Abstract
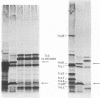
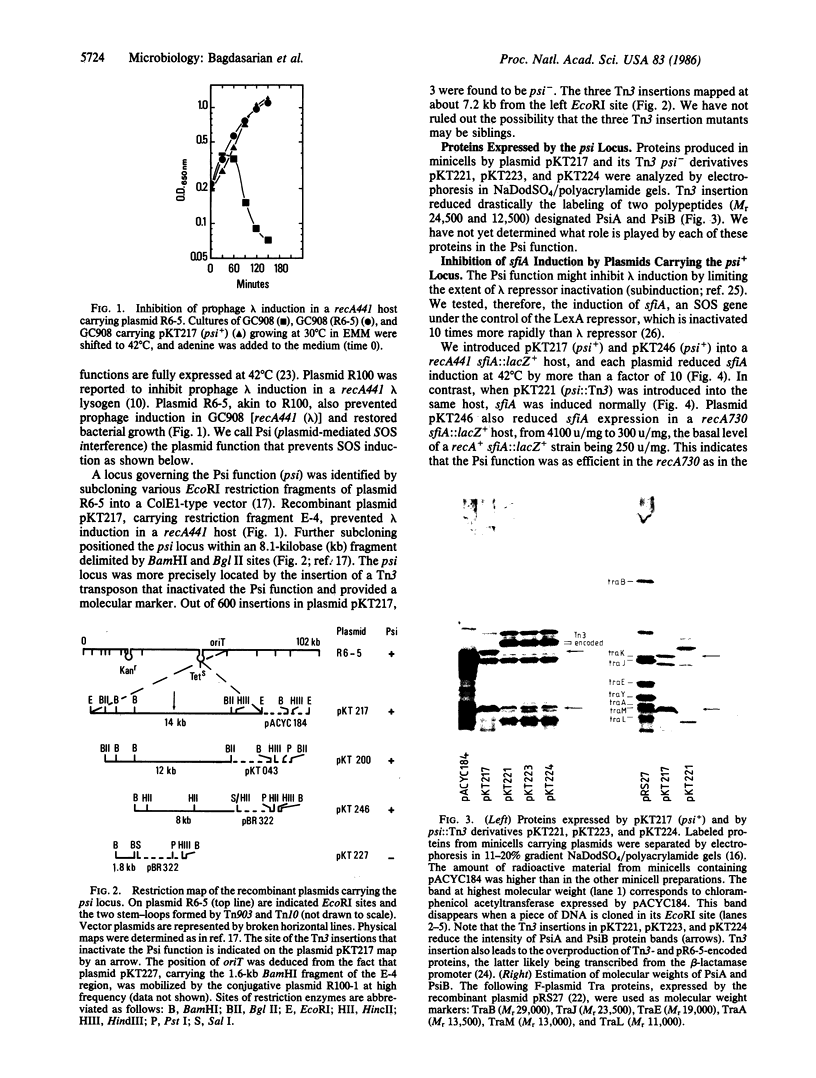
Plasmid R6-5 contains a locus whose product inhibits induction of sfiA and prophage lambda in a recA441 mutant at 42 degrees C and in a recA+ host after treatment with nalidixic acid. This plasmidic SOS-inhibition locus (psi) is situated on an 8.1-kilobase DNA fragment near oriT, the origin of plasmid R6-5 conjugational transfer. Loss of the Psi function, resulting from the insertion of Tn3 into psi+, greatly reduced the synthesis of two proteins, designated PsiA (Mr 24,500) and PsiB (Mr 12,500). Using host cells in which there was an inactive LexA repressor, we found that Psi function does not act by interfering with the expression of the SOS pathway. The Psi function may affect the generation of an SOS signal. We postulate that during the course of evolution, the Psi function has been selected in some conjugative plasmids so as to permit them to transfer single-stranded DNA without generating an SOS signal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailone A., Brandenburger A., Lévine A., Pierre M., Dutreix M., Devoret R. Indirect SOS induction is promoted by ultraviolet light-damaged miniF and requires the miniF lynA locus. J Mol Biol. 1984 Nov 5;179(3):367–390. doi: 10.1016/0022-2836(84)90071-8. [DOI] [PubMed] [Google Scholar]
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Bailone A., Sommer S., Devoret R. Mini-F plasmid-induced SOS signal in Escherichia coli is RecBC dependent. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5973–5977. doi: 10.1073/pnas.82.17.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brandenburger A., Bailone A., Lévine A., Devoret R. Gratuitous induction. J Mol Biol. 1984 Nov 5;179(3):571–576. doi: 10.1016/0022-2836(84)90082-2. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A. M., Smith G. R. Role of Escherichia coli RecBC enzyme in SOS induction. Mol Gen Genet. 1985;201(3):525–528. doi: 10.1007/BF00331350. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Devoret R., Pierre M., Moreau P. L. Prophage phi 80 is induced in Escherichia coli K12 recA430. Mol Gen Genet. 1983;189(2):199–206. doi: 10.1007/BF00337804. [DOI] [PubMed] [Google Scholar]
- Dutreix M., Bailone A., Devoret R. Efficiency of induction of prophage lambda mutants as a function of recA alleles. J Bacteriol. 1985 Mar;161(3):1080–1085. doi: 10.1128/jb.161.3.1080-1085.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. Effect of suppressors of SOS-mediated filamentation on sfiA operon expression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):169–175. doi: 10.1128/jb.153.1.169-175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Little J. W. The SOS regulatory system: control of its state by the level of RecA protease. J Mol Biol. 1983 Jul 15;167(4):791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Roberts J. W. RecA protein--promoted lambda repressor cleavage: complementation between RecA441 and RecA430 proteins in vitro. Mol Gen Genet. 1984;198(2):25–34. doi: 10.1007/BF00328696. [DOI] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo J. E., Moreau P. L., Blanco M., Devoret R. Restoration of RecA protein activity by genetic complementation. Mol Gen Genet. 1984;195(1-2):83–89. doi: 10.1007/BF00332728. [DOI] [PubMed] [Google Scholar]
- Resnick J., Sussman R. Escherichia coli single-strand DNA binding protein from wild type and lexC113 mutant affects in vitro proteolytic cleavage of phage lambda repressor. Proc Natl Acad Sci U S A. 1982 May;79(9):2832–2835. doi: 10.1073/pnas.79.9.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S., Bailone A., Devoret R. SOS induction by thermosensitive replication mutants of miniF plasmid. Mol Gen Genet. 1985;198(3):456–464. doi: 10.1007/BF00332939. [DOI] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Slocombe P. M. Plasmid incompatibility: cloning analysis of an incFII determinant of R6-5. Nature. 1978 May 4;273(5657):27–32. doi: 10.1038/273027a0. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisemann J. M., Funk C., Weinstock G. M. Measurement of in vivo expression of the recA gene of Escherichia coli by using lacZ gene fusions. J Bacteriol. 1984 Oct;160(1):112–121. doi: 10.1128/jb.160.1.112-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. From Gainesville to Toulouse: the evolution of a model. Biochimie. 1982 Aug-Sep;64(8-9):549–555. doi: 10.1016/s0300-9084(82)80086-2. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Kogoma T. Involvement of the activated form of RecA protein in SOS mutagenesis and stable DNA replication in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7539–7543. doi: 10.1073/pnas.81.23.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]