraftment
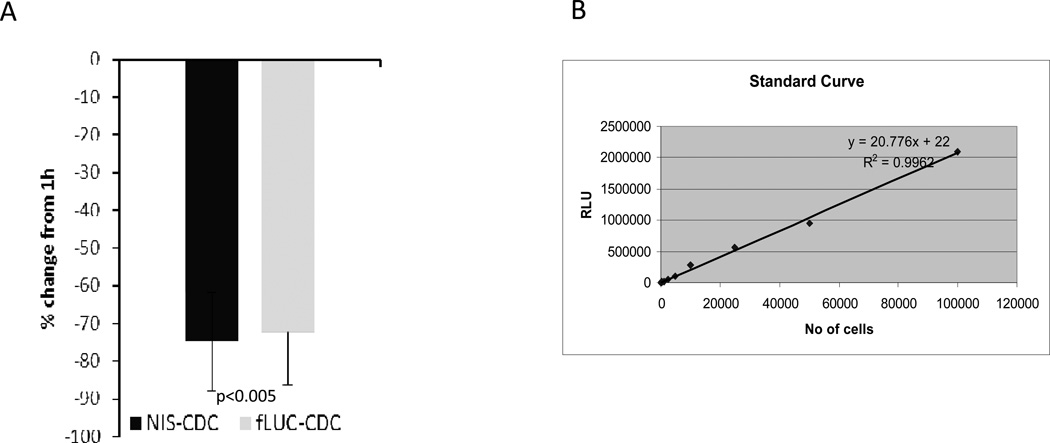
Ex vivo luciferase assay (n=12) using fluc+ rCDCs, and quantitative PCR for the male-specific SRY gene using NIS+ rCDCs (n=8) in separate sets of animals revealed a significant reduction in cell number (−72.39%±14.10% for fluc+ rCDCs and −74.77%±13.13% for NIS+ rCDCs) between 1hr and 24hrs post-transplantation (Fig 6A, B), confirming similar amounts of in vivo cell loss in both groups in the first 24hrs.
Fig 6. Confirmation of engraftment following intra-myocardial transplantation of fLuc+ and NIS+ rCDCs.
(A) Similar amounts of cell loss were observed between 1hr and 24hrs following intra-myocardial transplantation of fLuc+ and NIS+ rCDCs (P<0.005). (B) Standard curve correlating ex vivo luciferase activity in rat heart homogenates with known numbers of fLuc+ rCDCs.
DISCUSSION
This is the first study to report a link between stem cell adhesion, bioenergetics and the molecular imaging signal. The novel results of this study are as follows: 1) CDCs exhibit the Warburg effect (aerobic glycolysis); 2) cell adhesion is an important regulator of cellular metabolism and the molecular imaging signal derived from hNIS and luciferase; 3) in vivo 99mTc-pertechnetate uptake is higher at 24hrs following transplantation of cell suspensions (when compared to 1hr), despite fewer cells at this time point.
NIS imaging
Reporter gene strategies are useful in the longitudinal assessment of engraftment because transplanted cell viability is required for gene expression.1 We used a self-inactivating, 3rd generation lentivirus and the constitutively active cytomegalovirus virus (CMV) promoter which results in integration of the expression cassette into the host genome and high levels of transgene expression. Previous studies by our group have shown that expression of firefly luciferase or the human NIS gene in CDCs at an MOI of 20, did not affect CDC viability or function.4, 21 NIS promotes cellular uptake of iodide or 99mTc-pertechnetate and Na+, driven by the trans-membrane sodium gradient,14 which is generated by the Na+-K+-ATPase. Remarkably, after ectopic NIS expression, only cells over-expressing NIS will transport 99mTc-pertechnetate or iodine-124 (124I) after intravenous injection of these tracers, permitting noninvasive, longitudinal monitoring of stem cell engraftment by single-photon emission computed tomography (SPECT) and positron emission tomography (PET), respectively.4, 25, 26
In vivo 99mTc-pertechnetate uptake was low at 1hr following cell transplantation and increased at 24hrs, despite significant cell loss during this period. This finding is not due to impaired perfusion at the injection site at 1hr or due to cell proliferation, based on our perfusion results in non-infarcted animals at 1hr following transplantation (Fig 5B) and quantitative PCR for the SRY gene using NIS+ CDCs (Fig 6). We attribute this result to impairment of bioenergetics in suspended CDCs based on our in vitro studies in adherent and suspended cells.
For in vivo studies, hNIS+ rCDCs were dissociated using trypsin, suspended in IMDM and injected intra-myocardially. We used IMDM rather than PBS/saline as the vehicle for our in vivo experiments because in vitro studies revealed that 99mTc-pertechnetate uptake was higher when cells are suspended in IMDM, when compared to PBS/saline (Fig 3A). Our in vitro studies also confirmed that 99mTc-pertechnetate uptake was similar in CDCs dissociated using trypsin or non-enzymatic dissociation solution (Fig 3B), indicating that impairment of cellular bioenergetics, rather than trypsin-mediated degradation of NIS is the dominant mechanism underlying this phenomenon.
Cell dissociation and suspension resulted in significant down-regulation of glucose uptake, cellular metabolism and ATP levels (Fig 2A, B). Our in vitro studies also indicate a progressive increase in the cellular ADP/ATP ratio from 1hr to 3hrs in suspension, which would result in a lower Gibbs free energy availability from ATP hydrolysis, limiting Na+-K+ ATPase function, that in turn reduces trans-membrane Na+ gradient and consequently 99mTc-pertechnetate uptake – this could explain the very low in vivo 99mTc-uptake in the immediate post-transplantation period (the time interval between cell dissociation and completion of in vivo SPECT/CT (NIS) imaging was ~2–3 hrs). Transplanted cells would be expected to adhere to the cardiac extracellular matrix (ECM) over the 24hr period post-transplantation, resulting in improved bioenergetics which would translate into improved 99mTc-pertechnetate uptake-in vitro studies confirmed increased 99mTc-pertechnetate uptake in NIS+ CDCs, cultured on adhesive surfaces for 18–24 hrs, when compared to suspended cells. Cells that do not attach in vivo would undergo anoikis, which would explain some of the cell loss in the first 24hrs. Other mechanisms underlying cell loss could be progressive cell egress from the transplantation site via lymphatics and coronary veins5 (an effect that is more pronounced when cells are injected into contractile, non-infarcted myocardium) and cell death due to oxidative stress, inflammation or other mechanisms.27 Increase in the in vivo SPECT signal despite significant cell loss probably occurred because the magnitude of the increase in 99mTc-pertechnetate uptake of CDCs surviving at 24hrs greatly exceeded the effects of cell loss over the same time period.
Bioluminescence imaging
BLI-fluc imaging is based on the oxidation of the substrate D-luciferin by luciferase, a reaction that requires oxygen, magnesium, and ATP, and results in a red-shifted light emission (wavelength: 500–700nm), which can be detected using a camera.28 Since the luciferase reaction is directly dependent on ATP and Mg2+, composition of the cell suspension solution had a profound influence on the BLI signal. We found that the BLI signal generated by cells suspended in IMDM was higher than when suspended in PBS/saline at 1hr and 24hrs following transplantation. These differences could be related to differences in the in vivo cell survival and/or metabolism between the 2 groups. Further studies are needed to test whether cell suspension in solutions that contain substrates, Mg2+ and Ca2+ are superior to PBS/saline with respect to cell engraftment.
As in the case of 99mTc-pertechnetate uptake, the in vitro BLI signal was also higher in fluc+ rCDCs that were adherent for 18–24 hrs, compared to suspended cells in our in vitro studies. Hence, transplanted fluc+ rCDCs would also be expected to attach to cardiac ECM and improve cellular bioenergetics and light generation by luciferase over the first 24hrs following transplantation. However, the BLI signal was higher at 1hr, compared to 24hrs post-transplantation. This discrepancy in the in vivo molecular imaging signal at 24hrs, between in vivo SPECT and BLI is not due to differences in the in vivo survival of fluc+ CDCs and NIS+CDCs because cell loss in the first 24hrs was similar in the 2 groups (Fig 6A). We attribute the different results at 24hrs, using NIS and luciferase, to the dependence on O2 for generation of the luciferase signal, but not the NIS signal. Our in vitro metabolism studies reveal that stem cells rely primarily on glycolysis for ATP generation, a phenomenon referred to as the Warburg effect. Furthermore, inhibition of the mitochondrial F1-F0 ATP synthase did not affect ATP levels (Fig 2F), in contrast to cell dissociation which rapidly depressed ATP levels (Fig 2B). Since signal derived from NIS relies on the trans-membrane Na+ gradient, which is dependent on cellular ATP levels, it would be expected to increase with cell adhesion at 24hrs, following in vivo cell transplantation. However, the luciferase reaction is dependent on O2 as well as ATP. Low O2 tension at the transplantation site due to tissue damage from injection29, 30 combined with cell loss during this interval would be expected to impair recovery of the BLI signal but not the NIS signal at 24hrs.
Future implications for stem cell imaging
We chose 1hr as the first time point for our in vitro and in vivo studies because 1hr post-transplantation is often chosen as the baseline time point for longitudinal assessment of engraftment in experimental models of cell transplantation. We picked 24hrs as the 2nd time point, because it is unclear from the literature whether the signal at 1hr or 24hrs should be used as the baseline signal for longitudinal follow up of engraftment using molecular imaging.
With in vivo BLI, the BLI signal was higher at 1hr, compared to 24hrs post-transplantation and hence, more accurately represented cell fate in the first 24hrs following transplantation. However, the NIS-derived signal, using suspended cells was higher at 24hrs when compared to 1hr following cell transplantation despite significant cell loss. This result has important implications if NIS is used for longitudinal assessment of engraftment. Based on our results, the NIS-derived signal immediately post-transplantation, using suspended cells, is more reflective of cellular bioenergetics than actual cell number, when compared to the signal at 24hrs. Hence, it could confound quantification of engraftment in the first 24hrs, using SPECT-CT imaging of NIS+ rCDCs. This phenomenon may be harnessed to monitor the effects of small molecules and tissue engineered scaffolds on transplanted cell bioenergetics in the immediate post-transplantation period.
Study limitations
This is the first report of impaired bioenergetics by cell dissociation/suspension-further in depth studies are needed to understand the signaling pathways underpinning this phenomenon. Tissue engineered matrices containing Arg-Gly-Asp (RGD) motifs31, 32 that promote encapsulated cell attachment to the matrix,33 or cell-cell adhesion by generation of cell aggregates may abrogate the down-regulation of cellular metabolism in stem cell suspensions, and need to be tested.
We limited this study to rat CDCs because we have extensive experience with stem cell isolation, gene transduction and molecular imaging using this cell type. However, we believe that these results are not specific to CDCs and will be reproducible in other adherent cell types, e.g. mesenchymal stem cells (MSCs), embryonic stem cells and endothelial progenitor cells. Unfortunately, we were unable to transduce commercially available human MSCs using this 3rd generation lentivirus and hence were unable to test this clinically important cell type.
Technical limitations precluded cell sorting to exclude dead cells, in cell suspensions, prior to in vitro experiments at 1hr and measurement of transplanted cell bioenergetics in vivo, to verify our in vitro results. However, using flow cytometry, we consistently observe an ~15% reduction in cell viability which is much less than the reduction of cellular ATP levels, respiratory measurements, 99mTc uptake and in vitro BLI, indicating that cell suspension rather than decrease in cell viability is the primary cause of the observed depression of cellular bioenergetics.
CONCLUSION
Cell dissociation impairs cellular bioenergetics, resulting in reduced 99mTc-pertechnetate uptake and BLI signal. Since cell survival, proliferation and function are intimately linked to metabolism,34 BLI and NIS imaging could be useful tools for in vivo optimization of bioenergetics and engraftment of transplanted cells.
Supplementary Material
Novelty and Significance.
What Is Known?
Stem cell engraftment in the heart is low and results in small functional benefit.
Molecular imaging is useful to study in vivo stem cell biology and optimize engraftment.
Cell survival, proliferation and function are intimately linked to metabolism.
What New Information Does This Contribute?
Stem cell dissociation and suspension impairs cellular bioenergetics, resulting in reduction of the molecular imaging signal obtained by Single photon emission computer tomography (SPECT) and bioluminescence imaging (BLI) of cardiosphere-derived cells (CDCs) labeled with the sodium-iodide symporter (NIS) and luciferase, respectively.
Impairment of cellular bioenergetics in dissociated NIS+ cells can confound quantification of engraftment by in vivo SPECT imaging in the first 24h after transplantation.
SPECT imaging of NIS-labeled cells may be useful for in vivo optimization of bioenergetics and engraftment in transplanted stem cells.
Cell transplantation in the heart is limited by low engraftment. We used in vivo molecular imaging by SPECT and BLI to examine engraftment of CDCs in the first 24h after transplant. Using a combination of in vitro studies of cell viability, metabolism and function, and in vivo longitudinal imaging, we demonstrate a link between CDC dissociation/suspension, bioenergetics and the molecular imaging signal obtained by NIS (SPECT imaging) and firefly luciferase labeling (BLI) of transplanted cells. Specifically, we found that cell dissociation/suspension reversibly impairs cellular metabolism and ATP levels, resulting in decreased 99mTc-pertechnetate uptake and BLI signal in suspended cells, when compared with adherent cells. This translated into increased 99mTc-pertechneteate uptake (SPECT signal) at 24h, compared with 1h post-transplantation, despite considerable cell loss during this interval. However, the BLI signal decreased between 1h and 24h post-transplantation, and was reflective the cell loss during this period. Because the BLI signal depends on ATP, Mg2+ and O2, while the NIS-derived signal depends only on ATP, we propose that SPECT imaging of NIS+ cells may be useful for in vivo optimization of cellular bioenergetics and engraftment in small/large animal models and humans.
ACKNOWLEDGEMENTS
We are grateful to Jianhua Yu, Catherine Foss, PhD and Miguel Aon, PhD for helpful advice, James Fox and Gilbert Green for technical assistance.
SOURCES OF FUNDING
This study was funded by AHA-BGIA and NIH RO1 HL092985. Dr. Angel Chan was supported by NIH T32HL07227 Training Grant. Dr. Xiaoping Lin was partially supported by the China Scholarship Council. Dr. Styliani Vakrou was supported by a grant from the Hellenic Society of Cardiology. Kirubel Woldemichael was supported by an NIH diversity fellowship.
Non-standard Abbreviations
- CDCs
Cardiosphere-derived cells
- HSV-tk
Herpes simplex virus thymidine kinase
- Fluc
Firefly luciferase
- hNIS
Human sodium-iodide symporter
- polyHEMA
poly-2hydroxyethyl methacrylate
- PBS
Phosphate buffered saline
- ROI
Regions of interest
- CF
Calibration factor
- OCR
Oxygen consumption rate
- ECAR
Extracellular acidification rate
- ECM
Extracellular matrix
- SPECT
Single-photon emission computed tomography
- BLI
Bioluminescence imaging
- MSCs
Mesenchymal stem cells
- CMV
Cytomegalovirus virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Chan AT, Abraham MR. Spect and pet to optimize cardiac stem cell therapy. J Nucl Cardiol. 2012;19:118–125. doi: 10.1007/s12350-011-9485-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 3.Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, Phelps ME, Larson SM, Balatoni J, Finn R, Sadelain M, Tjuvajev J, Blasberg R. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terrovitis J, Kwok KF, Lautamaki R, Engles JM, Barth AS, Kizana E, Miake J, Leppo MK, Fox J, Seidel J, Pomper M, Wahl RL, Tsui B, Bengel F, Marban E, Abraham MR. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamaki R, Yu J, Fox J, Pomper M, Wahl RL, Tsui BM, O'Rourke B, Bengel FM, Marban E, Abraham MR. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiosphere-derived cells. J Nucl Cardiol. 2011;18:443–450. doi: 10.1007/s12350-011-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobert N, Britten M, Assmus B, Berner U, Menzel C, Lehmann R, Hamscho N, Schachinger V, Dimmeler S, Zeiher AM, Grunwald F. Transplantation of progenitor cells after reperfused acute myocardial infarction: Evaluation of perfusion and myocardial viability with fdg-pet and thallium spect. Eur J Nucl Med Mol Imaging. 2004;31:1146–1151. doi: 10.1007/s00259-004-1490-4. [DOI] [PubMed] [Google Scholar]
- 7.Bindslev L, Haack-Sorensen M, Bisgaard K, Kragh L, Mortensen S, Hesse B, Kjaer A, Kastrup J. Labelling of human mesenchymal stem cells with indium-111 for spect imaging: Effect on cell proliferation and differentiation. Eur J Nucl Med Mol Imaging. 2006;33:1171–1177. doi: 10.1007/s00259-006-0093-7. [DOI] [PubMed] [Google Scholar]
- 8.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 9.Schop D, Janssen FW, van Rijn LD, Fernandes H, Bloem RM, de Bruijn JD, van Dijkhuizen-Radersma R. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng Part A. 2009;15:1877–1886. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 10.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide symporter (nis): Characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, Welling A, Hofmann F, Oostendorp RA, Gansbacher B, Nekolla SG, Bengel FM, Botnar RM, Schwaiger M. Combined reporter gene pet and iron oxide mri for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med. 2009;50:1088–1094. doi: 10.2967/jnumed.108.060665. [DOI] [PubMed] [Google Scholar]
- 16.Bonios M, Chang CY, Pinheiro A, Dimaano VL, Higuchi T, Melexopoulou C, Bengel F, Terrovitis J, Abraham TP, Abraham MR. Cardiac resynchronization by cardiosphere-derived stem cell transplantation in an experimental model of myocardial infarction. J Am Soc Echocardiogr. 2011;24:808–814. doi: 10.1016/j.echo.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 19.Chirila TV, Constable IJ, Crawford GJ, Vijayasekaran S, Thompson DE, Chen YC, Fletcher WA, Griffin BJ. Poly(2-hydroxyethyl methacrylate) sponges as implant materials: In vivo and in vitro evaluation of cellular invasion. Biomaterials. 1993;14:26–38. doi: 10.1016/0142-9612(93)90072-a. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 21.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 1077 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth AS, Kizana E, Smith RR, Terrovitis J, Dong P, Leppo MK, Zhang Y, Miake J, Olson EN, Schneider JW, Abraham MR, Marban E. Lentiviral vectors bearing the cardiac promoter of the na+-ca2+ exchanger report cardiogenic differentiation in stem cells. Mol Ther. 2008;16:957–964. doi: 10.1038/mt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 24.Zvibel I, Smets F, Soriano H. Anoikis: Roadblock to cell transplantation? Cell Transplant. 2002;11:621–630. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi T, Anton M, Seidl S, Huisman M, Reder S, Bengel F, Botnar R, Schwaiger M. Localization and viability assessment of transplanted cells using genetic labeling with the human sodium/iodine symporter gene and magnetic labeling with iron oxides. J NUCL MED MEETING ABSTRACTS. 2007;48:1P. [Google Scholar]
- 26.Higuchi T, Anton M, Saraste A, Ahrens K, Seidel S, Pelisek J, Nekolla S, Bengel F, Botnar R, Schwaiger M. Assessment of stem cell enhancement strategy utilizing reporter gene imaging. J NUCL MED MEETING ABSTRACTS. 2008;49:29P-b. [Google Scholar]
- 27.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Almeida PE, van Rappard JR, Wu JC. In vivo bioluminescence for tracking cell fate and function. Am J Physiol Heart Circ Physiol. 2011;301:H663–H671. doi: 10.1152/ajpheart.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ. Incomplete retention after direct myocardial injection. Catheter Cardiovasc Interv. 2002;55:392–397. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- 30.Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: Implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 31.D'Souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (rgd): A cell adhesion motif. Trends Biochem Sci. 1991;16:246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti E. Rgd and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 33.Chang CY, Chan AT, Armstrong PA, Luo HC, Higuchi T, Strehin IA, Vakrou S, Lin X, Brown SN, O'Rourke B, Abraham TP, Wahl RL, Steenbergen CJ, Elisseeff JH, Abraham MR. Hyaluronic acid-human blood hydrogels for stem cell transplantation. Biomaterials. 2012;33:8026–8033. doi: 10.1016/j.biomaterials.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerman PS, Fox CJ, Thompson CB. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem Sci. 2004;29:586–592. doi: 10.1016/j.tibs.2004.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.